Abstract
This work studied the enzymatic synthesis of fatty acid ethyl esters (FAEE) for potential use as biodiesel via simultaneous esterification and transesterification of acid oil from macaúba in a solvent-free system. A fermented and dry babassu cake with lipase activity from Rhizomucor miehei was used as biocatalyst. FAEE content above 85% was achieved after 96 h of reaction with enzyme loading of 13 U per g of oil, 120 mmol of hydrous ethanol (95% ethanol and 5% water)/20 mmol of oil (molar ratio ethanol:oil of 6:1), at 40 °C. After two consecutive enzymatic reactions, 90.8 wt% FAEE content was obtained. These results demonstrate a promising transesterification/esterification method for FAEE production from an acid and low-cost oil and the process has potential to decrease the costs of enzymatic biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fatty acid mono-alkyl esters, currently produced in large scale by alkali-catalyzed transesterification of vegetable oil and fats with short chain alcohols such as methanol and ethanol, commonly serve as biodiesel [1, 2]. However, this conventional technology has negative aspects such as several downstream processes (to remove the alkaline catalyst from the product). Biodiesel industry has already making extensive use of low-cost/low-quality feedstocks [3]. However, most of the fatty acid alkyl esters that are possible source of biodiesel are still obtained from refined vegetable oils such as soybean and rapeseed oils. In this way the price of the raw material can correspond up to 80% of total biodiesel production cost, which contributes for the high biodiesel price [2].
Lipase-catalyzed processes have the potential to overcome the drawbacks of alkaline catalysis and have received growing interest because of the higher quality of products generated and the environmental benefits [4, 5]. Besides advantages of mild operating conditions and easier product purification [2], lipases catalyze both transesterification of triglycerides (TAG) and esterification of free fatty acids (FFA), which allows the application of cheaper feedstocks with high FFA content, including waste oils, unrefined vegetable oils, and animal fats. However, the high costs of the biocatalyst, especially when commercial enzymes are used, and the longer reaction times of enzymatic reactions, remain as drawbacks for the application of enzymes in biodiesel production [5]. Maximization of the biocatalyst reuse [6], use of whole-cells systems [7], and direct use of fermented solids as biocatalysts [8–11] have been studied as alternatives to overcome this drawback and make enzymatic biodiesel more cost-competitive. The third strategy has advantages over the first two because it may use inexpensive and plentiful agro-industrial residues as solid substrate for the microorganism growth and lipase production, and it also avoids expensive steps of lipase extraction, purification and immobilization [8–11].
Macaúba (Acrocomia aculeata) is a palm native from South American tropical forests, and its productivity reaches between 1500 and 5000 kg of oil per hectare per year [4]. The fruit pulp oil is rich in oleic acid [4] which contributes to generate a good quality biodiesel. But this oil generally has a high acid value, which makes it inappropriate for use as food or feedstock in conventional biodiesel production process [1, 4]. However, the exploration of this alternative non-edible oil crop for biodiesel can offer opportunities for big and small-scale farmers and rural oil producers, improving the use of regional resources and bringing environmental, economic, and social benefits [1, 4].
In a previous work, our group has reported an enzyme/enzyme hydroesterification process for biodiesel production from acid oil from macaúba pulp. The hydrolysis reaction was catalyzed by an enzyme obtained from dormant castor seedsand the esterification step was catalyzed by lipase from Rhizomucor miehei in the form of dry fermented solid [8]. The best result obtained in this system was 91% of esterification conversion after 8 h in a solvent-free system [8]. In the present study, we explore another approach for fatty acid esters production which consists in the direct addition of alcohol to the acid oil using this dry fermented solid with lipase activity from R. miehei obtained by SSF as biocatalyst. The simultaneous transesterification of TAG and esterification of FFA allows obtaining methyl or ethyl fatty esters (for potential use as biodiesel) in a single reaction step, which represents an improvement of the previous process. The composition of the final product was performed according to the standard methodologies published by ASTM, International, and ABNT (Associação Brasileira de Normas Técnicas—Brazilian Technical Standards Association).
Materials and Methods
Raw Material and Reagents
The acid oil from macaúba (A. aculeata) pulp was obtained from the fruits processing company PETROVASF (Montes Claros, Brazil). Babassu (Orbignya oleifera) cake, a solid residue from the babassu oil industry, was kindly provided by Tobasa S.A. (Tocantinópolis, Brazil). Anhydrous ethanol (99.8%), hydrous ethanol (95%), and methanol (99.8%), with analytical grade, were supplied by Sigma-Aldrich (St. Louis, MO, USA).All other reagents were of analytical grade.
Analysis of the Acidity, Water Content, and Fatty Acid Profile from Macaúba Acid Oil
The acidity, i.e., the percentage of FFA (wt%) in the oil, was analyzed by titration with NaOH 0.04 mol/L using a Mettler DG 20 autotitrator. The acidity was established according to equation:
where M is the NaOH concentration, V the volume of NaOH (mL), mm the molecular mass of the predominant fatty acid (g), and m is the sample mass (g).
The water content was determined by titration using a coulometric Karl Fischer titrator.
The fatty acid profile of macaúba oil was determined by gas chromatography after chemical methylation of oil. Fatty acid methyl esters (FAMEs) (1µL) were analyzed on a GC-2010 (Shimadzu Co., Kyoto, Japan) equipped with a flame ionization detector (FID) and an Omegawax capillary column (30 m × 0.25 mm × 0.25 µL). The detector and injector were set at 250 and 260 °C, respectively. The oven program was as follows: 200 °C for 5 min, then heated at 20 °C min−1 to 260 °C and kept constant at 260 °C for 6 min. Helium was used as carrier gas at a 2 mL.min−1 flow rate. The ester content (in mass percent) was determined by relative peak areas. A standard solution with 37 FAMEs of 37 different fatty acids (Supelco 37, Sigma-Aldrich) was used to identify the peaks in the chromatograms.
Biocatalyst Production
The fermented solid was obtained by solid-state fermentation (SSF) of babassu cake, using a strain of R. miehei (IDAC accession number 071113-01). Fermentations were carried out according to Aguieiras et al. [8], during 72 h. The fermented solids were dried in a lyophilizer until achieving a moisture content of less than 3 wt%, stored at 4 °C until use and named “dry fermented solid”. Before use the solids were macerated to reduce the incidence of large particles.
Enzyme Activity Determination
Hydrolytic activity of the biocatalyst was measured using p-nitrophenyl-laurate (pNP-laurate) as substrate according to Gutarra et al. [12]. After fermentation and lyophilization, enzymes were extracted with phosphate buffer (0.1 mol.L−1, pH 7.0) as described by Gombert et al. [13]. The supernatant was used for hydrolytic activity determination. One unit (U) of hydrolytic activity was defined as the amount of enzyme that releases 1 µmol of p-nitrophenol per minute under the assay conditions. The hydrolytic activity of the fermented solid used in this work was 43 U g−1.
Simultaneous Alcoholysis and Esterification Reactions
Reactions were carried out in closed 20 mL batch reactors magnetically stirred and thermostated. The medium was solvent-free and composed of substrates (macaúba oil and alcohol) and biocatalyst. Reaction progress was monitored by taking samples at fixed intervals that were analyzed for methyl/ethyl ester content. Reactions were carried out in duplicateusing 15 g of macaúba oil and13 U of dry fermented solid per g of oil, at 40 °C during 96 h. This temperature was chosen due to good results obtained in previous work [8]. The influences of the following parameters were studied: molar ratio ethanol to oil, type of alcohol, strategy of ethanol addition (single or stepwise addition), and amount of water in the reaction medium.
Fatty Acid Ethyl/Methyl Esters Content Determination
The fatty acid ethyl/methyl esters content resulted from the enzymatic alcoholysis and esterification was determined on a GC-2010 (Shimadzu Co.) equipped with a flame ionization detector (FID) and an Omegawax capillary column (30 m × 0.25 mm × 0.25 µL). The CG conditions were the same used in the section “Analysis of the Acidity, Water Content and Fatty Acid Profile from Macaúba Acid Oil”. Samples of 20 µL were diluted in 480 μL of an internal standard solution of methyl heptadecanoate (9 mg.mL−1) in heptane and 1 μL was injected with a split ratio of 1:20. The ester content was quantified using the peak area of the internal standard. The analyses were performed in duplicate and the standard deviation was less than 5%.
Medium Scale Fatty Acid Ethyl Esters Production
Reactions were carried out in two steps in 200 mL closed and thermostated reactors under mechanical stirring. The first reaction was carried out for 96 h and the medium was composed of anhydrous ethanol and macaúba oil in a molar ratio of 6:1 (added ½ at 0 h and at 24 h). At the end of the reaction, the product was extracted with hexane, filtered in filter paper, dried over anhydrous sodium sulfate, and concentrated in a rotary evaporator to remove both hexane and ethanol. This product was then used in a second transesterification/esterification reaction, carried out in the same conditions of the first reaction and using a fresh fermented solid as biocatalyst. After 96 h, the product was extracted with hexane and was subjected to the same purification steps of the first reaction. At fixed intervals, acidity and ethyl ester yield were determined. The composition of the final product was determined according to the following parameters: esters, free glycerol, total glycerol, monoglycerides, diglycerides, and triglycerides contents. These analyses were performed at Laboratory of Green Technologies (GREENTEC) located in the Federal University of Rio de Janeiro that is a laboratory licensed by ANP (National Agency of Petroleum, Natural Gas and Biofuels). FAEE-enriched product analyses were carried out according to the standard methodologies published by ASTM and ABNT. FFA content (acidity) was determined according to Sousa et al. [14] and water content was determined by titration using a coulometric Karl Fischer titrator. The results were compared with the values established by ANP Resolution Nº45 of 25/08/2014 [15] which establishes the specifications necessary for the sale of biodiesel in Brazil.
Results and Discussion
Analysis of the Acidity, Water Content, and Fatty Acid Profile from Macaúba Acid Oil
Macaúba oil contained 8 wt% FFA was used for all experiments except for the FAEE-enriched product characterization, in which an oil with acidity of 29 wt%, from a different harvest, was used. Water content in the oils with 8 and 29 wt% FFA were 929 ± 7.12 and 568 ± 6.8 mg.kg−1, respectively.
Table 1 shows that the macaúba acid oil had mainly oleic acid (67.3%).
The fatty acid composition of the raw material influences the physicochemical properties of the generated biodiesel fuel [16]. High concentrations of esters derived from saturated fatty acids improve the oxidation stability. However, high amount of long chain fatty acids leads to increase in cloud point and cold-filter plugging point. On the other hand, a biodiesel with high quantities of unsaturated bonds is more chemically unstable, which causes low oxidation stability, degradation, and polymerization. A biodiesel with high quantities of monounsaturated esters presents better results as a fuel [16–18]. In view of this, macaúba oil can be considered a good choice as feedstock for biodiesel production, due to it high content of oleic acid.
The average molecular weight of macaúba oil, calculated from the fatty acid composition, was 870.5 g.mol−1. This molecular weight neglects the FFA content of the oil.
Influence of Anhydrous Ethanol (99.8%) Concentration in Reaction Medium
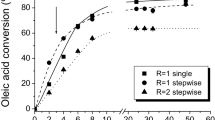
The influence of the molar ratio of ethanol to oil using a fermented solid as biocatalyst was studied using the oil with 8 wt% of acidity and the following molar ratios: 3:1, 4:1, and 6:1. Anhydrous ethanol (99.8%) was added at the beginning of the reaction. As can be seen in Fig. 1, although the ester yield obtained after 96 h has been similar for the molar ratios 3:1 and 4:1, the initial rate obtained using the lowest molar ratio was higher. This result may be related to the denaturation of the lipase in the presence of high concentrations of this polar alcohol due its interaction with the water molecules necessary for the maintenance of the native lipase structure [5, 19, 20]. The lowest rate and final ester yield were obtained in the molar ratio 6:1.
To avoid lipase deactivation, and at the same time maintain an excess of alcohol to shift the reaction equilibrium toward FAEE synthesis, the addition of ethanol in steps was evaluated. Four feed conditions were studied: (1) ethanol addition in equal parts at times 0 and 6 h, with a final molar ratio equal to 4:1, (2) ethanol addition in equal parts at times 0 and 6 h, with a final molar ratio equal to 6:1, (3) ethanol addition in equal parts at times 0 and 24 h, with a final molar ratio equal to 6:1 and (4) 1/6 of the total ethanol volume added at 0, 1, and 2 h and 1/2 added at 4 h, with a final molar ratio equal to 6:1. The latter condition was chosen based on previous results obtained by our group in the esterification of FFA with ethanol [8]. The initial rates obtained in the conditions 1, 2, and 4 were similar, and ethyl esters contents above 75% were attained after 96 h. The addition of the total amount of ethanol at 0 and 6 h (condition 2) resulted in lower ester yields. The final molar ratio ethanol to oil of 6:1 with addition of ethanol at 0 and 24 h (condition 3) was chosen for further studies, since the rates obtained in this condition were slightly higher in relation to those obtained in the other conditions evaluated (Fig. 2).
Influence of the Type of Alcohol
The use of methanol and hydrous ethanol (5% water) as acyl acceptors was studied, and the results were compared with those previous results obtained using anhydrous ethanol (99.8%) (Fig. 3). The ester content obtained in the reactions carried out with methanol was 28% lower than that attained using ethanol as acyl acceptor; a behavior also observed for other biocatalysts [20, 21]. These results can be explained considering that methanol is a more polar solvent and can promote greater enzyme inactivation [21].
Kinetic of transesterification reaction of macauba acid oil (8 wt% acidity) with methanol (99.8%), anhydrous ethanol (99.8%), and hydrous ethanol (95%). The reactions were conducted with 13 U of fermented solid per g of oil and alcohol:oil molar ratio of 6:1 (1/2 of alcohol added at 0 h and 1/2 added at 24 h), at 40 °C. Standard deviation <5%
The reaction conducted with hydrous ethanol showed higher ester yields (85% in 96 h) than that with anhydrous ethanol (76% in 96 h). This result can be related to the high formation of the organic/aqueous interface required for enzyme activity in the presence of hydrous ethanol. Similar results were attained by Deng et al. [22] who reported that the immobilized lipases from R. miehei and Thermomyces lanuginosus showed better activity when ethanol (96% v/v) was used as acceptor in ethanolysis of sunflower oil when compared to anhydrous ethanol.
The use of homogenous alkaline catalysts to produce fatty acid alkyl esters requires water-free alcohol in order to avoid saponification and consequently obtain good separation of glycerol co-product. Industrially, most of the biodiesel produced worldwide is made using methanol, since the anhydrous methanol is relatively cheaper and more reactive when compared to the anhydrous ethanol [23]. Ethanol produced from biomass is an azeotropic mixture (95% ethanol and 5% water). So, its use in conventional biodiesel production requires dehydration, an expensive process that increases overall product costs and disfavors its application in the alkaline transesterification route [23]. Moreover, hydrous ethanol could be a good alternative for enzymatic biodiesel production because it is less toxic than methanol and is produced on a large scale from renewable sources, such as corn or sugarcane [2].
Influence of the Water Concentration in the Reaction Medium
Some studies reported that the addition of water in the reaction medium can improve the enzymatic transesterification rates [10, 24, 25]. The optimal concentration of water in enzymatic transesterification reactions varies widely according to the type of enzyme and the composition of the reaction medium. The previous results obtained with hydrous ethanol (Fig. 3) showed a positive influence of water in the enzymatic transesterification/esterification reactions. Therefore, the influence of this parameter in the reactions carried out with fermented solid as biocatalyst was studied in more details in reactions carried with anhydrous ethanol (99.8% of ethanol). Water was added in the reaction medium in the following concentrations: 1, 2, 3, 5, 10, and 15 wt% (in relation to the mass of oil). The results are shown in Fig. 4.
Kinetic of transesterification reaction of macauba acid oil (8 wt% acidity) with different water concentrations (1, 2, 3, 5, 10, and 15 wt%). The reactions were conducted with anhydrous ethanol (99.8%), 13 U of fermented solid per g of oil and molar ratio ethanol:oil of 6:1 (1/2 of alcohol added at 0 h and 1/2 added at 24 h), at 40 °C. Standard deviation <5%
The addition of 1, 2, and 3 wt% of water improved the ester yields compared to the reaction carried out using anhydrous ethanol without addition of water. An ester yield above 84% was obtained in 96 h, which was similar to that obtained using hydrous ethanol.
Water contents higher than 5 wt% promoted a reduction in the reaction rates and in the final ester yield. Water is substrate for hydrolysis reaction, thus when it presented in high concentrations the reaction equilibrium is shifted towards enzymatic hydrolysis of the ethyl esters. Thus, the results obtained showed that the presence of small amounts of water (<3%) has a positive effect in the transesterification/esterification reactions carried out using fermented solid as biocatalyst and macaúba acid oil (acidity of 8%) as raw material.
A macaúba oil with higher acidity (29 wt%) from a different harvest was also used for enzymatic FAEE-enriched product synthesis. A comparative study using anhydrous ethanol and hydrous ethanol showed that the results obtained in these two conditions were similar; contrary to the observed, when a raw material with lower acidity was used (additional data are given in the Electronic Supplementary Materials). Considering this result, for a raw material with higher FFA content, it is better to use an alcohol free of water since the water, by-product of the esterification reaction, is produced in higher amount.
Medium Scale Fatty Acid Ethyl Esters Production
The acid oil from macaúba pulp (29 wt% FFA) was used in two consecutive enzymatic transesterification/esterification reactions with anhydrous ethanol. After the first reaction (96 h), a product with 79% of ethyl ester content and acidity of 3% was attained.
The product of the first step was used as raw material in a second reaction to consume the residual TAG and FFA. The results of both reactions are presented in Fig. 5.
Kinetic of transesterification reaction (ethyl ester content and acidity, i.e., FFA) of macauba acid oil (29 wt% acidity) with anhydrous ethanol (99.8%) in the first reaction (R1) and second consecutive reactions (R2). The reactions were conducted with 13 U of fermented solid per g of oil and molar ratio ethanol:oil of 6:1 (1/2 of alcohol added at 0 h and 1/2 added at 24 h), at 40 °C. Standard deviation <5%
After 96 h of reaction 90.8% ester content was obtained. This result was similar to that attained by Fernandes et al. [9] and Liu et al. [10]. in a medium with co-solvent using fermented solid as catalyst. Although the total reaction time is long (two reactions of 96 h), it can be seen that, in accordance with reaction rates, the first reaction could have been carried out in 24 h, and the second stage, in 48 h (total of 72 h).
Although the transesterification route can be carried out in just one step and, therefore, is apparently simpler, the ester content obtained by simultaneous transesterification/esterification was lower than that achieved in our previous study that employed a hydroesterification route [8]. Generally enzymatic transesterification reactions are slower and require long reaction times to obtain high yields compared to enzymatic esterifications. Fernandes et al. [9] obtained an ester yield of 94% after 18 h in esterification reactions of oleic acid with ethanol in organic reaction media catalyzed by lyophilized fermented solids containing Burkholderia cepacia lipase. The same fermented solid was also used as biocatalysts in transesterification reactions (ethanolysis) of soybean oil, and 40% conversion was obtained after 48 h, in a medium with co-solvent [26].
Figure 5 also shows that the reactions rates of the first reaction carried out using an oil with 29 wt% FFA were better than one showed the Fig. 2 (using an oil with 8 wt% of acidity). After 24 h, the ester yields obtained employing anhydrous ethanol were 42 and 70% for the reactions carried out using the oils with 8 (Fig. 2) and 29 wt% FFA (Fig. 5), respectively. In Fig. 5, the contribution of esterification to the formation of FAEE was higher, since it was used an oil with higher amount of FFA, which can explain this result. Moreover, some studies have reported that lipases exhibit higher activity in substrates with high FFA content due to the better solubility of alcohol in FFA, which prevents the toxic effects of the alcohol on the enzyme [27–30].
Furthermore, the effect of the glycerol by-product on the enzyme should be considered. Glycerol has low solubility in oil and high tendency to adsorb onto the enzyme surface, causing problems of mass transfer and leading to a decrease in reaction rate and operational stability of the biocatalyst [20]. Similarly, the accumulation of glycerol on the biocatalyst surface may reduce the activity of the lipases in the fermented solid.
The final product had 1.4% FFA and a water content of 310 mg.kg−1. Table 2 shows the composition of the crude FAEE-enriched product obtained by enzymatic transesterification/esterification and the respective specifications published by ANP Resolution 45 from 25/08/2014 [15].
Glycerol, monoglycerides, and diglycerides contents did not meet ANP specification, contrarily to the product obtained by the enzyme/enzyme hydroesterification [8]. It is important to note that, although the ASTM D6584 is concerned with analyzing of methyl esters, the same methodology can be used to analyze glycerides from ethyl esters, as previously reported [33]. The presence of glycerides (mono- and diglycerides) was expected, since ethyl esters were obtained by transesterification route and the crude product was not purified by downstream steps. Thus, a purification process would contribute to a better biodiesel quality.
Considering the current soybean oil price (US$1100 per ton) and macaúba oil price (US$600 to US$800 per ton) and the well-developed ethanol industry in Brazil, besides the use of a low cost enzymatic biocatalyst [34], the process of this work would favor the use of the enzymatic method and of entirely renewable raw materials for obtaining FAEE-enriched product serving as a potential biodiesel source, which could be named a 100% green biodiesel [4].
Conclusions
This work demonstrated an enzymatic process for the FAEE production from a low-cost raw material substrate (acid oil) and enzymatic biocatalysts (fermented solid) through simultaneous esterification of FFA and transesterification of TAG. The biocatalyst was able to convert oils with different acidities into ethyl esters (biodiesel) in a single reaction step. The use of hydrous ethanol is an economically viable alternative, since reduces costs with dehydration of ethanol, and provides the use of this biobased alcohol that is widely produced in countries such as Brazil and the USA. A FAEE content of 90% was reached and the composition of the final product was determined. Purification of the final biodiesel would help to meet the required quality for the product and to reduce the need of expensive additives. Since the high price of the commercial biocatalysts is one of the major obstacles for industrialization of enzymatic biodiesel synthesis, the process developed in this work shows promising results for FAEE production using a biocatalyst produced over an agro-industrial residue.
References
Lopes DC, Neto AJS, Mendes AA, Pereira DTV (2013) Economic feasibility of biodiesel production from Macaúba in Brazil. Energy Econ 40:819–824
Robles-Medina A, González-Moreno PA, Esteban-Cerdán L, Molina-Grima E (2009) Biocatalysis: towards ever greener biodiesel production. Biotechnol Adv 27:398–408
Monthly Biodiesel Production Report (2017) U.S. Energy Information Administration.http://www.eia.gov/biofuels/biodiesel/production/table3.pdf. Accessed 16 Jan 2017
Navarro-Díaz HJ, Gonzalez SL, Irigaray B, Vieitez I, Jachmanián I, Hense HJ, Oliveira V (2014) Macaúba oil as an alternative feedstock for biodiesel: characterization and ester conversion by the supercritical method. J Supercrit Fluid 93:130–137
Aguieiras ECG, Cavalcanti-Oliveira ED, Freire DMG (2015) Current status and new developments of biodiesel production using fungal lipases. Fuel 159:52–67
Rodrigues RC, Volpato G, Wada K, Ayub MAZ (2008) Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. J Am Oil Chem Soc 85:925–930
Yan J, Zheng J, Li S (2014) A novel and robust recombinant Pichia pastoris yeast whole cell biocatalyst with intracellular overexpression of a Thermomyces lanuginosus lipase: preparation, characterization and application in biodiesel production. Bioresour Technol 151:43–48
Aguieiras ECG, Cavalcanti-Oliveira ED, de Castro AM, Langone MAP, Freire DMG (2014) Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel 135:315–321
Fernandes MLM, Saad EB, Meira JA, Ramos LP, Mitchell DA, Krieger N (2007) Esterification and transesterification reactions catalysed by addition of fermented solids to organic reaction media. J Mol Catal B Enzym 44:8–13
Liu Y, Li C, Wang S, Chen W (2014) Solid-supported microorganism of Burkholderia cenocepacia cultured via solid state fermentation for biodiesel production: optimization and kinetics. Appl Energ 113:713–721
Soares D, Pinto AF, Gonçalves AG, Mitchell DA, Krieger N (2013) Biodiesel production from soybean soapstock acid oil by hydrolysis in subcritical water followed by lipase-catalyzed esterification using a fermented solid in a packed-bed reactor. Biochem Eng J 81:15–23
Gutarra MLE, Godoy MG, Maugeri F, Rodrigues MI, Freire DMG, Castilho LR (2009) Production of an acidic and thermostable lipase of the mesophilic fungus Penicillium simplicissimum by solid-state fermentation. Bioresour Technol 100:5249–5254
Gombert AK, Pinto AL, Castilho LR, Freire DMG (1999) Lipase production by Penicillium restrictum in solid-state fermentation using babassu oil cake as substrate. Process Biochem 35:85–90
Sousa JS, Cavalcanti-Oliveira ED, Aranda DAG, Freire DMG (2010) Application of lipase from the physic nut (Jatropha curcas L.) to a new hybrid (enzyme/chemical) hydroesterification process for biodiesel production. J Mol Catal B Enzym 65:133–137
Resolução ANP Nº45, de 25/08/2014. https://www.legisweb.com.br/legislacao/?id=274064. Accessed 17 Jan 2017
Ribeiro BD, de Castro AM, Coelho MAZ, Freire DMG (2011) Production and use of lipases in bioenergy: a review from the feedstocks to biodiesel production. Enzyme Res 2011:1–16
Knothe G, Gerpen JV, Krahl J (2005) The biodiesel handbook. AOCS Press, Champaign
Cremonez PA, Feroldi M, Nadaleti WC, Rossia E, Feidena A, Camargo MP, Cremonez FE, Klajn FF (2015) Biodiesel production in Brazil: current scenario and perspectives. Renew Sustain Energy Rev 42:415–428
Shimada Y, Watanabe Y, Samukawa T, Sugihara A, Noda H, Fukuda H, Tominaga Y (1999) Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. J Am Oil Chem Soc 76:789–793
Hernández-Martín E, Otero C (2008) Different enzyme requirements for the synthesis of biodiesel: Novozym-35 and Lipozyme-TL IM. Bioresour Technol 99:277–286
Al-Zuhair S, Ling FW, Jun LS (2007) Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochem 42:951–960
Deng L, Xu X, Haraldsson GG, Tan T, Wang F (2005) Enzymatic production of alkyl esters through alcoholysis: a critical evaluation of lipases and alcohols. J Am Oil Chem Soc 82:341–347
Antczak MS, Kubiak A, Antczak T, Bielecki S (2009) Enzymatic biodiesel synthesis—key factors affecting efficiency of the process. Renew Energy 34:1185–1194
Cesarini S, Diaz P, Nielsen PM (2013) Exploring a new, soluble lipase for FAMEs production in water-containing systems using crude soybean oil as a feedstock. Process Biochem 48:484–487
Nie K, Xie F, Wang F, Tan T (2006) Lipase catalyzed methanolysis to produce biodiesel: optimization of the biodiesel production. J Mol Catal B Enzym 43:142–147
Salum TFC, Villeneuve P, Barea B, Yamamoto CI, Côcco LC, Mitchell DA, Krieger N (2010) Synthesis of biodiesel in column fixed-bed bioreactor using the fermented solid produced by Burkholderia cepacia LTEB11. Process Biochem 45:1348–1354
Véras IC, Silva FAL, Ferrão-Gonzales AD, Moreau VH (2011) One-step enzymatic production of fatty acid ethyl ester from high-acidity waste feedstocks in solvent-free media. Bioresour Technol 102:9653–9658
Watanabe Y, Shimada Y, Baba T, Ohyagi N, Moriyama S, Teria T, Tominaga Y, Sugihara A (2002) Methyl esterification of waste fatty acids with immobilized Candida antarctica lipase. J Oleo Sci 51:655–661
Watanabe Y, Pinsirodom P, Nagao T, Yamauchia A, Kobayashi T, Nishida Y, Takagi Y, Shimada Y (2007) Conversion of acid oil by-produced in vegetable oil refining to biodiesel fuel by immobilized Candida antarctica lipase. J Mol Catal B Enzym 44:99–105
Du W, Wang L, Liu D (2007) Improved methanol tolerance during Novozyme 435-mediated methanolysis of SODD for biodiesel production. Green Chem 9:173–176
EN (2003) Fat and oil derivatives. Fatty acid methyl esters (FAME). Determination of ester and linolenic acid methyl ester contents. Designated EN 14103:2003
ASTM (2013) Standard test method for determination of total monoglycerides, total diglycerides, total triglycerides, and free and total glycerin in B-100 biodiesel methyl esters by gas chromatography. Designated ASTM D6584-13e1
Dias AN, Kurz MHS, Fagundes CAM, Caldas SS, Clementin RM, D’Oca MGM, Primel EG (2014) Evaluation of ASTM D6584 method for biodiesel ethyl esters from sunflower oil and soybean/tallow mixture and for biodiesel methyl esters from tung oil and soybean/tung mixture. J Braz Chem Soc 25:1161–1165
Castilho LR, Polato CMS, Baruque EA, Sant’Anna GL Jr, Freire DMG (2000) Economic analysis of lipase production by Penicillium restrictum in solid-state and submerged fermentations. Biochem Eng J 4:239–247
Acknowledgements
This research received financial support from FAPERJ, CNPq, ANP, and PETROBRAS. The authors are also grateful to Dr. Donato Alexandre Gomes Aranda, of the “Laboratory of Green Technologies (GREENTEC)” located in Federal University of Rio de Janeiro, to kindly provide his laboratory for biodiesel analyses.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Aguieiras, E.C.G., Cavalcanti-Oliveira, E.D., de Castro, A.M. et al. Simultaneous Enzymatic Transesterification and Esterification of an Acid Oil Using Fermented Solid as Biocatalyst. J Am Oil Chem Soc 94, 551–558 (2017). https://doi.org/10.1007/s11746-017-2964-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-2964-4