Abstract
Epoxidized vegetable oils are desirable chemicals due to their eco-friendly characteristics and their being a major source of many green products. Ring opening is one of the ways to convert these epoxidized oils to some new intermediates. The use of mono-functional amines, alcohols, acid anhydrides and thioethers for epoxy ring opening has been reported in the literature. In this study, thioglycolic acid (TGA) bearing thiol and carboxylic acid as two different functional groups and methyl ester of thioglycolic acid (TGAME) were used. Currently, there is no reported literature describing epoxy ring opening using chemicals
bearing two different functional groups simultaneously. In this way, two new polyols were synthesized, one with TGA (polyol 1) and one with TGAME (polyol 2). FTIR and 1H- and 13C-NMR spectroscopy confirmed that the ring was opened by the carboxylic acid group of TGA, and the thiol group was not involved in the ring opening whereas the ring was opened by the thiol group in the case of TGAME.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to public interest and strict government regulations over petroleum based chemicals, there has been a constant demand for renewable and eco-friendly products [1]. Examples of such products involve lubricants, bio-based polymers [2], composites [3, 4] and nanocomposites [5]. Vegetable oils are good candidates for renewable and eco-friendly products after having been modified. One useful reaction for the modification of vegetable oils is the epoxidation of double bonds with hydrogen peroxide in acetic acid [6–8]. Epoxidized soybean oil is provided commercially at reasonable cost and is a starting material for chemical modifications due to its reactive epoxy groups. Ring opening reaction is utilized to introduce one or more hydroxyl groups into fatty acid chain of vegetable oils [9]. In this way, polyols with two or more hydroxyl groups are synthesized and these polyols are used as raw materials for the synthesis of polyurethanes where double or multiple hydroxyl group (functionality) is usually required [10]. Vegetable oil based polyols are synthesized by epoxy ring opening reactions which are carried out with amines, alcohols, carboxylic acids, acid anhydrides and thioethers [11, 12].
In this study, thioglycolic acid (TGA), which has two different functionality, i.e. thiol and carboxylic acid, was used for the ring opening of epoxidized soybean oil. In addition, methyl ester of TGA was used for comparison. Ring opening was confirmed using FTIR and 1H- and 13C-NMR spectroscopy. Differential scanning calorimetry (DSC) was performed for investigating the thermal behavior of the resulting products.
Experimental Procedures
Materials
Soybean oil was purchased from Sigma-Aldrich and used without any further purification. Glacial acetic acid (100 %) and hydrogen peroxide (30 % w/w in H2O) for epoxidation, thioglycolic acid (98 %) and methyl thioglycolate (95 %) for ring opening, perchloric acid (30 %), toluene, methylene chloride, sodium sulfate anhydride and sodium bicarbonate were purchased from Merck KGaA, Germany. All these chemicals were used as received.
Methods
FTIR spectra were recorded on a Shimadzu IR prestige-21 spectrophotometer equipped with an ATR device having a diamond crystal, in a scanning range of 650–4000 cm−1 for 25 scans at a spectral resolution of 4 cm−1. Data were collected and processed using IR solution software. 1H- and 13C-NMR spectra were recorded using a Shimadzu Prestige-21 (200 VCE) spectrophotometer operating at 300 and 75 MHz, respectively for data collection and processing, VNMR 6.1 C software was used. Solutions were prepared with deuterated chloroform (CDCl3, 99.8 atom % D) at 15 % concentration by volume.
Thermal properties were investigated using Mettler Toledo DSC1 200 W. Approximately 3 mg of samples were weighed in aluminium pans (40 µL) and were subjected to cooling-heating cycles from −90 to 25 °C at 10 °C min−1 under nitrogen with 50 mL min−1 as the flow rate.
The hydroxyl number was determined according to ASTM D-1957-86. In this method, a solution of acetic anhydride in pyridine was used to esterify the hydroxyl groups of polyol quantitatively and the released acetic acid is titrated with KOH. High hydroxyl value of polyol 1 indicated that thiol group is involved in hydroxyl determination as well, since they can also react with the anhydride and generate acetic acid. The epoxide number was measured according to ASTM D 1652-04, Test Method B. In this method, the solution of ESBO in methylene chloride was titrated with standard perchloric acid in the presence of an excess amount of tetraethylammonium bromide. A SV-10 Series Sine-wave Vibro Viscometer of the N&D Company was used to measure dynamic viscosity. The Tuning Fork Vibration Method with 30 Hz was applied and a two points calibration was made before measurements. Gel permeation chromatographic (GPC) analysis was performed at 30 °C on a Shimadzu prominence GPC system equipped with a RID-10A refractive index detector, an LC-20AD solvent delivery unit, a CTO-10AS column oven and a set of two columns, PSS SDV 5 µL 1000 Å and PSS SDV 5 µL 50 Å. THF (HPLC grade) was used as the mobile phase at 1.0 mL/min. The sample concentration was 2 mg/mL, and the injection volume was 50 µL. The calibration curve was made with seven polystyrene standards covering the molecular weight range from 162 to 33,500 Da (MW: 162, 690, 1470, 3250, 8900, 19,100 and 33,500 Da).
Epoxidation of Soybean Oil
Soybean oil was epoxidized according to the procedure reported in the literature [13].A solution of soybean oil (1.0 × 102 g, 0.12 mol), glacial acetic acid (25 g, 0.42 mol), amberlite (25 g) and toluene (40 ml) were heated to a constant temperature of 55 °C by stirring in a four-necked round-bottomed flask equipped with a thermometer, a stirrer and a reflux condenser. Then, 30 % H2O2 (79 g, 0.70 mol) was added slowly from an addition funnel and allowed to react at 55 °C for 7 h. The solution was then filtered and washed with distilled water to pH 7.0. The oil phase was dried with anhydrous sodium sulfate and then filtered. Finally toluene was removed in a vacuum oven at 80 °C. The yield of the reaction was determined as 91 %. The hydroxyl value and epoxide number of starting epoxidized soybean oil were measured before reaction with TGA and TGAME and were found as 14 mg KOH/g and 6.2 % oxirane oxygen in weight, respectively.
Ring Opening Reaction with Methyl Thioglycolate
The ring opening reaction of epoxidized soybean oil with methyl thioglycolate was carried out under a nitrogen atmosphere in a 500-ml, three-necked, round-bottomed flask equipped with a thermometer, a stirrer and a reflux condenser. Epoxidized oil (12 g, 0.013 mol) and methyl thioglycolate (6.9 g, 0.065 mol) were added to 250 mL of methylene chloride and heated to 45 °C. Then, perchloric acid (1.3 g) was added slowly from an addition funnel and allowed to react for 6 h. The solution was then washed with aqueous (5 %) sodium bicarbonate and the organic phase was dried with anhydrous sodium sulfate. Solvent was removed in a vacuum oven and a viscous liquid was obtained. Any unreacted TGAME was removed with a rotary evaporator at 10 mm Hg (45 °C for 1 h, followed by 80 °C for 1.5 h, respectively). The hydroxyl value of this polyol 2 was 165.93 mg KOH/g; and the yield was 80.4 %.
Polyol 2. 1 H NMR (500 MHZ, CDCl 3 , ppm): δ 5.3 ppm (OH proton), δ 4.9–5.0 ppm (CH proton adjacent to –O–CO–R), δ 4.0–4.2 ppm (CH2 protons of CH 2 CHCH 2 glycerine backbone), δ 3.6–3.8 ppm (CH3 protons at the acyl end), δ 3.4–3.6 ppm (CH proton of CH–OH), δ 3.2–3.4 ppm (CH 2 protons adjacent to –S–), δ 2.7 ppm (protons –CH–S–), δ 1.2–1.8 ppm (CH 2 protons of fatty acid chain), δ 0.8 ppm (CH3 protons at the end of fatty acid chain).
13 C NMR (125 MHZ, CDCl 3 , ppm): δ 173.2 ppm (carbonyl carbon of triacylglycerol), 68.9 and 62.1 ppm (CH and CH2 carbons of –CH2 CHCH2– glycerol backbone, respectively), 62.8 ppm (–CH– carbon adjacent to –OH group), 56.8 ppm (CH carbon α to –S–), 54.1 ppm (quaternary carboxylic carbon), 33.5 ppm (CH2 carbon adjacent to –S–), 22.6–34 ppm (multiple singlets from the fatty carbon chain), 26.8 ppm (CH2 carbon adjacent to –SH), 14.0 ppm (CH3 end carbon of the fatty acid chain), FTIR: 3416 cm−1 (characteristic absorption peak due to hydroxyl groups), 2855–2926 cm−1 (methylene asymmetric stretching), 1739 cm−1 (triglycerides carbonyl stretching), 1458 cm−1 (CH2 bending vibration), 1378 cm−1 (CH3 bending vibration), 1150–1265 cm−1 (stretching vibrations of C–O group in esters), 734 cm−1 (CH2 rocking vibration).
Ring Opening Reaction with Thioglycolic Acid
Modification of epoxidized soybean oil was carried out in a 50-ml, three-necked, round-bottomed flask equipped with a thermometer, a stirrer and a reflux condenser. Epoxidized oil (12 g, 0.013 mol) was added to thioglycolic acid (6.0 g, 0.065 mol) in a flask and heated to 120 °C under a nitrogen atmosphere [14]. Then, perchloric acid (1.3 g) was added slowly from an addition funnel and allowed to react at 120 °C for 18 h. Upon completion of the reaction, the solution was washed with deionized water until the pH of the water reached 7 and the organic phase was dried with anhydrous sodium sulfate. A viscous liquid was obtained. The hydroxyl value of this polyol 1 was 280 mg KOH/g; and the yield was 84 %. The higher hydroxyl value of polyol 1 compared to polyol 2 can be attributed to the thiol group since polyol 1 has pendant thiol groups which also react with acetic anhydride to form acetic acid during hydroxyl determination. It must be pointed out that mild reaction conditions (45 °C, 6 h) used for ring opening reaction with TGAME, was not enough to open the epoxy ring with TGA. After several experiments, by raising temperature, increasing time and removing the solvent, the required conditions were optimized. Each time ring opening reaction was checked by the disappearance of the characteristic epoxy peak of ESBO at 823–833 cm−1 in the FTIR spectrum.
Polyol 1. 1 H NMR (300 MHZ, CDCl 3 , ppm): δ 5.3 ppm (OH proton), δ 4.9 ppm (CH proton adjacent to –O–CO–R), δ 4.2 ppm (CH2 protons of CH 2 CHCH 2 glycerine backbone), δ 3.4–3.6 ppm (CH proton of CH–OH), δ 3.2 ppm (CH2 protons α to –SH), δ 1.2–1.8 ppm (CH 2 protons of fatty acid chain), δ 0.8 ppm (CH 3 protons at the end of fatty acid chain).
13 C NMR (75 MHZ, CDCl 3 ): δ 173.2 ppm (carbonyl carbon of triacylglycerol), δ 73.3 ppm (–HC–O–), 68.9 and 62.1 ppm (CH and CH2 carbons of –CH2 CHCH2– glycerol backbone respectively), δ 62.8 ppm (–CH– carbon adjacent to –OH group), 54.1 ppm (quaternary carboxylic carbon), 22.6–34 ppm (multiple singlets from the fatty carbon chain), 26.8 ppm (CH2 carbon adjacent to –SH), 14.0 ppm (CH3 end carbon of the fatty acid chain).
FTIR: 3480 cm−1 (characteristic absorption peak due to hydroxyl groups), 2855–2926 cm−1 (methylene asymmetric stretching), 1734 cm−1 (triglycerides carbonyl stretching), 1463 cm−1 (CH2 bending vibration), 1376 cm−1 (CH3 bending vibration), 1159–1265 cm−1 (stretching vibrations of C–O group in esters), 734 cm−1 (CH2 rocking vibration).
Results and Discussion
Soybean oil is a triglyceride mainly comprised of five different fatty acids, i.e. linoleic acid (51 %, two double bonds), oleic acid (25 %, one double bond), palmitic aid (11 %, saturated), linolenic (9 %, three double bonds) and stearic acid (4 %, saturated).
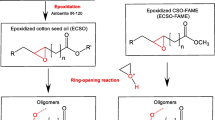
Our aim in this study was to open epoxidized soybean oil (ESBO) using chemicals bearing thiol and carboxylic acid functionalities simultaneously. For this purpose, soybean oil was epoxidized using peroxyacetic acid generated in situ by the reaction of glacial acetic acid with hydrogen peroxide in the presence of amberlite as the catalyst. In the next step, ring opening reactions of ESBO with TGA and TGAME were carried out. The reaction took place as shown in Fig. 1.
In the case of polyol 1, the epoxy ring was opened by the carboxylic acid group of TGA whereas it was opened by the thiol group of TGAME in the case of polyol 2.
FTIR Analysis
The FTIR spectra of ESBO and the soy-based polyols are shown in Fig. 2. Characteristic epoxy peaks of ESBO at 823 and 833 cm−1 (indicated by a circle), disappeared after the epoxy ring opening reaction. The absorption peaks at 3480 cm−1 in the spectrum of polyol 1 and 3419 cm−1 in the spectrum of polyol 2 (indicated by a circle) were attributed to the newly formed hydroxyl groups [15].
NMR Analysis
1H-NMR spectra of SBO, ESBO and the polyols are shown in Fig. 3. In the spectrum of ESBO new peaks corresponding to the –CH– protons of the epoxy ring and –CH2– protons alpha to the epoxy ring appeared at δ 2.8–3.2 and δ 1.4–1.6 ppm, respectively. Moreover, the intensity of the peak at δ 5.4 ppm corresponding methine protons in soybean oil decreased notably upon epoxidation [16]. However, a low epoxy number (6.2 %) indicated that some double bonds remained unchanged. The other peaks in the spectrum of ESBO, corresponded to terminal –CH3 groups (at 0.8–0.1 ppm), saturated –CH2 groups (at 1.1–1.4 ppm) and the protons of (–CH2–CH–CH2–) (at 4.1–4.3 and 5.2 ppm).
The disappearance of the characteristic multiple peaks of epoxy groups at 2.8–3.2 and 1.4–1.6 ppm in the spectrum of ESBO and the appearance of the large peak of OH at 5.3 ppm in the spectra of both polyol 1 and polyol 2 confirmed the quantitative epoxy ring opening reaction. The peak at 3.1–3.2 ppm in the spectrum of polyol 1 which was attributed to CH2 protons of –CH 2–SH, the –CH– proton of –CH–O–CO– Rester group appearing at 4.9–5.0 ppm and the peak at 5.3 ppm confirmed that the ring was opened by carboxylic acid group of TGA. In the spectrum of polyol 2, the new peaks at 3.3–3.4 and 3.4–3.6 ppm were attributed to –C(O)–O–CH3 of TGAME and the protons adjacent to hydroxyl group (–CH–OH), respectively. The new peaks appearing at 3.2–3.4 and 2.7 ppm which were attributed to –CH2 and –CH protons alpha to –S–, respectively, and terminal CH3 peak at 3.6–3.8 ppm [17], confirmed that the epoxy ring was opened via SH group of TGAME.
Figure 4 shows 13C-NMR spectra of SBO, ESBO, polyol 1 and polyol 2. The appearance of a new peak at 56 ppm confirmed epoxidation of SBO. New peaks in the spectra of polyol 1 and polyol 2 at 62.8 ppm were due to –CH– carbons adjacent to hydroxyl groups. In the spectrum of polyol 1, the appearance of new carbon peak at 73.3 ppm was attributed to –HC–O– and this confirmed that the ring opened by carboxylic acid group of TGA. In the case of polyol 2, the new peak at 56.8 ppm corresponding to –CH carbon alpha to –S– confirmed that the ring opened via the SH group of TGAME. The carbon peaks at 26.8 and 33.5 ppm were attributed to –H2C– adjacent to –SH– and –S– of polyol 1 and polyol 2, respectively. New carbon peak, which appeared at 176.1 ppm and did not exist on ESBO spectrum, can be attributed to carbonyl carbon of –C(O)–O–CH3 while the peak at 172.2 ppm can be attributed to –C(O) peak of soybean oil.
FTIR, 1H-NMR and 13C-NMR spectra are in agreement with the reported literature describing the ring opening reactions with chemicals that have thiol and carboxylic acid functional groups in their structures [6, 15, 16].
Differential Scanning Calorimetry Analysis
DSC analysis of polyols are shown in Fig. 5. Multiple melting point peaks of polyol 1 can be attributed to different crystal line structures. From Table 1, it is seen that melting points of polyol 1 shifted to higher temperatures compared with ESBO, due to increased intramolecular hydrogen bonding of OH groups and its melting point peaks were clearly defined [18]. The melting peak of polyol 2 at higher temperature (i.e. 16.2 °C) can be explained by increased regularity and higher hydrogen bonding of OH groups. Polyol 2 has also no SH groups to interact with OH groups.
GPC Analysis
The molecular weights were determined as: Polyol 1: 13500 g/mol (30.0 %, 15.3 min), 6400 g/mol (11.9 %, 14.3 min), 3900 g/mol (20.2 %, 13.7 min), 1800 g/mol (37.7 %, 12.5 min); polyol 2: 4970 g/mol (10.1 %, 14.2 min), 2995 g/mol (28.8 %, 14.6 min), 1787 g/mol (59.1 %, 15.4 min). The retention time of the samples in minutes decreased in the following order: ESBO (15.7), polyol 2 (15.4, 14.6, 14.2), polyol 1 (15.3, 14.3, 13.7). The GPC chromatograms of ESBO, polyol 1 and polyol 2 are shown in Fig. 6. It was observed that high molecular weight products formed indicating the presence of epoxy ring opening polymerization and chain coupling. The formation of higher molecular weight products in polyol 1 was probably due to higher extent of coupling which might result from the reaction of epoxy rings with thiol groups some of which remained intact during the reaction of TGA with ESBO. Due to its acid-catalyzed nature, epoxy ring opening would take place by protonation and therefore it is more favorable from the carboxylate moiety of TGA rather than thiol. The observed coupling, therefore, might be an outcome of those thiols reacting during and/or post reaction of carboxylate (Fig. 7).
Viscosity Measurement
It can be seen from the viscosity data (Table 2) that polyol 1 and polyol 2 have higher viscosities than ESBO. This viscosity increase may be due to a higher molecular weight resulting from the ring opening polymerization of polyols and may also be due to the free hydroxyl groups. Polyol 1 has a much higher viscosity than polyol 2 due to its higher molecular weight resulting from the high degree of chain coupling. GPC analysis confirms this higher molecular weight compared to polyol 2.
Conclusion
By the ring opening reaction of ESBO with chemicals containing two different functional groups, novel polyols were synthesized. When TGA was used, the epoxy ring was opened by the carboxylic acid group. When TGAME was used, the ring was primarily opened by the thiol group. Polyols were characterized by spectroscopic methods and different melting points and regularities were observed by thermal analysis. GPC and viscosity measurements revealed that high molecular weight products formed probably due to the polymerization of the epoxy groups. The presence of higher molecular weight products in polyol 1 as compared to polyol 1 might stem from some of the thiol groups of TGA initially remaining intact and then being involved in ring opening of other epoxy groups resulting in chain coupling.
References
Rhee I (1996) Evolution of environmentally acceptable hydraulic fluids. NLGI Spokesman 60:28–35
Thames SF, Blanton MD, Mendon S, Subramanian R, Yu H (1998) Surfactants and fatty acids: plant oils. In: Kaplan DL (ed) Biopolymers from renewable resources. Springer, Berlin, pp 267–271
Williams GI, Wool RP (2000) Composites from natural fibers and soy oil resins. Appl Compos Mater 7:421–432
Liu ZS, Erhan SZ, Calvert PD (2007) Solid free form fabrication of epoxidized soybean oil/epoxy composite with bis or polyalkyleneamine curing agents. Compos Appl Sci Manuf 38:87–93
Lu Y, Larock RC (2006) Novel biobased nanocomposites from soybean oil and functionalized organoclay. Biomacromolecules 7:2692–2700
Campanella A, Rustoy E, Baldessari A, Baltanas MA (2010) Lubricants from chemically modified vegetable oils. Bioresour Technol 101:245–254
Fogassy G, Ke P, Figueras F, Cassagnau P, Rouzeau S, Courault V (2011) Catalyzed ring opening of epoxides: application to bioplasticizers synthesis. Appl Catal General 393:1–8
Scala L, Wool RP (2002) Effect of FA Composition on epoxidation kinetics of TAG. J Am Oil Chem Soc 79:373–378
Lin Yang B, Yang L, Dai H, Yi A (2008) Kinetic Studies on oxirane ring cleavage of epoxidized soybean oil by methanol and characterization of polyols. J Am Oil Chem Soc 85:113–117
Narine SS, Kong X, Bouzidi L, Sporns P (2007) Physical properties of polyurethanes produced from polyols from seed oils: elastomers. J Am Oil Chem Soc 84:55–63
Biswas A, Adhvaryu A, Gordon SH, Erhan SZ, Willet JL (2005) Synthesis of diethylamine functionalized soybean oil. J Agric Food Chem 53:9485–9490
Lee KW, Hailan C, Yinhua J, Kim YW, Chung KW (2008) Modification of soybean oil for intermediates by epoxidation, alcoholysis and amidation. Korean J Chem Eng 25:474–482
Park SJ, Jin FL, Lee JR (2004) Synthesis and thermal properties of epoxidized vegetable oil. Macromol Rapid Commun 25:724–727
Sharma BK, Advaryu A, Liu Z, Erhan SZ (2006) Chemical modification of vegetable oils for lubricant applications. J Am Oil Chem Soc 83:129–136
Sharma BK, Advaryu A, Liu Z, Erhan SZ (2006) Synthesis of hydroxy thio-ether derivatives of vegetable oil. J Agric Food Chem 54:9866–9872
Sharma BK, Liu Z, Adhvaryu A, Erhan SZ (2008) One-pot synthesis of chemically modified vegetable oils. J Agric Food Chem 56:3049–3056
Desroches M, Caillol S, Auvergne R, Boutevin B, David G (2012) Biobased cross-linked polyurethanes obtained from ester/amide pseudo-diols of fatty acid derivatives synthesized by thiol-ene coupling. Polym Chem 3:450–457
Dai H, Yang L, Lin B, Wang C, Shi G (2009) Synthesis and characterization of the different soy-based polyols by ring opening of epoxidized soybean oil with methanol, 1,2-ethanediol and 1,2-propanediol. J Am Oil Chem Soc 86:261–267
Acknowledgments
The authors acknowledge the financial support of The Research Fund of Sakarya University (BAP) (Project no: 2013-02-04-054).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Karadeniz, K., Akı, H., Sen, M.Y. et al. Ring Opening of Epoxidized Soybean Oil with Compounds Containing Two Different Functional Groups. J Am Oil Chem Soc 92, 725–731 (2015). https://doi.org/10.1007/s11746-015-2638-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2638-z