Abstract
The effects of sodium bisulfite on the electrophoresis profile; turbidity; and thermal, surface, and adhesive properties of soy β-conglycinin protein were studied. Sodium bisulfite dissociated high-molecular-weight aggregates in the protein, and the aggregate percentage decreased with increasing sodium bisulfite concentration. Denaturation temperature of sodium-bisulfite-treated β-conglycinin increased as sodium bisulfite increased. However, at high sodium bisulfite concentration (i.e. 36 g/L), denaturation enthalpy decreased significantly. Sodium bisulfite caused changes in the β-conglycinin secondary structure and promoted ionization of lysine residues as indicated by FT-IR results. A sudden drop in turbidity at pH 4.8 was observed at the same salt level. The contact angle of β-conglycinin on cherry wood reached its minimum at 6 g/L sodium bisulfite and 24 g/L on glass. Water resistance of β-conglycinin was improved but not significantly by 6 g/L sodium bisulfite at pH 9.5. An obvious increase in adhesion strength of the protein occurred at 3 and 6 g/L sodium bisulfite at pH 4.8. A high sodium bisulfite concentration at 36 g/L sharply reduced the adhesive performance of β-conglycinin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proteins constitute about 40% of the soybean seed, and 90% of the proteins are extractable with water or salt solutions. Soy proteins have been extensively used as functional or nutritional components in a wide range of foods. Since being used as adhesives in the early 1920s, soybean proteins have also shown potential for use as alternatives to formaldehyde-based resins for wood products.
Globulins, the dominant storage protein, account for about 50–90% of soybean seed proteins. Major globulins in soybean are β-conglycinin (7S) and glycinin (11S). β-conglycinin is a trimer with a molecular weight of 150–200 kDa composed of the subunits α, α′, and β. The α and α′ subunits are composed of the core regions and extension regions, whereas the β subunit has only the core region. β-Conglycinin is a glycoprotein. The α and α′ subunits have two carbohydrate moieties and the β subunit has one [1]. Subunits of β-conglycinin are not covalently linked, rather they are held together primarily by hydrophobic forces [2]. β-conglycinin is composed of a relatively large amount of arginine and amide-containing amino acids, but lack tryptophan and sulfur-containing amino acids [3]. β-Conglycinin trimers exhibit an association–dissociation phenomenon under different pH and ionic strength. β-conglycinin has a trimeric structure (7S) at pH 7.0 and ionic strength μ >0.5 or as pH <4.8. When ionic strength μ <0.2, β-Conglycinin exists in hexamer form (10S) in the pH range 4.8–11.0 [4]. The association–dissociation behavior affects the β-conglycinin structure, resulting in changes in chemical and functional properties.
Soy protein modified with sodium bisulfite (NaHSO3) behaves like latex adhesives and has an adhesive strength comparable to formaldehyde-based adhesives [5]. β-Conglycinin and glycinin are the major components of the adhesive system. A previous study on the effects of sodium bisulfite on glycinin showed that the adhesive performance of glycinin was not improved by NaHSO3 modification [6]. Glycinin modified with NaHSO3 did not possess the cohesive behavior of latex-like soy protein adhesive. Therefore, glycinin may not be the main contributor to the special characteristics of the soy latex adhesive. This gave rise to the hypothesis that the other major component (β-conglycinin) may play an important role in the NaHSO3 modified soy latex adhesive. The behavior of β-conglycinin in the presence of NaHSO3 needed to be investigated thoroughly to elucidate its function in the adhesive system, which is the aim of this study. Although only few disulfide bonds were found in 1 mol of β-conglycinin and these bonds seemed to be buried in the hydrophobic region of the molecule [7], NaHSO3 can affect the structure and function of glycinin in terms of ionic strength. It is possible that β-conglycinin has a unique contribution to adhesion performance. This present study investigated the effects of sodium bisulfite on the electrophoresis profile, thermal properties, turbidity, contact angle and adhesive properties of soy β-conglycinin.
Materials and Methods
Materials
Defatted soy flour obtained from Cargill (Cedar Rapids, IA) was used for isolation of soy β-conglycinin. The soy flour contained 52.4% protein with a protein dispersibility index of 90. Sodium bisulfite was obtained from Fisher Scientific (Fair Lawn, NJ). Cherry wood veneers with dimensions of 50 × 127 × 4.8 mm (width × length × thickness) were provided by Veneer One (Oceanside, NY).
Isolation of β-Conglycinin
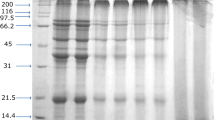
Crude β-conglycinin was separated from soy flour using the method described by Thanh and Shibasaki [8]. The ammonium sulfate fractionation method, as described by Iwabuchi and Yamauchi [9], was used to purify the β-conglycinin. Three grams of crude β-conglycinin were dissolved in 100 mL phosphate buffer (32.5 mM K2HPO4, 2.6 mM KH2PO4, 0.4 M NaCl, 10 mM mercaptoethanol, and 1 mM EDTA). Ammonium sulfate was added to the slurry to 75% saturation. Supernatant was obtained after the slurry was centrifuged. Ammonium sulfate was added further to the supernatant to achieve 90% saturation. After centrifugation, the precipitate was collected as β-conglycinin. Purified β-conglycinin was dialyzed against deionized water for 3 days and lyophilized. The β-conglycinin had about 91% purity (Fig. 1, lane A), as evaluated with SDS-PAGE.
SDS-PAGE pattern of native glycinin in the presence of 2-mercaptoethanol. In the absence of 2-mercaptoethanol: unmodified β-conglycinin (lane B); β-conglycinin modified by 3 g/L NaHSO3 (lane C); β-conglycinin modified by 6 g/L NaHSO3 (lane D); β-conglycinin modified by 12 g/L NaHSO3 (lane E); β-conglycinin modified by 24 g/L NaHSO3 (lane F); β-conglycinin modified by 36 g/L NaHSO3 (lane G)
NaHSO3 Treatment
The β-conglycinin was dispersed in deionized water at 5% solid content. The solution was adjusted to 9.5 with 1 N NaOH and stirred at room temperature for 1 h. NaHSO3 was added to the dispersion at 0, 60, 120, 240, 480, and 920 mg/g of β-conglycinin, equivalent to 0, 3, 6, 12, 24, and 36 g NaHSO3/L of solution. The pH of the resulting solution was maintained at 9.5 by adding 1 N NaOH and the modification was conducted with mild stirring at room temperature for 2 h.
SDS-PAGE
SDS-PAGE was performed on a 4% stacking gel and a 12% separating gel with a discontinuous buffer system as described by Laemmli [10]. Protein samples were mixed with a sample buffer containing 2% SDS, 25% glycerol, and 0.01% bromphenol blue. To prevent disulfide bond breakage not induced by NaHSO3, SDS-PAGE was performed in the absence of 2-mercaptoethanol for protein samples. To estimate purity of the β-conglycinin, 2-mercaptoethanol was added to the sample buffer to perform the reducing SDS-PAGE. The gel was stained in 0.25% Coomassie brilliant blue R-250 and destained with a solution containing 10% acetic acid and 40% methanol. Molecular weight standards (21.5–97.4 kDa) were run with the samples. Densitometry was obtained by analyzing the gel image using the Kodak 1D Image Analysis software, version 4.6 (Kodak, Rochester, NY).
Differential Scanning Calorimetry
The thermal properties of β-conglycinin samples were studied using a differential scanning calorimeter (DSC) (DSC7, Perkin-Elmer, Norwalk, CT) calibrated with indium and zinc. Protein solutions (about 50 μL) were hermetically sealed in the large-volume stainless steel pan. All samples were held at 20 °C for 1 min and then scanned from 20 °C to 150 °C at a heating rate of 10 °C/min. Peak temperatures (i.e., denaturation temperature) and denaturation enthalpies were calculated from the thermograms. Duplicates were made for each sample and the average values were reported.
Infrared Spectroscopy
β-Conglycinin samples were freeze-dried and ground for analysis. Fourier-transform infrared (FT-IR) spectroscopy was performed with a Nicolet Nexus 670 FT-IR spectrometer (Nicolet Instrument Corporation, Madison, WI). About 150 mg of ground β-conglycinin samples were made into a disc. Each disk was scanned 64 times at a resolution of 4 cm−1.
Turbidity
The turbidity of β-conglycinin samples was determined as the absorbance at 600 nm using the method described by Thanh and Shibasaki [8]. Modified β-conglycinin samples were diluted to 0.1% with deionized water and maintained at pH 9.5. The pH of diluted protein samples was adjusted to 4.8 with 0.1 N HCl. All protein samples at pH 9.5 and 4.8 were stirred for 1 h before being analyzed with a spectrometer (UV-1650PC, Shimadzu Scientific Instruments, Columbia, MD). All measurements were done in duplicate and the average was reported.
Contact Angle Measurement
The contact angle was measured with an Optical Contact Angle Meter (CAM100, KSV Instruments, Helsinki, Finland). A droplet of β-conglycinin solution (2 μL) was placed on the surface of the substrate. Two substrates were used in this test: cherry wood and glass (plain microscope slides, Fisher Scientific, Fair Lawn, NJ). Contact angles for cherry wood were measured every 3 s from 0 to 147 s. For glass, the contact angles were measured every 1 s from 0 to 49 s. Five replicates were made for each sample, and average values were used for analysis.
Morphology Properties
β-Conglycinin samples were diluted to 1% with deionized water for imaging. Diluted samples were absorbed onto Formvar/carbon-coated 200-mesh copper grids (Electron Microscopy Science, Fort Washington, PA) and stained with 2% (w/v) uranyl acetate (Ladd Research Industries, Inc., Burlington, VT). A Philips CM 100 (FEI Company, Hillsboro, OR) transmission electron microscope (TEM) was used to investigate the microstructure of β-conglycinin samples. The morphology of β-conglycinin was observed with operation conditions at an accelerating voltage of 100 kV.
Wood Specimen Preparation
Cherry wood veneers were preconditioned in a chamber (Electro-Tech System, Inc., Glenside, PA) for 7 days at 23 °C and 50% relative humidity. The extracted β-conglycinin was tested for adhesion quality. Since the soy latex adhesive was prepared by adjusting the pH of the NaHSO3-modified soy protein from 9.5 to 4.8 [5], the pH of β-conglycinin was also adjusted from 9.5 to 4.8 by using 1 N HCl to simulate the processing procedure for soy latex adhesives. A volume of 350 μL β-conglycinin solution was brushed onto a marked area of 127 mm × 20 mm (length × width). Two brushed wood pieces were left at room condition for 15 min then assembled and hot-pressed (model 3890 Auto M; Carver, Inc., Wabash, IN) at 4.9 MPa and 170 °C for 10 min. The glued wood assemblies were cooled, conditioned at 23 °C and 50% relative humidity for 3 days, and cut into five pieces with dimensions of 80 × 20 mm (glued area of 20 × 20 mm). The cut wood specimens were conditioned for another 4 days before measurement.
Shear Strength
Wood specimens were tested using an Instron Tester (Model 4465, Canton, MA) according to ASTM Standard Method D2339-98 [11]. Crosshead speed was 1.6 mm/min, and stress at maximum load was recorded as shear strength. The average of five replicates was reported.
Water Resistance
Water resistance was measured following ASTM Standard Methods D1183-96 and D1151-00 [11]. Wood specimens were soaked in tap water at 23 °C for 48 h and tested immediately after soaking for wet strength. Soaked strength was assessed after specimens were dried and conditioned at 23 °C and 50% humidity for another 7 days. Shear strength was tested as described previously.
Results and Discussion
SDS-PAGE Analysis
In reducing SDS-PAGE, β-conglycinin gave three major bands representing α′ (~82 KDa), α (~77 KDa) and β (~47 KDa) subunits, respectively (Fig. 1, lane A). In nonreducing SDS-PAGE, a high-molecular-weight band appeared in all unmodified β-conglycinin (Fig. 1, lane B) and NaHSO3-modified β-conglycinin samples (Fig. 1, lanes C–G). Intensity of the new band decreased gradually with increasing NaHSO3 concentration (Fig. 1, lanes C–G). In β-conglycinin, the α and α′ subunits have a small amount of cysteine [11], so it is possible that these two subunits took part in the formation of the aggregation as suggested by Petruccelli [12]. NaHSO3, which could break disulfide bonds, helped to dissociate the aggregates in the unmodified β-conglycinin.
However, the main SDS-PAGE patterns of unmodified and modified β-conglycinin were similar in the range of NaHSO3 from 0 to 36 g/L (Fig. 1, lanes B–G), which implied that NaHSO3 had no effect on cleavage of the protein. Two moles of disulfide bond are present in a mole of β-conglycinin, and these bonds are buried in the hydrophobic region [7]. It is very difficult for the reducing agent NaHSO3 to reach the buried disulfide bonds. This result is in agreement with the findings on the effect of reducing agents such as 2-mercaptoethanol on β-conglycinin [13]. No significant effects of 2-mercaptoethanol were observed on ultracentrifugation, optical rotatory dispersion, and fluorescence emission of β-conglycinin.
Effects of NaHSO3 on Thermal Properties
The denaturation temperature (T d) and enthalpy (ΔH) of denaturation of β-conglycinin treated with different NaHSO3 concentrations are shown in Table 1. β-conglycinin with 0 g/L NaHSO3 had an ΔH of 4.1, which is only about 57% of the reported value 7.25 [14]. Because ΔH is the quantity of the heat energy required to denature the protein, higher ΔH indicates a more stable and ordered structure. The decrease in ΔH suggests the β-conglycinin was partially denatured at pH 9.5 during stirring for 3 h even without NaHSO3 treatment.
The NaHSO3-treated β-conglycinin had significantly higher T d and ΔH than the untreated protein. The T d of β-conglycinin increased with higher NaHSO3 concentration and reached its maximum value at 36 g/L (Fig. 2). T d is the transition temperature at which the ordered protein structure is disrupted and unfolded. Its value reflects the stability of protein structure. The increase in thermal stability of β-conglycinin could be due to neutralization of negative charges on the protein surface by salt, which reduce electrostatic repulsion and stabilize the protein.
β-Conglycinin achieved a 1.5-fold increase in ΔH after modification with 3 g/L NaHSO3. The ΔH kept increasing up to 24 g/L NaHSO3, and dropped significantly at 36 g/L NaHSO3 (Table 1). Deak et al. [15] also observed the increase in ΔH of β-conglycinin after addition of NaHSO3, but ΔH were relatively constant at different concentrations of NaHSO3 in their research. Treatment with NaHSO3 enhanced the forces (e.g. hydrogen bonding and hydrophobic force) that stabilize β-conglycinin conformation, so the destabilized β-conglycinin in alkaline condition gained stability after adding NaHSO3. In the pH range 4.8–11, β-conglycinin (7S) dissociates to 5.6S and 2S at high ionic strength [16]. The decrease in ΔH at 36 g/L NaHSO3 might be a result of this dissociation.
FT-IR Spectroscopy
Absorption bands in FT-IR spectra, corresponding to amide I and amide II, are frequently used to characterize the backbone conformation of protein. Unmodified β-conglycinin showed the amide I band at 1,697 cm−1, and a small shift of the band to 1,695 cm−1 was observed in protein modified by 6 g/L NaHSO3 (Fig. 3). In β-conglycinin modified by 36 g/L NaHSO3, the amide I band was centered at 1,685 cm−1 with a shoulder around 1,650 cm−1 (Fig. 3). The amide I band is composed of protein secondary structure components including α-helix, β-sheet, and random coil. The shift of the amide I band maxima indicates that the secondary structure of β-conglycinin was changed by NaHSO3 modification, especially at high salt concentration. β-sheets, α-helix, and random coils give bands at around 1,690, 1,652, and 1,660 cm−1, respectively [17]. The shift of the amide I band from 1,697 to 1,685 cm−1 suggests that the β-sheet content in β-conglycinin decreased with NaHSO3 modification. At 36 g/L NaHSO3, the shoulder at 1,650 cm−1 implied that there was an increase in α-helix or random coil components in β-conglycinin.
The NH3 + stretching vibrations of amino acids consist of multiple combination and overtone bands at about 2,000 cm−1. Also, NH3 + ions have a sharp bending band around 1,400 cm−1 [18]. With the addition of 6 g/L NaHSO3, absorption bands at 1,928 and 1,435 cm−1 appeared, and these peaks became more intense with increasing NaHSO3 concentration from 6 to 36 g/L (Fig. 3). These results suggested that NH3 + groups were present in modified β-conglycinin. The NaHSO3 modification may provide a favorable environment for ionization of amino groups to NH3 +. At pH 9.5, β-conglycinin attains negative charges and the quantity of NH3 + should be very limited. Because lysine ε-amino groups have a pK a at around 10.5, it is most likely that the NH3 + ion groups are derived from the lysine ε-amino groups. However, NaHSO3 has a strong absorption band at 1,440 cm−1. The band at 1,435 cm−1 may be partially due to presence of NaHSO3 in β-conglycinin.
Effects of NaHSO3 on Turbidity
Figure 4 shows the turbidity of the β-conglycinin at pH 9.5 and 4.8 as a function of NaHSO3 concentration. At pH 9.5, the turbidity of β-conglycinin was independent of NaHSO3 concentration in the range from 0 to 36 g/L (Fig. 4). β-Conglycinin has a negative surface charge at pH 9.5, so the addition of salt suppresses protein electrostatic interaction and promotes aggregation. However, as shown by SDS-PAGE, NaHSO3 dissociated intermolecular disulfide-bond-linked polymers and potentially lowered turbidity. Moreover, the relatively high surface charge density of β-conglycinin determines that a large amount of salt is needed to neutralize electrostatic repulsion [19]. It is possible that the greatest NaHSO3 concentration, 36 g/L, was not high enough to significantly decrease turbidity by shielding surface charges.
β-Conglycinin had much higher turbidity at pH 4.8 than at pH 9.5, which is due to favorable protein–protein interaction when the pH approached its isoelectric point (Fig. 4). Turbidity increased slowly with increasing NaHSO3 concentration up to 12 g/L, almost leveled off at 24 g/L, and then decreased significantly from 1.065 to 0.689 as NaHSO3 concentration reached 36 g/L. As discussed in the thermal properties section, the ordered β-conglycinin structure was partially disrupted under the alkaline conditions. The altered conformation will cause changes in the surface charge of β-conglycinin; e.g., the isoelectric point might shift away from 4.8. Turbidity of β-conglycinin increased slightly because of electrostatic shielding upon addition of NaHSO3 at pH 4.8. When NaHSO3 concentration exceeds the level that makes protein possess a net zero charge, the salting-in effect progressively dissociates aggregates and increases solubility [2].
Effects of NaHSO3 on Contact Angle
Figure 5 shows the contact angle of β-conglycinin on cherry wood (Fig. 5a) and glass surfaces (Fig. 5b) as a function of NaHSO3 concentration. The contact angle is a parameter indicating the affinity of a liquid for a solid. A lower contact angle is an indication of better wettability of a liquid on a solid surface. On the wood surface, 3 g/L NaHSO3 did not significantly decrease the contact angle. The contact angle of β-conglycinin reached its minimum at 6 g/L NaHSO3 and then progressively increased with increasing NaHSO3 concentration. The TEM images of β-conglycinin demonstrate the difference in morphology of unmodified and modified β-conglycinin (Fig. 6). In the absence of NaHSO3, β-conglycinin was composed mainly of large aggregates of various sizes (Fig. 6a). With 6 g/L NaHSO3, almost all the aggregates fragmented to uniform-sized granules dispersed in water (Fig. 6b). Fragmentation of aggregates increased the surface area of β-conglycinin protein polymer, resulting in an increase in the effective contact area between the protein molecules and wood surface.
However, the existence of salt (NaHSO3) could decrease the effective protein–wood interfacial area. For β-conglycinin with a high NaHSO3 concentration (i.e., 36 g/L), a thin layer of salt sedimentation on the wood surface was observed after a drop of protein solution was absorbed by the wood. The presence of NaHSO3 could prevent intimate contact between β-conglycinin and the wood surface, inhibit molecular attraction between them and increase the contact angle extensively.
Glass has a high-energy hydrophilic surface. The contact angle is greatly affected by the surface free energy of the solid substrate. A surface with a higher surface energy has a lower contact angle. Therefore, β-conglycinin solutions have a much lower contact angle on glass than on wood. Similar to a wood surface, the contact angle of β-conglycinin decreased with increasing NaHSO3 concentration. However, the lowest contact angle was obtained at 24 g/L NaHSO3 (Fig. 5b). In addition to dispersing aggregates, NaHSO3 could make the protein solution more polar, which favors the attraction between the protein solution and the hydrophilic glass surface. On glass, the reinforced positive effect of NaHSO3 dominates the contact process over the negative effect up to 24 g/L NaHSO3. However, at high NaHSO3 concentration (36 g/L), the barrier formed between protein and glass significantly increased the contact angle.
Effects of NaHSO3 on Adhesive Shear Strength
The adhesive strength of β-conglycinin treated with different concentration of NaHSO3 at pH 9.5 and 4.8 is shown in Table 2. At pH 9.5, NaHSO3 had insignificant effects on the dry strength in the range from 0 to 24 g/L, but a notable reduction was observed at 36 g/L NaHSO3. Good wetting is synonymous with intimate molecular contact between the adhesive and wood substrate, which must be compatible for good adhesion [20]. The relatively high contact angle of β-conglycinin with 36 g/L NaHSO3 on the wood surface resulted in poor wetting of the protein adhesive on wood. In addition, proteins and peptides containing more β-sheet structure tend to have a higher adhesion strength [21]. The FT-IR results show that the decrease in the amount of β-sheet structure in β-conglycinin may result in a lower dry strength of β-conglycinin at 36 g/L NaHSO3.
The wet strength of β-conglycinin at pH 9.5 decreased as dramatically as dry strength (Table 2). During soaking, water could take away soluble components in the protein adhesive and salts, and compete with the protein to form hydrogen bonds with the wood. The result is a weakening of the bond strength. β-conglycinin treated with 6 g/L NaHSO3 showed a greater wet strength, but this increase was not significant. The small increase might be related to better wettability (low contact angle) at 6 g/L NaHSO3. When water dissolves salts in the protein adhesive, cavities would be generated in the adhesive that penetrated into wood surface and between the pieces of wood. These cavities disrupt the continuous adhesive matrix which is detrimental to its strength. The higher the NaHSO3 concentration, the more cavities are generated during water soaking. This effect could be the main reason for the reduced adhesive strengths observed at concentrations from 12 to 36 g/L NaHSO3.
At pH 4.8, the dry strength of β-conglycinin increased significantly as NaHSO3 concentration rose to 3 g/L, and gradually decreased from 12 to 36 g/L NaHSO3 (Table 2). As discussed in the turbidity section, protein interaction was strengthened in the presence of NaHSO3, which is favorable for protein entanglement during curing and gives high strength [22]. However, an excessive increase in protein aggregation can hinder penetration of the protein into the pores on the wood surface, restrain formation of mechanical interlocks, and weaken adhesion. At 36 g/L NaHSO3, dissociation of protein aggregates could result in too much penetration into the wood surface. If a large portion of small molecules penetrated deeply into the wood surface, then the relatively long distance between proteins would restrict protein interaction [23]. β-Conglycinin with 0 and 3 g/L NaHSO3 had the best wet strength, whereas a higher amount of NaHSO3 induced a decrease in the wet strength. A similar phenomenon was observed at pH 9.5, the wet strength was extensively affected by the negative effect of NaHSO3 as a salt (Table 2).
Conclusions
Our results indicated that NaHSO3 altered the ionic strength surrounding the protein molecules in the solution environment and changed surface charges of the protein, which subsequently affected the thermal stability and turbidity of β-conglycinin positively. The NaHSO3 treatment was also able to change the protein secondary structure and cause lysine ionization in β-conglycinin, e.g., the β-sheet structure in β-conglycinin decreased at 36 g/L NaHSO3. Excessive salt prohibited intimate contact between the protein and substrate. Moderate NaHSO3 concentration improved the adhesive strength of β-conglycinin at pH 4.8 and the water resistance at pH 9.5, but surplus NaHSO3 was not favorable for adhesive performance. This study revealed that the adhesive properties of β-conglycinin were only slightly improved by NaHSO3 modification, which cannot explain the strong adhesion of a soy latex-like adhesive. The working mechanism of the soy latex adhesive probably involves complicated interactions between β-conglycinin and glycinin, which needs to be studied further.
References
Thanh VH, Shibasaki K (1977) Beta-conglycinin from soybean proteins isolation and immunological and physicochemical properties of the monomeric forms. Biochim Biophys Acta 470:370–384
Kinsella JE, Damodaran S, German B (1985) Physicochemical and functional properties of oilseed proteins with emphasis on soy protein. In: Altschul AM, Wilcke HL (eds) New protein foods, vol 5. Seed storage proteins (Food Science and Technology). Academic Press, Orlando, FL, pp 107–179
Nielsen NC (1974) Structure of soy proteins. In: Altschul AM, Wilcke HL (eds) New protein foods. Academic Press, New York, pp 27–64
Lampart-Szczapa E (2001) Legume and oilseed proteins. In: Sikorski ZE (ed) Chemical and functional properties of food proteins. Technomic, Lancaster, pp 407–434
Sun XS, Zhu L, Wang D (2006) Latex based adhesives derived from soybeans. U.S. Patent PCT pub. No. 35091, pending
Zhang L, Sun XS (2008) Effect of sodium bisulfite on properties of soybean glycinin. J Agric Food Chem 56:11192–11197
Thanh VH, Shibasaki K (1976) Heterogeneity of beta-conglycinin. Biochim Biophys Acta 439:326–338
Thanh VH, Shibasaki K (1976) Major proteins of soybean seeds. J Agric Food Chem 24:1117–1121
Iwabuchi S, Yamauchi F (1987) Determination of glycinin and β-conglycinin in soybean proteins by immunological method. J Agric Food Chem 35:200–205
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Annual Book of ASTM Standards (2002) ASTM International, West Conshohocken, PA
Petruccelli S, Anon MC (1995) Soy protein isolate components and their interactions. J Agric Food Chem 43:1762–1767
Clara Sze KW, Kshirsagar HH, Venkatachalam M, Sathe SK (2007) A circular dichroism and fluorescence spectrometric assessment of effects of selected chemical denaturants on soybean (Glycine max L.) Storage proteins glycinin (11S) and β-conglycinin (7S). J Agric Food Chem 55:8745–8753
Mo X, Sun X, Wang D (2004) Thermal properties and adhesion strength of modified soybean storage proteins. J Am Oil Chem Soc 81:395–400
Deak NA, Murphy PA, Johnson LA (2006) Effects of reducing agent concentration on soy protein fractionation and functionality. J Food Sci 71:200–208
Ibuchi C, Imahori K (1978) Heterogeneity and its relation to the subunit structure of the soybean 7s globulin. Agric Biol Chem 42:31–36
Mizutani Y, Matsumura Y, Imamura K, Nakanishi K, Mori T (2003) Effects of water activity and lipid addition on secondary structure of zein in powder systems. J Agric Food Chem 51:229–235
Silverstein RM, Webster FX (1997) Characteristic group absorptions of organic molecules. In: Silverstein RM, Webster FX (eds) Spectrometric identification of organic compounds. Wiley, pp 81–109
Yuan YJ, Velev OD, Chen K, Campbell BE, Kaler EW, Lenhoff AM (2002) Effect of pH and Ca2+-induced associations of soybean proteins. J Agric Food Chem 55:4953–4958
Gollob L, Wellons JD (1989) Wood adhesion. In: Skeist I (ed) Handbook of adhesives. Van Nostrand Reinhold, New York, pp 598–610
Mo X, Hiromasa Y, Warner M, Al-Rawi AN, Iwamoto T, Rahman TS, Sun X, Tomich JM (2008) Design of 11-residue peptides with unusual biophysical properties: induced secondary structure in the absence of water. Biophys J 94:1807–1817
Mo X, Zhong Z, Wang D, Sun X (2006) Soybean glycinin subunits: characterization of physicochemical and adhesion properties. J Agric Food Chem 54:7589
Cheng E, Sun X (2006) Effects of wood-surface roughness, adhesive viscosity and processing pressure on adhesion strength of protein adhesive. J Adhes Sci Technol 84:997–1017
Author information
Authors and Affiliations
Corresponding author
Additional information
X. S. Sun: Contribution No. 09-060-J from the Kansas Agricultural Experimental Station, Manhattan, KS 66506.
About this article
Cite this article
Zhang, L., Sun, X.S. Sodium Bisulfite-Induced Changes in the Physicochemical, Surface and Adhesive Properties of Soy β-Conglycinin. J Am Oil Chem Soc 87, 583–590 (2010). https://doi.org/10.1007/s11746-009-1528-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1528-7