Abstract
Successful industrial applications of soy protein adhesives require high adhesion strength and low viscosity at high solid protein concentration. This study examined the effects of β-conglycinin (7S) and glycinin (11S) ratios on the physicochemical properties of soy protein adhesives. Soy protein adhesives with various 7S/11S ratios were extracted from soy flour slurry modified with sodium bisulfite using the acid precipitation method, which is based on the different solubilities of 7S and 11S globulins. Seven glycinin-rich soy protein fractions and six β-conglycinin-rich soy protein fractions were obtained. The external morphology of the samples changed from the viscous cohesive phase to the clay-like phase without cohesiveness. The viscous cohesive samples had good flowability and good water resistance with a wet adhesion strength of 2.0–2.8 MPa. They were stable for up to several months without phase separation at room temperature. Based on the results, we suggest that proper protein–protein interaction, hydration capacity (glycinin-rich soy protein fractions), and certain ratios of 7S and 11S (β-conglycinin rich soy protein fractions) in the soy protein sample are crucial to continuous protein phase formation. Hydrogen bonding, electrostatic forces, and hydrophobic interactions are involved in maintaining the protein viscous cohesive network, whereas disulfide bonds do not exert significant effects. This study describes a new way to investigate viscous cohesive soy protein systems with high solid protein content, thus alleviating the disadvantages of traditional methods for studying the adhesive properties of soy protein isolates, which tend to have poor water resistance, low solid contents, and short storage life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soy protein has been used widely in foods for its nutritional and functional properties. Biodegradable adhesives of soy protein also have been considered and studied extensively as potential alternatives to synthetic petrochemical adhesives. Two predominant storage proteins, glycinin (11S) and β-conglycinin (7S), constitute 80% of the total protein content in soybean, and they both have specific properties that contribute to the physicochemical properties of soy protein ingredients [1]. Glycinin is a hexameric protein with a molecular weight of about 360 kDa composed of five different types of subunits: A1aB1b, A2B1a, A1bB2, A5A4B3, and A3B4 [2]. Each subunit contains one acidic polypeptide and one basic polypeptide, linked by a disulfide bridge. Glycinin has a relatively high cysteine content, with 18–20 intra- and inter-molecular disulfide bonds [3]. β-Conglycinin (150–200 kDa) is a trimer, consisting of three subunits, α′, α, and β. The subunits are non-covalently associated by hydrophobic interaction and hydrogen bonding, and do not contain disulfide bonds [4]. Inherent differences in structure and molecular properties of the 11S and 7S globulins will lead to different functional properties of soy protein such as solubility, thermal and morphological properties, and adhesion performance [5–9].
Soy protein subunits have been studied for their adhesion properties on wood. Several types of soy proteins have been investigated, including pure 7S globulin and 11S globulin suspensions [8–10], mixtures of 7S and 11S with various ratios [7], or 7S subunits (α′, α, and β) and 11S subunits (acidic, basic subunits) [11, 12]. The β subunit showed greater water resistance than α′, α, and β-conglycinin [11]. Glycinin was found to improve wet adhesion strength [7, 10]. The basic subunits from glycinin had higher water resistance than acidic subunits due to the considerably greater number of hydrophobic amino acids in the basic subunits [12]. Previous studies also revealed that soy protein modified with sodium bisulfite had an adhesive strength comparable to formaldehyde-based adhesives [13, 14]. However, Zhang and Sun [8, 9] reported that the adhesive performance of pure 11S and 7S globulin was not improved by sodium bisulfite modification. Therefore, we hypothesized that the interaction between 7S and 11S components in the soy protein adhesive system would dominate adhesion performance. The ratio of unmodified 11S to 7S has been shown to have a significant influence on adhesion [7]. In this study, we examined the interaction of 7S and 11S globulins at various ratios that were extracted directly from soy protein slurries modified with sodium bisulfite using the acid precipitation method. Because 7S and 11S have different solubilities under different pHs, we expected to obtain samples with various ratios of 7S and 11S by using different pHs during extraction. The objective of this study was to examine the effects of 7S/11S mixtures modified by sodium bisulfite on adhesive properties on wood, and to characterize the interactions and physiochemical properties, such as electrophoresis profiles and rheological, thermal, and morphological properties, of modified 7S and 11S.
Materials and Methods
Materials
Defatted soy flour (Cargill, Cedar Rapids, IA) was used as the starting material. The soy flour contained about 50% protein and 10% moisture with a dispersion index of 90. Sodium bisulfite (NaHSO3), sodium chloride (NaCl), and urea were obtained from Fisher Scientific (Fair Lawn, NJ). Sodium thiocyanate (NaSCN), 2-mercaptoethanol (Me-SH), and propylene glycol (PG) were purchased from Sigma-Aldrich (St. Louis, MO). All reagents were of analytical reagent grade (AR). Cherry wood veneers with dimensions of 50 × 127 × 5 mm (width × length × thickness) were provided by Veneer One (Oceanside, NY).
Soy Protein Adhesive Preparation
The aqueous protein extract was prepared by mixing defatted soy flour in water at 6.25% solids content at pH 9.5 with 2 N NaOH. NaHSO3 was added to the slurry at 6 g/L on the basis of water volume based on previous studies [13, 14]. The slurry was stirred for 2 h at room temperature, then the carbohydrates were removed from the soy protein using a Beckman Coulter Avanti J-25 centrifuge (Beckman Coulter, Brea, CA) at 12,000 g (8,235 rpm for JA 14 rotor). The resulting supernatant was divided into seven portions and each portion was adjusted to pH values of 6.0, 5.8, 5.6, 5.4, 5.2, 5.0, and 4.8, respectively, using 2 N HCl. The supernatants were centrifuged at 12,000 g to get the glycinin-rich fractions (labeled as Gly pH 6.0, Gly pH 5.8, etc.). Then, the pH of each resulting supersaturate was adjusted to pH 4.8, and the slurries were centrifuged at 8,000 g (6,724 rpm for JA 14 rotor) to obtain β-conglycinin-rich soy protein fractions (labeled as Cong pH 6.0, Cong pH 5.8, etc.). The flowchart in Fig. 1 illustrates the procedure, using the pH 6.0 sample as an example. The product yield, water content, and external morphology of all samples are summarized in Table 1.
SDS-Polyacrylamide Gel Electrophoresis
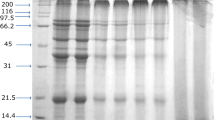
SDS-PAGE was performed on a 4% stacking gel and 12% separating gel with a discontinuous buffer system according to the method described by Laemmli [15]. NaHSO3-modified soy protein samples were mixed with a sample buffer containing 2% SDS, 25% glycerol, and 0.01% bromphenol blue for the non-reducing gel. SDS-PAGE under reducing conditions was carried out with 2-mercaptoethonal added to the protein loading buffer. A total of 8 μg protein was applied to each sample slot. Electrophoresis was preformed at 40 mA and 150 V for 120 min. The gel was stained in 0.25% Coomassie brilliant blue R-250 and destained in a solution containing 10% acetic acid and 40% methanol. Densitometry was obtained by analyzing the gel image using the Kodak 1D Image Analysis software, version 4.6 (Kodak, Rochester, NY).
Particle Size Analysis of Protein Precipitates Aggregates
The aqueous protein suspensions of pH 5.8, 5.4, 5.0, 4.8, and their resultant supersaturates after centrifugation at pH 4.8, as described in section “Soy protein adhesive preparation”, were used for the particle size distribution measurements. A LECOTRAC LTS-150 Particle Size Analyzer (LECO Corporation, Tampa, FL) was used for this study. Soy protein concentration was within the equipment’s recommend range, and ultrasonic was utilized to disperse the soy protein particles well in the sample cell. Two replicates were made with each sample. The mean diameter of volume distribution (mv) was recorded for each sample.
Determination of Protein Solubility
Two soy protein samples, Gly pH 5.4 and Cong pH 5.4, were selected to study their solubility in various reagents. Freeze-dried soy protein powder (1 g) was dispersed in 10 ml citric acid–Na2HPO4 buffer (pH 5.4 and 4.8, respectively) containing either 0.5 M NaCl, 0.5 M NaSCN, 3 M Urea, 0.2 M Me-SH, or 20% PG to make a suspension. After 2 h constant stirring at room temperature, each suspension was centrifuged for 15 min at 8,000 g. The supernatant was discarded gently and the precipitated protein was freeze-dried for the protein solubility calculation.
Rheological Properties
Apparent viscosity measurements of NaHSO3-modified soy protein samples were performed using a Bohlin CVOR 150 rheometer (Malvern Instruments, Southborough, MA) with a CP 4/40 cone and plate fixture (4 cone angle, 40 mm cone diameter). The distance between the cone and the plate was set to 150 μm. The apparent viscosity measurements were tested in the shear rate range of 0.1–100 s−1. The testing temperature was 23°C. A thin layer of silicone oil was spread over the circumference of the sample to prevent the sample from dehydrating during the test.
Thermal Properties
Thermal properties of NaHSO3-modified soy protein samples were evaluated by a differential scanning calorimeter (DSC) (Q200, TA instrument, Schaumburg, IL) calibrated with indium and zinc. Fresh soy protein samples (20 mg) were hermetically sealed in Tzero aluminum hermetic pans. Each sample was held at 20°C for 1 min, then scanned from 20 to 130°C at a heating rate of 10°C/min. Peak temperatures and denaturation enthalpies were calculated from thermograms by Universal Analysis 2000 software.
Morphological Properties
A Philips CM 100 (FEI Company, Hillsboro, OR) TEM was used to observe the microstructure of NaHSO3-modified soy protein adhesive. NaHSO3-modified soy protein samples were diluted to 1% in deionized water and then sonicated for 10 min in an L & R320 ultrasonic stirrer (L & R Manufacturing Company, Keary, NJ). Diluted samples were absorbed onto Formvar/carbon-coated 200-mesh copper grids (Electron Microscopy Science, Fort Washington, FA) and stained with 2% (w/v) uranyl acetate (Ladd Research Industries, Burlington, VT) for 60 s at room temperature.
Wood Specimen Preparation
NaHSO3-modified soy protein possessing flowable and brushable properties were selected to characterize their adhesion performance. Cherry wood veneers were preconditioned in a chamber (Electro-Tech Systems, Glenside, PA) for 7 days at 23°C under 50% relative humidity. The adhesives were brushed onto one end of the cherry wood piece with dimensions of 127 × 20 mm (length × width) until the entire area was completely wet. The amount of adhesive applied to each piece was about 0.3 g. Two brushed wood pieces were assembled immediately and conditioned for 20 min at room temperature. The assembled wood specimens were then pressed with a hot press (Model 3890 Auto M; Carver, Wabash, IN) at 1.4 MPa and 150°C for 10 min.
Shear Strength Measurements
The wood assemblies glued with NaHSO3-modified soy protein adhesive were cooled, conditioned at 23°C under 50% relative humidity for 2 days, and cut into 5 pieces with dimensions of 80 × 20 mm (glued area of 20 × 20 mm). The cut wood specimens were conditioned for another 4 days before measurements were taken. Wood specimens were tested with an Instron Tester (Model 4465, Canton, MA) according to ASTM Standard Method D2339-98 at a crosshead speed of 1.6 mm/min [16]. Shear adhesion strength at maximum load was recorded; reported values are the average of five specimen measurements.
Water resistance of the wood assemblies was measured following ASTM Standard Methods D1183-96 and D1151-00 [17, 18]. The preconditioned specimens were soaked in tap water at 23°C for 48 h, and the wet strength was tested immediately after soaking.
Results and Discussion
NaHSO3-Modified Soy Protein Samples
Thirteen NaHSO3-modified soy protein samples were obtained upon centrifugation: seven glycinin-rich soy protein fractions and six β-conglycinin-rich soy protein fractions. The products yield (wet weight basis), solid content, and external morphology are summarized in Table 1. The total protein yield of the glycinin-rich soy protein fractions were increased as the extraction pH decreased from 6.0 to 4.8, as more and more glycinin and β-conglycinin partitioned from the supernatant. Glycinin is known to precipitate in the pH range of 4.4–6.8, with the isoelectric point (IP) approximately at pH 5.4, while the β-conglycinin precipitates in the pH range of 4.0–5.6 with IP 4.8 [19]. However, as a reducing agent, NaHSO3 introduced extra charges on the surface of soy proteins (RS-SO3 −) and the minimum solubility range shifted to a lower pH value, which can be reflected by the very low extraction yield of Gly pH 6.0 (9%). The glycinin-rich soy protein displayed viscous cohesive properties in the pH range of 5.4–5.8, while the soy protein became a clay-state phase of non-cohesiveness when the pH was lower than 5.4. The β-conglycinin-rich soy protein fractions exhibited viscous cohesive properties for samples Cong pH 5.0, Cong pH 5.2, and Cong pH 5.4, whereas other sample fractionations obtained at pH > 5.4 lost the continuous phase. In addition, due to the reducing effects of NaHSO3, all samples had several months of storage life. The storage life was estimated in our laboratory using adhesion strength on wood veneer as an indicator. Sustainable shear adhesion strength of NaHSO3-modified soy protein was obtained within 3 months (unpublished data). Visual observation of mold was also used as an indicator. The unmodified soy protein suspension starts growing mold within 3 days at room temperature, while the modified soy protein (wet sample) could last for 3 months without mold because of the reducing effects of NaHSO3.
SDS-PAGE
Glycinin-rich Soy Protein Fractions
Reducing SDS-PAGE was performed to study the subunit distribution in each soy protein sample. The electrophoresis profiles of glycinin-rich soy proteins are presented in Fig. 2a. The predominant components were identified as α′, α, and β subunits of β-conglycinin and acidic (A) and basic (B) polypeptides of glycinin. The bands observed at about 32 kDa might be due to the non-covalently bonded acidic polypeptides (A4) in the G4 (A5A4B3) glycinin subunits [2]. The 30 kDa band belongs to the agglutinin subunits of 7S [20], and its isoelectric point was at pH 6.0 [21]. Therefore, its intensity attenuated gradually as pH decreased from 6.0 to 5.0. These bands disappeared under non-reducing SDS-PAGE (Fig. 2b), indicating that agglutinin was connected by the disulfide linkage [20]. A trace amount of the band at 18 kDa, corresponding to trypsin inhibitor of 7S, also intensified as the pH decreased [22]. The percentages of 7S increased from 9 to 15%, whereas 11S accounted for about 70%, as the pH decreased from 6.0 to 5.0 (Table 2), indicating a significant fractionation of 7S and 11S globulins in this pH range. The majority of 7S was precipitated at pH 4.8, accounting for about 30% of the total protein.
Reducing and non-reducing SDS-PAGE patterns of NaHSO3-modified soy protein adhesives. a Glycinin-rich fractions under reducing conditions. b Glycinin-rich fractions under non-reducing conditions. c β-conglycinin-rich fractions under reducing conditions. d β-conglycinin-rich fractions under non-reducing conditions
Moreover, trivial amounts of protein aggregates were observed on the top of the resolving gel, and the intensity decreased as the pH decreased. NaHSO3 breaks the disulfide linkage, releasing some acidic and basic polypeptides. The basic subunits were highly hydrophobic, which could facilitate the self-association and formation of protein aggregates [23]. Furthermore, the IP of the basic subunits from different soy flour species were reported to be at pH 8.0–8.5 or pH 4.5–8.0 [12]. Hence, we believe that those protein complexes were composed of basic polypeptides that interacted mainly through hydrophobic association, and the band intensity decreased as pH decreased.
Non-reducing SDS-PAGE profiles of glycinin-rich soy protein samples showed a major band at about 56 kDa, corresponding to the glycinin A–B complexes composed of the acidic and basic polypeptides linked by disulfide bonds (Fig. 2b). The appearance of the faint bands at 38 kDa (acidic) and 23 kDa (basic) resulted from the reduction of disulfide bond-linked A–B complexes, suggesting that the reducing effects of NaHSO3 on the soy protein were insignificant. The trace bands around 100 kDa could be the result of disulfide bond formation in aggregates induced by freeze-drying or thiol-disulfide exchange during the modification process [24]. Some protein aggregates remained on the top of the resolving and staking gels. The major force involved in these aggregates could be the disulfide bond formation as evidenced by the near disappearance of these bands in the reducing electrophoresis.
β-Conglycinin-rich Soy Protein Fractions
In the reducing SDS-PAGE, the subunits distribution of β-conglycinin exhibited significant differences among different pH fractions (Fig. 2c). As shown in Table 3, the content of 7S and 11S globulins was reciprocal: 23.5–72% increase for 7S and 64.2–23.9% decrease for 11S, respectively.
Non-reducing electrophoresis profiles revealed three types of protein aggregates linked by disulfide bonds (Fig. 2d). The aggregates at approximately 100 kDa were formed by the glycinin subunits as mentioned in Fig. 2b, and the bands faded as the glycinin content decreased. Other bands located at about 120 kDa were intensified as the β-conglycinin fraction increased in the soy protein samples. These bands could be formed by α′ and α subunits connected by disulfide bonds, as suggested by Petruccelli and Anon [20]. The even higher molecular weight protein complex remained on the top of the resolving and stacking gels. Individual polypeptides are known to be released from soy protein due to the reducing and unfolding effects of NaHSO3. Some of those polypeptides might re-associate into high molecular weight protein complexes through disulfide bond formation, as displayed in the gel.
Apparent Viscosity
The shear rate dependence of apparent viscosity of the soy protein samples are summarized in Fig. 3. Apparent viscosity decreased as the shear rate increased in the range of 0.1–100 s−1, revealing the shear thinning behavior of the soy protein. Under the same shear conditions, the apparent viscosity of the glycinin-rich soy protein fractions increased as the extraction pH decreased from 6.0 to 5.0; however, as the extraction pH reached 4.8, its viscosity decreased somewhat (Fig. 3a). Simultaneously, the external morphology of the samples transformed from a diluted state to a very viscous clay state as the pH decreased from 6.0 to 5.0. Similar phenomena were observed in a previous study [25]. The protein samples formed a solid plug rather than a viscous liquid upon centrifugation in the low-pH level (pH 4.0–5.3), and their morphology images displayed the promoted protein aggregates with larger particle size. As the extraction pH approached the isoelectric point, strong protein–protein intermolecular forces were achieved, resulting in high-density, viscous protein aggregates.
The strong molecular forces could also be reflected by the particle size of protein precipitates as shown in Fig. 4a. The acid precipitation of soy protein is generally thought to occur through the rapid formation of primary particles in the size range of 0.1–0.3 μm followed by aggregation of these particles via collision to aggregates 1–50 μm in size [25]. The size of aggregates can be manipulated by pH, salt concentration, temperature, etc., which could control the intermolecular forces of protein molecules. The mean diameter of volume distribution (mv) of glycinin-rich soy protein fractions increased gradually, with pH decreases from 5.8 (1.56 μm) to 5.4 (6.74 μm), and a large extended protein agglomeration (58.8 μm) occurred at pH 5.0. In the case of pH 4.8 (40 μm), a larger amount of 7S of lower molecular weight was precipitated from the supernatant, which could reduce the particle size of the final product, and, to some extent, the apparent viscosity. The bimodal size distribution occurred at pH 5.0 and 4.8, which was also observed by Liu et al. [25]. Furthermore, proteins at the isoelectric point exhibited minimum water hydration and swelling ability due to the zero net charge on the protein surface; therefore, more water would be expelled out of the protein structure, making the protein samples less cohesive (Gly pH 5.2, pH 5.0, pH 4.8).
As to the β-conglycinin-rich soy protein fractions, sample Cong pH 5.4 exhibited the highest apparent viscosity, while other samples possessed viscosity in a similar range (Fig. 3b). However, only slight differences in particle size were observed (5.8–5.0 μm) among all the samples (Fig. 4b). Interestingly, the ratio of 7S and 11S globulins in Cong pH 5.4 was approximately 1 based on densitometry analysis, suggesting that specific and stronger intermolecular interactions might be induced at certain 7S/11S ratios. Additionally, when the 7S component presented predominately in the protein fraction, the sample also displayed viscous cohesive properties (Cong pH 5.2, pH 5.0). In contrast, the glycinin-dominated soy protein system resulted in a non-continuous protein phase, which is reflected in the phase separation of water and protein for samples Cong pH 6.0 and Cong pH 5.8. This phenomenon also could be partially attributed to the fact that 11S has greater surface hydrophobicity than 7S, and could expel more water from the protein network [26, 27].
The soy protein structure was unfolded and the protein conformation was altered by NaHSO3 modification through the ion pair shielding effect and reducing effects [28]. This re-associated protein conformation could be manipulated by pH (glycinin-rich soy protein fractions) and protein composition (conglycinin-rich soy protein fractions). We propose that the protein aggregates could be coalesced upon centrifugation to form a homogeneous and continuous protein phase, exhibited as viscous cohesive substances when the re-formed protein network had the ability to retain the proper amount of water. These high protein content phases were stable for up to several months without phase separation when stored at room temperature.
Solubility of Soy Protein in Different Reagents
The effects of various reagents such as NaCl, NaSCN, urea, Me-SH, and PG on the solubilities of protein samples could reflect the molecular forces contributing to the maintenance of the soy protein’s viscous cohesive network. Protein samples Gly pH 5.4 and Cong pH 5.4 were dissolved in these reagents. The amount of protein solubilized from the sample was determined as shown in Table 4. Approximately 25% of these two proteins dissolved into the citric acid–Na2HPO4 buffer. More protein was solubilized in the presence of 0.5 M NaCl or 0.5 M NaSCN, indicating the salt-in effect of natural salts at low salt concentration. Urea increased the solubilities of Gly pH 5.4 and Cong pH 5.4 to 61 and 76%, respectively. Urea is known to destabilize the hydrophobic interactions and hydrogen bonding, which suggested that these forces are involved in maintaining the protein matrix. In the presence of Me-SH, about 35% of proteins were solubilized, indicating that the disulfide bond had limited effects in stabilizing the protein network. Propylene glycol could diminish the hydrophobic strength but enhance the hydrogen bonds and electrostatic interaction by lowering the dielectric constant [29]. Thus, the lowest solubility of soy protein in PG indicated that increasing hydrogen bonding and electrostatic interactions could stabilize the protein network. Therefore, the electrostatic force, hydrogen bond, and hydrophobic interactions are involved in maintaining the protein’s viscous cohesive network, whereas disulfide bonds do not play a significant role.
Thermal Properties
The thermal denaturation transition of proteins can be determined using differential scanning calorimetry (DSC) by measuring the amount of energy absorbed or released from protein samples. This energy is usually detected as an endothermic peak in the DSC thermogram, in terms of the parameters of denaturation temperature (T d) and total denaturation enthalpy (ΔH d). The typical DSC thermogram of glycinin-rich soy protein is shown in Fig. 5a. The major peak at T d of 97–98 °C was designated as the glycinin globulin, and the tiny shoulder peak was the β-conglycinin at T d of 80 °C. Gly pH 5.8 had the higher T d (98.84 °C) and ΔH d (16.6 J/g) than other samples (Table 5). As the pH decreased from 5.8 to 4.8, the denaturation temperature for both glycinin and β-conglycinin shifted gradually to a lower temperature, and the total ΔH d decreased from 16.6 to 8.68 J/g. These T d results seem to conflict with the theory that protein at IP had the highest thermal stability. However, as shown in Fig. 2, high molecular weight protein aggregates were detected, and the intensity of those high molecular weight proteins decreased as the pH decreased from 6.0 to 5.0. Therefore, we believe these small amounts of protein aggregates play a vital role in the thermal stability of the samples. In addition, glycinin is well known to be much more thermostable than β-conglycinin due to its existing disulfide linkage [30]. Although only a small amount of β-conglycinin was incorporated into soy protein extracted at lower pH, it could have decreased the unfolding enthalpy as the pH decreased.
In the thermogram of β-conglycinin-rich soy protein (Fig. 5b), T d of both glycinin and β-conglycinin increased slightly as the sample gradually become viscous cohesive phases, suggesting that more thermal stable states were formed (Table 5). Cong pH 5.4 had the highest denaturing temperature, suggesting strong protein interactions that may have resulted from equal amounts of 7S and 11S components in the sample. The total ΔH d of soy protein decreased by 3.3 J/g as fractionation pH decreased from 6.0 to 5.6. This might be the result of an increasing amount of less stable 7S globulin presented in the samples. While the ΔH d of Cong pH 5.4 improved suddenly to 8.07 from 6.30 J/g in Cong pH 5.6, and it then dropped around 0.6 J/g in samples Cong pH 5.2 and Cong pH 5.0, which agreed with the T d results.
Morphology Properties
As a salt, NaHSO3 could enhance protein hydrophobic interaction through ion shielding effects, which would lead to favoring protein aggregation and cluster formation [8]. However, as a reducing agent, NaHSO3 disrupts protein structure by breaking the hydrophobic and electrostatic interactions and hydrogen bonds. Consequently, individual polypeptides are formed. And the rearranged protein network and aggregates from those individual polypeptides would be dependent on pH, ionic strength, and protein composition.
The microstructures of glycinin-rich and β-conglycinin-rich soy protein samples are displayed in Fig. 6. Large numbers of spherical protein aggregates with similar diameters were observed in the Gly pH 5.8 sample (Fig. 6a). These aggregates connect with each other to form a uniform, continuous network. The thread-shaped protein peptides were enclosed inside the continuous network. As the pH decreased to 5.4, the network was composed mainly of the thread-shaped polypeptides, while the uniform chain-like network structures disappeared and spherical protein clusters with different diameters were observed (Fig. 6b). When the separation pH decreased to 5.0, the continuous phase disappeared from the network structure and numerous individual spherical protein aggregates formed in similar size (Fig. 6c). The continuous network in Gly pH 5.8 might be able to hold the proper amount of water inside the protein structure like a pocket, which would be beneficial to cohesive substance formation.
As to the β-conglycinin-rich protein, Cong pH 5.4 was characterized by the chain-like networks composed of spherical protein clusters (Fig. 6d), whereas Cong pH 5.8 displayed disintegrated protein clusters as the number of short chains increased. This indicates much stronger protein–protein interactions in the Cong pH 5.4 sample. Sun et al. [14] also reported that chain-like networks connected by protein aggregates would be formed when the electrostatic attraction force favors hydrophobic interaction in the cohesive soy protein samples with high solid protein content. Therefore, we assume that the chain-like protein aggregate structures favor the formation of viscous cohesive properties at high protein concentration, such as in the Cong pH 5.4 sample.
Shear Adhesion Strength
Several factors, including protein yield, solid protein content, and flowability, could affect the practicality of soy protein adhesives for industrial applications. Six NaHSO3-modified soy protein samples with viscous cohesive properties were chosen to study their tensile strength. Adhesion performance was characterized in terms of dry and wet shear adhesion strength (Table 6). All soy protein samples, except the Gly pH 5.8 sample (4.54 MPa), exhibited excellent dry strength with 100% wood cohesive failure. A similar trend was observed for the wet strength, in that viscous cohesive samples exhibited good water resistance with strength in the range of 1.9–2.8 MPa, but 1.2 MPa for Cong pH 5.8, compared to 1.6 MPa for the control soy protein isolate adhesive under the same curing conditions. The results also showed that the dry and wet adhesion performance of β-conglycinin-rich soy protein was much better than glycinin-rich soy protein adhesives.
Many factors are involved in affecting the strength of protein adhesives: pH, composition and structure of the protein, solid protein contents, flowability, and the interactions of hydrophobic and hydrophilic groups with the wood surface. Soy protein adhesives prepared at or close to their isoelectric point (IP = 4.5) were proven to have the highest shear strength due to the strong protein–protein interactions during the curing process [12, 31]. These interactions might contribute to better adhesion performance of the β-conglycinin-rich soy protein (pH 4.8) than that of the glycinin-rich soy fractions. And for the Gly pH 5.8 sample, which is far away from its IP, electrostatic repulsions between protein molecules occurred due to the redundant surface charges, which might, in turn, enhance protein–water interactions instead of protein–protein and protein–wood interactions, leading to reduced adhesion strength. In addition, the β-conglycinin-rich fractions were 5% higher in protein concentration than the glycinin-rich fractions (Table 1). This could also contribute to higher adhesion strength in the β-conglycinin-rich samples.
Furthermore, one of the hurdles in soy protein adhesives applications is their high viscosity; hence, the solid content commonly used in the lab and industrial field is in the range of 10–15%. In our study, the solid content of viscous cohesive soy proteins was 33%, about 15% higher than that used in normal industrial applications. High protein concentrations could favor the protein crosslink, entanglement, and protein-wood surface interactions in the curing process, leading to reduced protein-water interactions and improved water resistance. The most important factor affecting adhesive strength is the chemical modification of soy protein, as discussed previously. NaHSO3 could bring the hydrophobic amino groups outside of the protein molecules due to the breakage of inter-disulfide bonds, leading to improved surface hydrophobicity of the soy protein, which is beneficial for adhesion strength improvement, especially for water resistance.
Conclusion
A viscous cohesive soy protein system with good flowability could be produced by adjusting extraction pH and sodium bisulfite modifications during the acid precipitation process. The viscous cohesive samples were stable for up to several months without phase separation when stored at room temperature. The wet adhesion strength of the viscous cohesive soy protein adhesives could reach 2.8 MPa. Based on the morphology, viscosity, and particle size of the precipitates, we propose that proper protein–protein interaction, hydration capacity (glycinin-rich soy protein fractions), and certain ratios of 7S and 11S (β-conglycinin rich protein fractions) in protein systems are crucial to continuous protein phase formation. Hydrogen bonding, electrostatic forces, and hydrophobic interactions are involved in maintaining the protein latex network; however, the effect of disulfide bonds is insignificant.
References
Peng IC, Quass DW, Dayton WR, Allen CE (1984) The physicochemical and functional properties of soybean 11s globulin-a review. Cereal Chem 61:480–490
Staswick PE, Hermodson MA, Nielsen NC (1984) Identification of the cystines which link the acidic and basic components of the glycinin subunits. J Biol Chem 259:3431–3435
Kella NKD, Barbeau WE, Kinsella JE (1986) Effect of oxidative sulfitolysis of disulfide bonds of glycinin on solubility, surface hydrophobicity and in vitro digestibility. J Agric Food Chem 34:251–256
Thanh VH, Shibasaki K (1978) Major proteins of soybean seeds. Subunit structure of beta-conglycinin. J Agric Food Chem 26:692–695
Saio K, Watanabe T (1978) Differences in functional properties of 7S and 11S soy bean proteins. J Texture Stud 9:135–157
Ning L, Villota R (1994) Influence of 7S and 11S globulins on the extrusion performance of soy protein concentrates. J Food Process Preserv 18:421–436
Mo X, Sun X, Wang D (2004) Thermal properties and adhesion strength of modified soybean storage proteins. J Am Oil Chem Soc 81:395–400
Zhang L, Sun X (2008) Effect of sodium bisulfite on properties of soybean glycinin. J Agric Food Chem 56:11192–11197
Zhang L, Sun XS (2010) Sodium bisufite induced changes in the physicochemical, surface and adhesive properties of soy β-conglycinin. J Am Oil Chem Soc 87:583–590
Wang Y, Wang D, Sun XS (2005) Thermal properties and adhesiveness of soy protein modified with cationic detergent. J Am Oil Chem Soc 82:357–363
Mo X, Wang D, Sun XS (2011) Physicochemical properties of β and α′α subunits isolated from soybean β-Conglycinin. J Agric Food Chem 59:1217–1222
Mo X, Zhong Z, Wang D, Sun XS (2006) Soybean glycinin subunits: characterization of physicochemical and adhesion properties. J Agric Food Chem 54:7589–7593
Qi G, Sun XS (2010) Soy protein adhesive blends with synthetic latex on wood veneer. J Am Oil Chem Soc 88:271–281
Sun XS, Wang D, Zhang L, Mo X, Zhu L, Bolye D (2008) Morphology and phase separation of hydrophobic clusters of soy globular protein polymers. Macromol Biosci 8(4):295–303
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Annual Book of ASTM Standards (2002) D2339-98. ASTM International, West Conshohocken 15.06:158–160
Annual Book of ASTM Standards (2002) D1183-96. Vol. 15.06, ASTM International, West Conshohocken, pp 70–73
Annual Book of ASTM Standards (2002) D1151-00. Vol. 15.06, ASTM International, West Conshohocken, pp 67–69
Thanh VH, Okubo K, Shibasaki K (1976) Major proteins of soybean seeds. A straightforward fraction and their characterization heterogeneity of beta-conglycinin. J Agric Food Chem 24:1117–1121
Petruccelli S, Anon MC (1995) Soy protein isolate components and their interactions. J Agric Food Chem 43:1762–1767
Bogracheva TYa, Bespalova NYu, Leont’ev AL (1996) Isolation of 11S and 7S globulins from seeds of glycine max. Appl Biochem Microbiol 32:429–433
Iwabuchi S, Yamauchi F (1987) Electrophoretic analysis of whey proteins present in soybean globulin fractions. J Agric Food Chem 35:205–209
Lakemond CMM, Jongh HJ, Hessing M, Gruppen H, Voragen AGL (2000) Soy glycinin: influence of pH and ionic strength on solubility and molecular structure at ambient temperatures. J Agric Food Chem 48:1991–1995
Wolf WJ (1993) Sulfhydryl content of glycinin: Effect of reducing agents. J Agric Food Chem 41:168–176
Liu DYM, Litster JD, White ET (2007) Precipitation of soy proteins: particle formation and protein separation. Am Inst Chem Eng 53:514–522
Riblett AL, Herald TJ, Schmidt KA, Tilley KA (2001) Characterization of β-conglycinin and glycinin soy protein fractions from four selected soybean genotypes. J Agric Food Chem 49:4983–4989
Wool R, Sun XS (2005) Soy protein adhesives. In: Wool R, Sun XS (eds) Bio-based polymers and composites. Elsevier, Burlington, pp 327–368
Babajimopoulos M, Damodaran S, Rizvi Syed SH, Kinsella JE (1983) Effects of various anions on the rheological and gelling behavior of soy proteins. Thermodynamic observations 31:1270–1275
Utsumi S, Kinsella JE (1985) Structure function relationships in food proteins: subunit interactions in heat-induced gels of 7S, 11S and soy isolate proteins. J Agric Food Chem 133:297–303
Badley RA, Atkinson D, Hauser H, Oldani D, Green PJ, Stubbs MJ (1975) The structure, physical and chemical properties of the soybean protein glycinin. Biochim Biophys Acta 412:214–228
Wang D, Sun XS, Yang G, Wang Y (2009) Improved water resistance of soy protein adhesion at isoelectric point. ASABE 52:173–177
Acknowledgment
This article is contribution No. 11-292-J from the Kansas Agricultural Experimental Station, Manhattan, Kansas 66502.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Qi, G., Li, N., Wang, D. et al. Physicochemical Properties of Soy Protein Adhesives Obtained by In Situ Sodium Bisulfite Modification During Acid Precipitation. J Am Oil Chem Soc 89, 301–312 (2012). https://doi.org/10.1007/s11746-011-1909-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1909-6