Abstract
The kinetics of the oxirane cleavage of epoxidized soybean oil (ESO) by methanol (Me) without a catalyst was studied at 50, 60, 65, 70 °C. The rate of oxirane ring opening is given by k[Ep][Me]2, where [Ep] and [Me] are the concentrations of oxiranes in ESO and methanol, respectively and k is a rate constant. From the temperature dependence of the kinetics thermodynamic parameters such as enthalpy (ΔH), entropy (ΔS), free energy of activation (ΔF) and activation energy (ΔE a) were found to be 76.08 (±1.06) kJ mol−1, −118.42 (±3.12) J mol−1 k−1, 111.39 (±2.86) kJ mol−1, and 78.56 (±1.63) kJ mol−1, respectively. The methoxylated polyols formed from the oxirane cleavage reaction , were liquid at room temperature and had three low temperature melting peaks. The results of chemical analysis via titration for residual oxiranes in the reaction system showed good agreement with IR spectroscopy especially the disappearance of epoxy groups at 825, 843 cm−1 and the emergence of hydroxy groups at the OH characteristic absorption peak from 3,100 to 3,800 cm−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Utilization of renewable resources as an alternative to fuel and raw mineral materials has been a significant area of recent research. In particular, vegetable oils and fats are promising alternatives to petrochemicals because they are relatively inexpensive and a renewable resource [1]. Furthermore, a wide range of cost-competitive and environmentally friendly products have now become available due to advances in oleo-chemistry technology [2]. Among these, soybean derivatives have been used in many commercial applications, including as components of urethane foams and thermosetting plastics, as plasticizers and stabilizers in chlorine- containing resins, and as additives in lubricants [3–5].
Epoxidized soybean oil (ESO) is one of the raw materials used for the synthesis of polyurethane elastomers (PUE), where double or multiple hydroxyl functionality is usually required [6]. Hydroxy groups can be introduced into soybean oil in many ways, resulting in different polyol structures which, when converted to polyurethane, impart different properties to the final product [7].
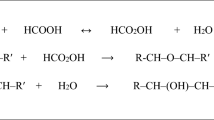
Assuming a single hydroxy group is introduced into each oxirane and 100% conversion of oxirane group to hydroxy group, the resulting polyol from this oxirane cleavage reaction is presented in Scheme 1. Because the oxiranes are in the middle of fatty acid chains, the hydroxy groups will also be located in the middle of the chains. These dangling chains (the short carbon chains after the last hydroxy group from left to right in each fatty acid carbon chain, Scheme 1) act as plasticizers that reduce polyurethane rigidity and improve polyurethane flexibility [8].
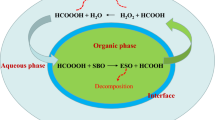
The preparation of polyols from ESO with various reactants has been the subject of many studies [8, 9]. A number of inorganic and organic reagents such as hydrogen, hydrochloric acid, formic acid, acetic acid, or methanol (Me) can be used as ring openers to prepare polyols from ESO. Among these ring openers, methanol can produce a polyol with the highest OH content and a low viscosity, both of which are highly desirable of a liquid polyol for the preparation of polyurethane materials [7]. Other advantages of polyurethanes synthesized from soy-based polyols include a higher thermal stability and improved dielectric properties [1, 7]. However, limited attention has been paid to the kinetic relationships of the ring-opening reaction of ESO with methanol. This work aims to understand the kinetic characteristics of the ring opening reaction of ESO, with methanol (Me) as the ring opener.
Materials
ESO with an oxygen content of 0.385 mol of oxygen per 100 g of oxirane was kindly provided by Nanhai Oil Co. Ltd. Other reagents (methanol >99.5%, isopropanol >99.7%, potassium bromide, sodium carbonate 99%, sodium hydroxide 96.0%, hydrogen peroxide 30%, hydrobromic acid 48%, and sulfuric acid 98%) were purchased from Guangzhou Chemical Reagent Co. Ltd.
Methods
ESO (40.0 g, 0.154 mol) was charged into a four-necked round-bottomed reaction flask equipped with a reflux condenser and was heated to the desired temperature in a constant-temperature water bath. Methanol, was pre-warmed to the same temperature and then quickly added to the flask. Samples of the reaction mixture were taken at 30 min intervals during the course of the experiment and analyzed for epoxy oxygen content (EOC) and hydroxyl content.
EOC was determined by the direct titration of epoxy groups with HBr according to the standard method for oils and fats [10]. The hydroxyl values were determined according to ASTM titration method (E 1899-02) standard test method for OH groups through a reaction with p-toluenesulfonyl isocyanate (TSI) and potentiometric titration with tetrabutylammonium hydroxide.
An IR Prestige-21 (Shimadzu, Japan) Fourier transform infrared (FT-IR) spectrometer was used to analyze the structure of the polyols and ESO. Samples were prepared as thin films on a KBr disk. The cleavage reactions were monitored as the disappearance of the oxirane peaks at 825 and 843 cm−1. Thermal analysis was performed on a small mass of sample (10.0 mg) under nitrogen using a differential scanning calorimeter (DSC 200-PC, Netzsch, Germany). A heating rate of 10 k min−1 was used from −70 to 100 °C.
Results and Discussions
Kinetic Model
In general, the kinetic form of the rate equation for the ESO oxirane cleavage reaction with methanol can be expressed as:
where r is the rate of oxirane ring opening, mol min−1; [Ep] is the molar concentration of oxiranes in ESO; [Me] is the molar concentration of methanol; m and n are the reaction orders with respect to oxirane and methanol, and k is the rate constant in the reaction system. If the molar concentration of methanol is in large excess compared to that of the oxirane oxygen the kinetic form can be reduced to:
where k′ is the apparent rate constant when methanol is used in large excess, i.e.,
The reaction order in oxirane was derived from measurements at 50, 60 and 70 °C with a fixed molar ratio of epoxy group to methanol of about 1:12. The plots of ln([Ep]o/[Ep]) versus reaction time for the oxirane cleavage of SBO by methanol at various temperature are shown in Fig. 1 It can be seen from these results that ln([Ep]o/[Ep]) has a linear relationship with the reaction time at each different temperature, suggesting a pseudo first reaction order in oxirane concentration. This result is in good agreement with those reported by Goud [11], Gan [12], and Zaher [13] et al. who studied the cleavage of the oxirane ring by acetic acid in vegetable oils. The value of pseudo rate constant, k′ (i.e., the slope of each linear regression) was 4.33 × 10−5 UNIT, 1.05 × 10−4 UNIT and 2.46 × 10−4 UNIT at 50, 60 and 70 °C, respectively.
A second series of experiments were conducted to calculate the reaction order with respect to methanol. Three concentrations of methanol (i.e., 0.997, 1.973 and 3.224 mol L−1) were used and the reactions were carried out at 65 °C. Samples were withdrawn at intervals and tested for residual epoxy oxygen content. The results of this series of experiments are shown in Fig. 2. The pseudo-first order rates of the reactions increased with methanol concentration (i.e., k′ = 1.90 × 10−5 UNIT, 7.43 × 10−5 UNIT and 1.99 × 10−4 UNIT, respectively). Fitting these data to Eq. 3 gives \( \ln \,k' = 2.01\,\ln {\left[ {{\text{Me}}} \right]} - 10.88. \) Therefore the oxirane cleavage reaction was second order with respect to methanol concentration.
Combining the results from these two sets of experiments the kinetic form of the rate equation for the ESO oxirane cleavage reaction by methanol is
Temperature Dependence of the Cleavage Reaction
The temperature dependence of the oxirane cleavage reaction was modeled by the Arrhenius equation (Table 1, Fig. 3). The activation energy of oxirane cleavage, E a was calculated from the slope of the Arrhenius as 78.56 (±1.63) kJ mol−1. According to Zaher et al. [13], the activation energy of oxirane cleavage of ESO by acetic acid is 66.21 kJ mol−1. Since acetic acid was used as the ring opener of that oxirane cleavage reaction, which is self-catalyzed, a decrease in the activation energy of the oxirane cleavage reaction system is expected. The enthalpy of activation, ΔH, entropy of activation, ΔS, and free energy of activation, ΔF, were 118.42 (±3.12) J mol−1 k−1 and 111.39 (±2.86) kJ mol−1 at 25 °C [13, 14].
FT-IR Spectroscopy
FT-IR spectra of the prepared soybean polyols based on the oxirane cleavage reaction are presented in Fig. 4. In comparison with the spectrum of the unreacted ESO, the disappearance of epoxy groups at 825, 843 cm−1, and the emergence of hydroxy group at 3,452 cm−1 are obvious. These data are similar to those presented by Guo et al. [7] in a similar system and provide evidence for the oxirane cleavage reaction of ESO by methanol without the presence of a catalyst to form the methoxylated hydrins of the triglycerides or polyols. The peak at 3,452 cm−1 is indicative of the hydroxyl content of the polyols formed and although it cannot be quantified because of interference from moisture in the air, the normalized (based on the area of ester carbonyl group) FT-IR spectra on the characteristic hydroxyl peak at the spectrum range from 3,100 to 3,800 cm−1 showed that the hydroxyl content of polyols gradually increased at a chosen reaction time when the reaction temperature was varied from 50 to 80 °C. This result suggested the higher the temperature is the faster the oxirane cleavage reaction goes.
Thermal Properties Analysis
Thermograms of the prepared soy-based polyols after one (ESO-Me/1 h) and 2 h (ESO-Me/2 h) reaction at 70 °C are presented in Fig. 5. Both polyols from the ESO had three melting peaks and a crystallization peak consistent with observations of similar systems reported by Guo et al [7]. Multiple peaks in the polyols and ESO are ascribed to different crystalline polymorphs that are also present in soybean oil [15]. ESO displayed crystallization before melting that was characterized by a sharp peak at −9.6 °C. The methoxylated polyol (ESO-Me/2 h) had three low temperature melting peaks at −45.4,−22.7 and −8.1 °C but is still liquid at room temperature.
Due to the size of melting peaks is controlled by the crystallization rate and the physical state is largely determined by hydrogen bonding. Decreasing the oxirane content caused an increase in hydroxyl content and viscosity and an eventual transition from the liquid state into the grease state. The melting peaks of the polyol at −8 °C was wider at longer reaction times. It suggest that the introduction of more hydroxy functional groups in ESO by the oxirane cleavage reaction with methanol caused the shift and deformation of the melting peaks.
Conclusion
In this work the polyol was synthesized by oxirane ring opening in ESO with methanol without a catalyst and a kinetic model r = k[Ep][Me]2 has been established. Thermodynamic parameters such as enthalpy, entropy, free energy of activation and activation energy in the oxirane cleavage reaction are found to be 76.08 kJ mol−1, −118.42 J·mol−1 k−1, 111.39 kJ mol−1, and 78.56 kJ mol−1, respectively. The higher activation energy of oxirane cleavage reaction compared to the report by the other authors studied on the oxirane cleavage reaction by acetic acid, is due to the fact that methanol was not used along with a proton acid catalyst in this work. The methoxylated polyol was also characterized by chemical and physical means. Differential scanning calorimetry revealed the crystalline structure and melting peaks of the polyol. The polyol displayed as a liquid at room temperature, with two melting peaks and behavior of crystallization before melting. The disappearance of epoxy groups in the spectrum of fingerprint region and the emergence of the hydroxy groups at its characteristic absorption peak in the FT-IR spectra showed good agreement with the results of residual epoxy oxygen content in the oxirane cleavage reaction system by the chemical analysis via titration.
References
Zlatanic A, Petrovic ZS, Dusek K (2002) Structure and properties of triolein-based polyurethane networks. Biomacromolecules 3:1048–1056
Guidotti M, Ravasio N, Psaro R, Gianotti E, Marchese L, Coluccia S (2003) Heterogeneous catalytic epoxidation of fatty acid and methyl esters on titanium-grafted silicas. Green Chem 5:421–424
Li F, Hanson MV, Larock RC (2001) Soybean oil-divinylbenzene thermosetting polymers: synthesis, structure, properties and their relationships. Polymer 42:1567–1579
Okieimen FE, Bakare OI, Okieimen CO (2002) Studies on the epoxidation of rubber seed oil. Ind Crops Products 15:139–144
Rangarajan B, Havey A, Grulke EA, Culnan PD (1995) Kinetic parameters of a two-phase model for in situ epoxidation of soybean oil. J Am Oil Chem Soc 72:1161–1169
Hoefer R, Daute P, Grutzmacher R, Westfechtel A (1997) Oleochemical polyols––a new material source for polyurethane coatings and floorings. J Coat Technol69:65–72
Guo A, Cho Y, Petrovic ZS (2000) Structure and properties of halogenated and nonhalogenated soy-based polyols. J Polym Sci [A] 38:3900–3910
Fedderly, Lee JJ, Lee GF, Hartmann JD, Dusek B, Duskova-Smrckova K, Somvarsky M (2000) Network structure dependence of volume and glass transition temperature. The Soc Rheol Inc J Rheol 44:961–972
Guo A, Javni I, Petrovic Z (2000) Rigid polyurethane foams based on soybean oil. J Appl Polym Sci 77:467–473
Paquot C, Hautfenne A (1987) IUPAC Standard methods for the analysis of oils, fats and derivatives. 7th edition, Blackwell, London
Goud VV, Patwardhan AV, Pradhan NC (2006) Studies on the epoxidation of mahua oil (Madhumica indica) by hydrogen peroxide. Bioresour Technol 97:1365–1371
Gan LH, Goh SH, Ooi KS (1992) Kinetic studies of epoxidation and oxirane cleavage of palm olein methyl esters. J Am Oil Chem Soc 69:347–351
Zaher FA, EI-Mallah MH, EI-Hetnawy MM (1989) Kinetics of oxirane cleavage in epoxidized soybean oil. J Am Oil Chem Soc 66:698–700
Okieimen FE, Bakare OI, Okieimen CO (2002) Studies on the epoxidation of rubber seed oil. Ind Crops Products 15:139–144
Hasenhuette GL (1994) Kirk Othmer Encyclopedia of Chemical Technology. In: Kirk RE, Othmer DF, Kroschwitz JI, Howe-Grant M (eds) Wiley, New York
Acknowledgments
This work was supported by Natural Science Foundation of Guangdong Province (No. 06025028).
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments on this Paper by Yijin Xu and Zoran S. Petrovic can be found at 10.1007/s11746-008-1306-y.
The Authors’ Response to the Comments on this Paper can be found at 10.1007/s11746-008-1309-8.
The Editor’s Response to the Comments on this Paper can be found at 10.1007/s11746-008-1308-9.
About this article
Cite this article
Lin, B., Yang, L., Dai, H. et al. Kinetic Studies on Oxirane Cleavage of Epoxidized Soybean Oil by Methanol and Characterization of Polyols. J Am Oil Chem Soc 85, 113–117 (2008). https://doi.org/10.1007/s11746-007-1187-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1187-5