Abstract
Aqueous extraction processing technologies, having advanced in recent years, may be a viable alternative to hexane extraction to separate oil and protein from soybeans. Different extraction strategies incorporating various modes of comminution, extraction buffers, and enzymes allow production of a range of oil and protein products, but also create different processing challenges. Processes capable of achieving high free oil yields often result in a soluble protein fraction difficult to isolate and dilute oil emulsions difficult to break. Other processes can achieve high yields and purities of native soy protein, but with reduced free oil yield or require a high osmotic and ionic strength extraction buffer. This review article discusses these various advanced processes and their relative advantages and disadvantages. In addition, the current understanding of the underlying fundamental concepts of aqueous extraction is discussed in order to help direct future investigations to improve these technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although a very small number of soybean crushing plants utilize mechanical screw presses with soybeans, the vast preponderance of today’s high-yielding vegetable oil extraction processes use organic solvents, the most prevalent being hexane to achieve crude oil yields greater than 95% [1, 2]. In a few operations, isohexane may have recently been used with soybeans [3, 4]. Hexane, a mixture of hexane isomers rich in n-hexane (≈60%), is derived from nonrenewable sources (petroleum distillates) and is highly flammable and explosive, posing hazards to plant personnel and property. Furthermore, hexane in the atmosphere can react with nitrogen oxides to form ground level ozone, causing off-site health hazards in surrounding communities. Therefore, hexane is classified as a volatile organic compound by the US. Environmental Protection Agency, and, as such, its release must be tightly controlled, monitored and reported, with excessive releases resulting in expensive fines. The combination of these factors makes hexane extraction of vegetable oil a capital-intensive and operationally complex process that is expensive and challenging to operate safely.
Alternatives to solvent extraction have existed for many years. Some methods predate solvent extraction by centuries [5], but none have matched the high oil yields of solvent extraction. With rising petroleum costs and increasing awareness of environmental issues, there is renewed interest in developing high-yielding alternatives to solvent extraction to produce edible oil from soybeans. Among the potential alternatives to solvent extraction, aqueous extraction processing (AEP) has received much interest. AEP has a significant advantage over hexane extraction besides safety and environmental issues in that both oil and protein are extracted simultaneously. However, AEP free oil yields are still less than hexane extraction. A review paper comparing AEP with the assistance of various enzymes of oilseeds to hexane extraction processing, published by Rosenthal et al. in 1996 [6], highlighted the advantages and disadvantages of this emerging technology over conventional processes. More recently and since the Rosenthal review, advances in AEP technologies applied to soybeans have shown particular promise [7–9].
Aqueous Extraction Processing and Enzyme-Assisted Aqueous Extraction Processing (EAEP)
In AEP/EAEP, water is used as an extracting medium to remove oil as an emulsion or free oil unlike organic solvents, which dissolve the oil. Centuries ago it was discovered that grinding soybeans in the presence of water freed the oil from the bean tissues and the oil would rise to the top and float, usually as a highly emulsified cream [10, 11]. Typical steps in AEP are: (1) mechanical disruption of soybean cells by grinding; (2) extraction of oil and protein with or without enzymes; (3) centrifugal separation of an oil-rich emulsion, insoluble solids, and a liquid containing water solubles; and (4) demulsification of the oil-rich cream fraction to recover free oil. A simplified diagram of an early AEP process proposal incorporating protein recovery and water recycle is shown in Fig. 1. AEP/EAEP results in three distinct fractions: an insoluble fraction (residual fraction) rich in cellulose, insoluble proteins and other insoluble material as well as entrained soluble material; a liquid fraction (skim) of soluble proteins, minerals, and carbohydrates as well as measurable amounts of dispersed oil droplets; and an oil-in-water emulsion stabilized by proteins and phospholipids (cream). Only occasionally would any free oil be present when used with soybeans; all recoverable oil had to come from demulsifying the very stable cream.
Overview of a proposed aqueous extraction process utilizing ultrafiltration for protein recovery and water recycling. Water is introduced into the process with the recycled water recovered from membrane filtration. From Lusas et al. [12]
Each of these three fractions presents a challenge to the economic viability of AEP/EAEP of soybeans. First, oil remaining in the residual insoluble solids and skim represents a significant oil loss compared to solvent extraction processes, frequently on the order or 35% of the total oil (Table 1). Second, the cream is a highly stable oil-in-water emulsion that must be demulsified to recover a free oil product. Third, the skim fraction, containing all the soluble material (much of the protein) and a significant portion (~20%) of the total oil dispersed as an oil-in-water emulsion, contains a large fraction of the total soybean mass. Economic viability, therefore, depends upon employing better separation technologies to recover more of the oil in the cream and/or creating value-added products from this novel material; the skim fraction has properties unlike any other by-product of current soybean processing methods, so recovering value from the skim presents an important problem to be addressed. Because these challenges are interconnected, a solution to one challenge often creates complications with another. Therefore, an overall economically optimal process must address each of these three challenges in combination. The scope of this review paper is to provide a synopsis of literature since the Rosenthal et al. review [6] advancing these three areas for soybeans, pointing out interconnections where appropriate, and discussing relative advantages and disadvantages of various techniques.
Soybean Cotyledon Microstructure
A good understanding of microstructural characteristics of soybeans is important for understanding extraction principles. Soybean plants are dicots, with seeds consisting primarily of cotyledon cells (collectively known in the trade as meat) surrounded by a seed coat and pericarp layer. Typical soybean composition is 20% oil, 40% protein, 35% carbohydrate (16% soluble and 19% insoluble), and 5% ash on dry basis [13]. Most of the soybean protein and oil are stored in the cotyledon tissue in organelles called protein bodies and oil bodies (also called oleosomes), respectively. A transmission electron micrograph of the soybean cotyledon tissue is shown below in Fig. 2. Typical cotyledon cells are cylindrical in shape, about 30 μm in diameter and 70–80 μm long [14]. To achieve a high degree of disruption of cells by size reduction, particle sizes must be of this length scale.
TEM of soybean seed and cotyledon cells. Reproduced from Campbell and Glatz [15]. CW cell wall, PB protein body, N cell nucleus, OB oil body. Copyrighted figure courtesy of the Journal of Agricultural and Food Chemistry, ACS publishing
Because the cell wall is the primary barrier to extraction or to washing the oil from the cell, it must be ruptured for any significant extraction to occur [6, 15–17]. In the crushing industry, this is called cell distortion. Like most plant cells, the soybean primary cell wall is constructed of pectins, hemicelluloses, and microfibrils of cellulose cross-linked with protein. Within the primary cell wall is a secondary cell wall of cellulose and hemicelluloses [18]. The cells are held together by a middle lamella composed mostly of pectins [19]. Mass transfer across the cell wall barrier occurs through plasmodesmata, small openings in the cell wall ranging from 20 to 80 nm in diameter. These will allow transfer of molecules with molecular weights up to 900 Da [20], establishing the importance of disrupting the cell wall for extraction of oil and protein to take place.
About 80% of the total protein in soybeans is stored in organelles called protein bodies, which occupy most of the cotyledon cell volume [14]. Therefore, removal of the protein bodies may be a prerequisite for oil release. Protein bodies range in size from 10 to 50 μm in diameter. In aqueous media, large protein body organelles are disrupted more easily than smaller protein bodies [21]. Above neutral pH, protein bodies dissolve quite readily in water provided there is adequate solvent for protein dissolution and adequate cellular disruption for effective mass transfer of solutes and solvents [22].
Soybean oil bodies are much smaller than protein bodies, ranging from 0.2 to 0.5 μm in diameter [14]. They fill the space between protein bodies and are enmeshed in a matrix of cytoplasmic proteins [23]. Oil bodies are stabilized by a delimiting membrane composed of a phospholipid membrane interspersed with an amphipathic protein, oleosin [24, 25]. These proteins cover oil bodies with specific coverage of about 3.2 mg/m2, a number consistent over a broad range of oilseeds [25] and, in conjunction with phospholipid, play an important role in stabilizing the oil bodies both in situ and in aqueous environments [24]. Microscopic studies showed that oil bodies have an apparent affinity for cell walls, protein bodies, plasmalemma, and endoplasmic reticulum, but not for other organelles [14, 26], which may be an important consideration when trying to liberate oil from the confines of a disrupted cell.
In general, oil bodies are very stable toward coalescence in aqueous suspension and can survive the relatively high shearing and centrifugation speeds (10,000×g) typically used in their isolation [24, 25, 27]. Oil bodies remain intact in 9 M urea and after direct contact with hexane [27, 28] but coalescence can be induced by hydrolyzing the exterior protein portion of the droplet membrane with a protease [24]. While extrusion can also destroy the integrity of oil bodies, they largely remain intact following flaking or grinding with only minor coalescence, although unextracted oil after AEP of soybean flour (oil remaining in the residual fraction) was mostly in the form of coalesced oil droplets, and not native oil bodies [15].
Effects of Physical Treatments on Soybean Microstructure
Soybean Flour
Physical comminution can be an effective method for disrupting most, but often not all, soybean cell walls. The three most studied modes of comminution are grinding, flaking, and extruding.
Grinding soybeans into full-fat flour has little effect on the morphology of oil and protein bodies, but grinding can achieve substantial cellular disruption affecting ca. 95% of all cotyledon cells [15]. Both grinding and flaking followed by grinding releases much of the intracellular material from highly disrupted cells; however, larger multi-cell flour particles remain after grinding as well as after flaking followed by grinding, with disrupted cells close to the particle’s surface and intact cells at the interior of the particle.
Flaking
Flaking is the predominant comminution method used for commercial hexane extraction today. Typically, soybeans are cracked into approximately 4–6 fragments per bean, conditioned at 60 °C, and then passed through smooth-surfaced roller mills, resulting in flakes approximately 1 cm across by 250 μm thick [29]. Flaking cracked and conditioned soybeans causes extensive cell distortion and a high degree of disruption of cell walls with little change in morphology of the protein and oil bodies [30] as has been seen for other oilseeds, as well [31]. This disruption allows solutes and solvents to pass easily through the cellular matrix, which explains why flaking enhances hexane extraction. Images of flakes after solvent extraction of the oil indicated some oil coalescence; however, the cytoplasmic matrix around the oil bodies and protein bodies remains intact, and protein body morphology is unchanged, even after removing the oil [30]. In AEP, however, the removal of oil from the cells depends on the length and tortuosity of the oil release pathway. Even though flaking is effective in disrupting cells, it may not succeed in providing oil that can be easily separated from the cells.
Extrusion
A detailed study on the effects of extrusion on the morphology of untoasted defatted soybean flakes ground into grits was conducted by Aguilera et al. [32]. In the screw arrangement they studied, each extruder element increased cellular disruption, with complete disruption resulting from the effects of the rapid expansion as the material passed through the die. Extrusion substantially denatures soy proteins and destroys the cytoplasmic network [33]. Upon cooling, the proteins form a new network by disulfide cross-linking [34] and covalent bonding between lysine and glutamine or asparagines residues [35] although denaturation is incomplete. Differential scanning calorimetric studies of isoelectric precipitates of AEP extracts from extruded flakes did not indicate significant denaturation of glycinin and β-conglycinin, the two major storage proteins in soybean [36]. It is likely that denatured proteins were not extracted and remained in the residual fraction. Still, extrusion of full-fat soybean flakes results in complete destruction of the integrity of soybean protein bodies and oil bodies, with the oil remaining in the form of large coalesced oil droplets after extrusion [15].
While the complete cellular disruption achieved by extrusion would be advantageous for AEP, the formation of a new insoluble protein network sequesters oil within this network, adversely affecting oil extraction yields (Fig. 3) [15]. Furthermore, autocatalytic lipid oxidation can result in a polymerization reaction with proteins, where the peroxyl radical of a lipid peroxide reacts with susceptible amino acid residues and incorporates the protein in a lipid peroxide-protein polymer as shown below [37]
where L is lipid, P is protein, and O is oxygen. Extrusion is known to cause the formation of lipid complexes with proteins as well as with starch [38], and such complexing is consistent with observations noted in oil analyses of extruded full-fat soybean flakes [7]. Depending on the extent of covalent bonding between lipid and protein, this polymerization reaction may be a limiting factor to high yield of free oil.
Images of a extruded soy flakes before extraction b residual after AEP; and c residual after EAEP with protease. From Campbell and Glatz 2009 [15]. CO coalesced oil, CW cell wall fragment. Figure was modified from the copyrighted figure courtesy of the Journal of Agricultural and Food Chemistry, ACS publishing
Extraction of Oil
General Extraction Parameters for AEP/EAEP
Regardless of whether oil ends up as free oil, cream, or skim emulsion, oil must first be released from the solid material. This section will discuss the effects of processing conditions on oil extraction yield, defined here as oil released from the initial solids during AEP. Partitioning of oil into skim and cream fractions, as well as overall yield of free oil will be discussed in subsequent sections. In general, the extraction parameters that affect the release of oil from soybean are pH, solids-to-liquid ratio (S/L), agitation rate, particle size and/or type of cellular disruption, enzyme type, enzyme concentration, extraction time, and temperature [12, 39]. The effects of some of these parameters will be discussed in the context of various modes of comminution used for soybean extraction below. Regardless of mechanical treatment, however, the link between protein solubility and oil extraction is ubiquitous for soybeans [7, 8, 17, 39]. It is well known that soy proteins have affinity for oil; oil-holding capacity and emulsifying capacity and emulsion stability are commonly studied functional properties of soybean proteins. It is hypothesized that the oil is physically entrapped by insoluble protein [40] or adsorbed, as indicated by microscopy of protein-bound oil showing oil droplets wetting protein surfaces [41]. The oil-holding capacities of soy protein isolates and soy protein concentrates are 119–156 ml oil/100 g and 74–101 ml oil/100 g, respectively [40]. Because the mass of protein outweighs the mass of oil in soybeans by two to one, it is apparent that even if oil is released from a cell, it can still be entirely sequestered by insoluble protein. Rosenthal et al. [17] saw nearly identical extraction behavior for oil and protein between pH 2 and 10, with very low extraction yields near pH 4.5, the isoelectric point of soybean proteins, where protein solubility is at a minimum. Because protein solubility is highest at basic pH explains why most AEP/EAEP extractions achieve greatest yields at high pH. Likewise, extraction yields of oil and protein were reduced after heating soy flour to 121 °C [17], a temperature likely high enough to cause protein denaturation and reduced solubility.
For investigations intended to develop practical industrial applications using AEP/EAEP technologies, the protein extraction yield is, logically, often defined as the total protein separated from the residual fraction, which excludes dissolved protein entrained in the liquid portion of the residual fraction from the yield measurement. Considering the relationship between oil and insoluble protein, however, it should be pointed out that insightful information regarding fundamental extraction mechanisms can also be gained by reporting the total dissolved protein (protein in the skim plus the dissolved protein entrained in the liquid portion of the residual fraction). For example, accounting for total dissolved protein Campbell and Glatz [15] showed that, for soy flours between 0.05 and 0.20 S/L, there was no S/L dependency of protein dissolution, and total dissolved protein was also a reliable indicator of the extent of cellular disruption. This is a measure that future investigators should take into consideration.
Regardless of protein solubility, the release of any protein or oil requires the disruption of the soy cotyledon cell wall. This is illustrated by images of extracted residue published by Campbell and Glatz [15], showing no noticeable change in microstructure after extraction for those cells still intact. The most studied methods of cellular disruption of soybean are grinding, flaking, and extruding, as well as various combinations of the three methods. These mechanical treatments result in very different microstructure of the residual fraction, which will determine how oil is sequestered and liberated as droplets from the residual fraction.
Extraction from Extruded Soybean Flakes
Typical oil extraction yields and distributions of oil between the three fractions are summarized in Table 1. The most advanced AEP/EAEP processes utilize extruded full-fat soybean flakes as starting material [7–9, 15, 36, 42, 43]. Countercurrent two-stage extraction strategies (discussed in detail below) using extruded soybean flakes have achieved oil extraction yields of 97–99%, with S/L as high as 0.20 (Table 1) [9, 42]. Because of consistent oil levels in skim amounting to 10–20% of the total oil, overall free oil yields after demulsification still range from 75 to 85%, although these values represent the highest free oil yields reported in the literature. Cream oil yields of 85% have also been achieved from soy flours (Table 2) but with greater water usage, which will be discussed below.
High AEP/EAEP oil extraction yields, ranging from 88 to 99%, from extruded ground and/or flaked soybeans have only been achieved with proteases [7–9, 42, 43]. Proteases increase oil yield by dissolving the insoluble denatured protein, disrupting the insoluble matrix resulting from extrusion and releasing otherwise sequestered oil [15, 34, 36], as seen in Fig. 3. Cellulases have also been tested on extruded materials, but without achieving increased oil yield, [7, 43] indicative of the extent of cellular disruption achieved by extrusion. Conditioning moisture, but not temperature, prior to flaking/extrusion affects oil extraction yield, with maximum yield at 10% conditioning moisture [44]. Both conditioning temperature and moisture also affect the distribution of extracted oil between the cream and free oil fractions, although maximizing free oil yield by altering conditioning parameters is not necessarily advantageous. Cream from conditions that give greater free oil yields are more difficult to demulsify than creams from lower free-oil yielding conditions [44]. As with soy flour extraction, increasing S/L decreases oil extraction yield [9], although probably for reasons related to the ability of the extraction medium to break apart the extrudate matrix or protein-oil interactions, which is different than explanations proposed for similar effects in soy flours, discussed below. Although AEP of extruded ground and/or flaked soybeans has given the most success, few studies have been conducted to understand the more fundamental aspects of these processes. A better understanding may lead to process efficiency improvements and innovations to reduce the amount of oil remaining in the skim fraction.
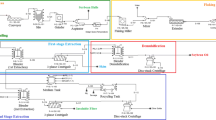
The most advanced AEP/EAEP process uses a countercurrent two-stage strategy (Fig. 4) utilizing extruded soybean flakes and protease enzymes [9, 42], which was developed to reduce water usage while maintaining the high yields achieved in earlier single-stage EAEP. In addition to increasing S/L from 0.10 to 0.17 (reducing water usage), two-stage EAEP increases oil extraction from 96% for single-stage EAEP to 99% for two-stage EAEP and protein extraction from 85 to 96% [8, 42]. Although two extraction stages achieved oil extraction levels similar to conventional hexane extraction (>97%) [42], overall free oil recovery in two-stage EAEP was only 78–80% because of oil remaining in the skim fraction [44].
The process in Fig. 4 reduces enzyme cost by using protease, which maintains activity under EAEP conditions, for both demulsification (discussed in demulsification section below) and extraction. Countercurrent two-stage extraction and cream demulsification were fully integrated and demonstrated on the laboratory scale (2 kg soybeans), with demulsification step yields of 81% [45]. Incomplete demulsification typically results in the formation of new cream and skim fractions, where the smallest oil droplets remain homogeneously distributed in the continuous phase, referred to in Fig. 4 as the third and fourth skim, and the larger droplets are concentrated in the floating oil-rich cream fraction. With continuing enzyme activity, the interfacial conditions continue to change, and so demulsification and oil separation is increased by additional separation steps (Fig. 4). By controlling enzyme activity between stages, countercurrent two-stage extraction also allows better control of the extent of hydrolysis of soy proteins, the usefulness of which will be discussed in “Recovering value from the skim fraction section” below.
Recently, de Moura et al. [46] demonstrated proof of concept of this same process at pilot scale using active enzyme in both stages. Extractions were scaled-up in the pilot plant at the Iowa State University Center for Crops Utilization Research using 75 kg of extruded flakes and 480 kg of slurry (0.17 S/L). Approximately 98% of the oil and protein were extracted. Recovery of free oil was approximately 76%, with 19% of the oil being retained in the skim. At pilot-plant scale, 91.6% demulsification could be achieved. Remaining cream and skim from the demulsification step were recycled to enable additional oil recovery.
Extraction from Flaked Soybeans
Two studies report oil yields from AEP/EAEP of flaked soybean between 56 and 60% at 0.1 S/L with marginal or no effect from the addition of protease using a relatively aggressive or mild protease, respectively [36, 47]. There was a modest but significant 6% increase in protein extraction yield for flake extraction using the aggressive protease [47].
Jung and Mahfuz [36] tested the effects of high pressure treatment on extraction from flakes, with consistent correlation between oil and protein extraction. At 200 MPa, protein extraction increased significantly compared to unpressurized controls, with a statistically insignificant increase in oil. At 500 MPa, denaturation reduced protein solubility and, consequently, oil extraction yield. Microscopic images of the residual fraction from the 500 MPa extraction showed oil entrained in an insoluble protein matrix [34], similar to that seen in extruded material. The use of proteases increased both protein and oil extraction for 500 MPa treated flakes, but yields were still less than 200 MPa treated flakes. Extraction of undenatured protein ranged between 70 and 77%.
Extraction from Soy Flours
Single-stage oil extraction yield from soybean flours are typically between 60 and 80% (Table 1), depending on extraction conditions [17, 39]. In addition to pH, temperature and protein solubility, oil and protein extraction yields from flour are affected by agitation rate, S/L, protease, cellulase, ultrasonication [48], and number of successive extraction stages.
Proposed models incorporating flour particle size have offered some insight into the mechanisms through which the parameters listed above affect yield [15, 17]. Major principles of oil and protein extraction from soybean flour are illustrated in Fig. 5. Campbell and Glatz [15] proposed a model based on microscopic images of soy flour and extraction data whereby oil is readily released from particles <55 μm in diameter (hence, highly disrupted cells since this is the average dimensional length of a soy cell). Likewise, cells >55 μm from the edge of large flour particles are, on average, completely intact and are therefore unextractable. Between these two extremes, oil release is dependent upon extraction conditions (i.e., agitation rate, S/L, extraction time and enzyme). Rosenthal et al. [17] also presented an extraction model based on the principle of disrupted cells at the surface of flour particles. They postulated that oil and protein yields should be proportional to the specific surface area of flour particles, and confirmed this experimentally by showing a linear relationship between oil yield and the inverse of flour particle radius. It is worth pointing out that the slope of this line was 27 μm, indicating that 100% oil yield would be obtained from a particle with a radius of 27 μm (or diameter of 54 μm), coinciding well with the 55 μm deduced by Campbell and Glatz [15].
Unextracted oil in disrupted cells of flour particles consists mostly of coalesced oil droplets [15], suggesting that agitation increases extraction yields by decreasing the average size of coalesced oil droplets, increasing droplet mobility and the likelihood of passing out of the cellular matrix into the bulk medium. Campbell and Glatz [15] supported this hypothesis by adding sodium dodecyl sulfate, a common surfactant, to the extraction medium, resulting in 13% more oil being extracted. The energy imparted by agitation may also increase yields by increasing cellular disruption [17]. Similarly, ultrasonication can increase oil and protein extraction yields [48, 49]. For flour particles between 125 and 150 μm, the increase was very large, from 62 to 90% at 0.17 S/L [48]. While the authors attributed the effects of ultrasonication to cellular disruption, it is likely that ultrasonication increases oil emulsification as well, which would be another explanation for the ultrasonication effects on yield.
The S/L may affect oil and protein extraction yields through several mechanisms. Small oil droplets may pass freely in and out of disrupted cells, resulting in some fraction of total oil entrained in the void volume of these cells (Fig. 5). Increased S/L increases droplet concentration as well as the size of the disrupted cell void volume relative to total liquid volume, decreasing extraction yield. This would explain how successive extractions achieve higher yields. Rosenthal et al. [17] achieved >90% oil extraction yields with successive extractions from mono-disperse soy flour (100 μm), with no further yield increase after four extractions. Increased yields with successive extractions indicate that countercurrent multi-stage extraction techniques similar to those used in increasing yields in extruded flake extractions may also be successful in increasing oil yields from soy flour. Increasing S/L may also decrease yields by increasing the formation of inelastic protein films at the oil–water interface of coalesced oil droplets, preventing droplet disruption by turbulent forces, and also by increasing the continuous phase viscosity, which slows droplet diffusion allowing more time for droplet coalescence inside disrupted cells [15].
The use of proteases can increase extraction yields by up to 25% [2–4], depending on S/L [15], particle size [39], and agitation rate. For small particle size (100 μm) at high agitation rate (2,000 rpm), proteases have no effect on yields [39]. Two explanations for the yield increase from protease have been proposed. Yoon et al. [48] suggested that protease disrupts the protein network in which oil bodies are enmeshed, a statement supported by increases in protein yields upon hydrolysis. Campbell and Glatz [15] proposed that protease may increase yield by disrupting or preventing the formation of viscoelastic films at the oil–water interface, or by creating small polypeptides that diffuse to and along the interface faster than large native proteins, either of which assists in the disruption of coalesced oil droplets by weakening the interface.
Another enzymatic approach to AEP of flour is to increase cellular disruption with cellulases. The use of cellulases in EAEP of soybean flour has improved oil extraction yields, but only under certain conditions. Rosenthal et al. [39] observed decreased extraction yields of both protein and oil after cellulase treatment, an effect most likely a result of less favorable extraction pH. Even though the extraction was carried out at pH 9, the cellulase treatment prior to extraction was done at pH 5, which resulted in irreversible precipitation of protein. Using a process intended to extract intact oil bodies from flour (discussed in detail below), Kapchie et al. [49] extracted significantly more oil (as intact oil bodies) from soy flour after incubating 20 h with a mixture of cellulase, hemicellulase, and pectinase at a pH between 4.6 and 5.0 [50], increasing the oil extraction yield from 92 to 98% after four successive extractions without and with enzymes, respectively, the highest yield seen in the reviewed literature of soy flours. One notable difference with the work of Kapchie et al. is that the extraction medium contained 0.5 M NaCl and 0.4 M sucrose. Supernatant fractions from these extractions were rich in solubilized protein, even at isoelectric pH, which was due to the high salt content of the extraction medium.
Extraction of Intact Oil Bodies
There are many food, cosmetic, pharmaceutical and other applications requiring highly stable oil-in-water emulsions, for which AEP/EAEP fractions are inherently suitable. Seed oil bodies themselves could be considered to be a form of emulsion that, in situ, protects the seed’s lipid stores from endogenous plant enzymes during storage and provides the surface area for enzyme access to lipid for metabolism during germination [51]. Some recent research has focused on developing aqueous extraction technologies with the intention of recovering stable intact oil bodies [49, 52–57]. Most of these methods were adapted from the wide range of earlier oil body characterization studies [24, 25, 27, 58, 59]. Three prominent methods are shown in Fig. 6. Typically, the processes involve imbibing whole seeds or flour overnight in extraction buffer, followed by homogenizing/extracting, filtering, and floating of the oil bodies by centrifugation. The simplified method (Fig. 6 c) closely resembles conventional EAEP methods except for the use of high salt and sucrose concentrations and the addition of an incubation period prior to extraction to allow time for cellulolytic action. Extraction buffers for oil body EAEP range from alkaline tris buffers with moderate salt concentrations [56] to buffers with 0.5 M NaCl and 0.4 M sucrose, under acidic conditions [54].
Various extraction processes designed to recover intact oil bodies a Iwanaga et al. [51] starting from whole soybean b Kapchie et al. [49, 57] starting from soy flour with and without enzymes c simplified procedure with cellulase starting from soy flour [57] Dashed lines represent processing variant. *Enzyme cocktail consisting of 1% each of: (in Ref. [49]) Multifect Pectinase FE, Cellulase A, and Multifect CX 13L, or (in Ref. [57]) Multifect Pectinase FE, Multifect CX B, and Multifect GC
Oil yields from oil body extraction processes are summarized in Table 2. For single-stage extraction, yields of oil in the floating fraction are about 36% of the total in the starting whole seed (Process A) [56] and, for soybean flours (Processes B and C), range from 30% with filtration and without the use of cell wall-degrading enzymes (cellulase and pectinase) [55], to 64% with enzymes and with filtration [49, 55], although the effect of the enzymes on oil yield as cream is not clear. In Process B, which used a filtration step to remove the incubation buffer prior to extraction, a cellulase/pectinase enzyme cocktail had profound effects on oil yields as cream, increasing yields as cream from 39 (no enzyme) to 64% (with enzyme) [49]. Eliminating the filtration step by Process C, however, increased oil yield as cream to ca. 60% with or without enzyme [55]. Cellulase/pectinase enzymes used with Process C did result in a modest increase in extraction yield compared to Process C without enzymes, but only after four successive extractions, with yields of about 68 and 78% for extractions without and with enzymes, respectively [55]. A general absence of full mass balance data, as well as poor mass balance closures make evaluation and comparison of these alternative processes difficult. If poor mass balance closures in Table 2 are attributable to error in determining the fraction of oil in the cream fraction (which is typically a difficult measurement to make) then the oil body process may be the highest oil yield EAEP process.
Since AEP/EAEP are water-intensive processes, high solute concentration in the aqueous effluent could pose major problems to process economics, and elimination or recovery of such solutes would likely be necessary. Recycling up to three uses of extraction medium (skim) without enzyme makeup in Process C (Fig. 6C) attained extraction yields between 70 and 80% of oil in cream before yields began to decrease [54]. It is believed that high sucrose and salt concentrations are necessary to preserve the oil body organelle integrity during extraction. In addition to this, sucrose may assist in oil body flotation by increasing the continuous phase density, and also may increase cellular disruption by osmotic effects [55] although the hard cell wall of plant cells typically prevents lysis from osmotic shock, and any osmotic effects on cellular integrity during EAEP of soybeans have not been satisfactorily demonstrated. Using high salt concentration, on the other hand, increases protein solubility at low pH allowing cellulase-assisted extraction to occur near soy protein pI, as discussed previously. The aqueous fraction from oil body EAEP using cell wall-degrading enzymes with supernatant recycling contains 62% of the total soybean proteins in an undenatured form [54], and therefore contains much of the potential value from the soybean. Separation of these proteins from the sucrose and salt in solution, probably using ultrafiltration or dilution so isoelectric precipitation can occur would have to be achieved in order to realize these values.
Nature of Cream from AEP of Flour and Oil Body EAEP
Considering the high stability of oil bodies and the similarities between the processes used in oil body AEP/EAEP and conventional soybean flour AEP, it would seem possible that cream extracted from conventional soybean flour AEP could also be composed of native oil bodies. SDS-PAGE profiles of surface proteins from conventional AEP of soy flour cream [60] closely resemble surface protein profiles of soybean oil bodies at early stages of isolation, with oleosins as well as many other proteins associated with the oil droplets [27]. After heat treatment of conventional AEP cream, most non-oleosin proteins disappear from the protein profiles, indicating that non-oleosin proteins are bound to a membrane surface rather than an integral part of the interfacial membrane [60]. While this observed change highlights the possibility of conventional AEP creams being native oil bodies, heating cream does result in an increase in particle size, either from droplet coalescence or aggregation, although cream stability is not affected [60]. It is difficult to compare properties of conventional AEP and EAEP creams to those of oil body EAEP creams because few studies provide detailed analyses, such as droplet size-distribution and surface protein profiles, of the resulting creams. Reported most often are the oil and protein contents, which give oil-to-protein ratios that range from 2.5:1 to 5:1 for oil body AEP [56, 57], and from 12:1 to 21:1 for conventional soybean flour AEP [60, 61]. By comparison, oil-to-protein ratio of purified oil bodies of 0.5 μm in diameter containing an oleosin layer of 3.2 mg/m2 [25] would be 20:1. Endogenous proteins have particular affinity for oil bodies even after extensive rinsing treatments, especially in soybeans [27, 62]. The soybean thiol protease P34 (gly m Bd 30K), a known soybean allergen [63], has a particular affinity for the oil body membrane [28, 64]. Extraneous, non-membrane protein material is evident in images of the floating fractions from oil body EAEP [55, 57]. More work needs to be done to improve our understanding of the nature of conventional AEP creams from soybean flour and oil body EAEP.
Demulsification of AEP Cream
Demulsification of Cream from Extruded Soybean Flakes
Recovering free oil using AEP/EAEP has been the predominant goal of researchers in most of the published literature. Cream from extruded soybean flakes is easier to demulsify than cream from flour or from flakes [65] as indicated in Table 3, which summarizes demulsification efforts for a variety of AEP/EAEP processes. AEP of extruded flakes with or without protease produces free oil without any demulsification [65–67], but in general, protease addition during extraction not only increases the quantity of free oil, it also facilitates demulsification of the cream. Proteases used for cream demulsification also retain between 85 and 100% of their original activity, allowing the same protease to be used for both demulsification and extraction [61, 65]. Still, the poorer stability of cream from extruded flakes allows for a number of successful demulsification strategies, including reduction of pH to 4.5 (average isoelectric pH of soy proteins), and treatment with phospholipase. The success of these treatments indicates that the cream from extruded soybean flakes is stabilized by electrostatic repulsion between surface proteins and phospholipids, and elimination of either of these species results in complete coalescence of cream oil droplets.
Phospholipids play an important role in cream emulsion stability; however, eliminating phospholipid stabilization of cream from extruded soybean flakes is not completely straightforward. Adding small amounts of LysoMax (an A2 lysophospholipase) actually increases cream stability of cream from extruded soybean flakes [65, 66], probably a result of the creation of lysophospholipids, which are more effective emulsifiers than other phospholipids [61]. Phospholipase D, an endogenous phospholipase in soybeans, converts phosphatidylcholine (PC) and phosphatidylethanolamine (PE), two major soybean phospholipids, to phosphatidic acid (PA) [68]. Jung et al. [65] tested storage conditions of extruded soybean flakes to determine if endogenous phospholipase activity could affect cream stability. Neither temperature (between 4 and 30 °C) nor storage time (up to 96 days) affected cream stability, which was attributed to phospholipase D deactivation during extrusion. While extrusion may deactivate phospholipase D, it is active during AEP of soy flours as well as at the higher moisture content (typically ca. 15%) necessary for effective extrusion of full-fat soy flakes [69]. Converting PE and PC to PA before and during AEP/EAEP is associated with reduced free oil yield [69], and therefore could play an important role in determining emulsion stability.
Demulsification of Cream from Soybean Flour and Flakes
For cream and oil body isolates [58] from AEP and EAEP of soybean flour or flakes, stability toward coalescence is significantly greater than cream from extruded soybean flakes. The most successful demulsification treatments were extensive proteolysis, or proteolysis followed by hydrolysis of the interfacial phospholipid with a phospholipase [61]. Interestingly, the phospholipase that was used by Chabrand and Glatz [61] with success was a lysophospholipase with only an A1 activity, meaning it could cleave the sn 1 fatty acid of the phosphoglyceride only in the absence of a fatty acid at the sn 2 position. This suggests that the primary stabilizer of the cream oil droplets after proteolysis were lysophospholipids, as was confirmed by the analysis of the cream phospholipids. Lysophosphatidylcholine (LPC) and lysophosphatidic acid (LPA), present in the cream before demulsification, were eliminated after phospholipase treatment while other phospholipid species remained. This result may not be generally applicable, as lysophospholipids were probably created by endogenous enzymes during flour storage or during extraction itself. Observations of the formation of a precipitate following treatment with phospholipase at pH 4.5, which resulted in complete demulsification, may indicate interaction between proteins and phospholipids. Treatment at pH 4.5 with no phospholipase yielded neither a precipitate nor complete demulsification. At isoelectric pH, proteins may be able to pack much more densely at the oil–water interface because electrostatic repulsion is at a minimum. In the absence of any additional protein adsorption/desorption, changes in protein surface density would lead to uneven surface coverage, allowing coalescence until the total surface area allowed an optimal surface protein density, and, hence, only partial demulsification. Hydrolyzing phospholipid at the interface apparently resulted in protein desorption and complete droplet coalescence, indicating possible interaction between phospholipid and protein. Protein adsorption to an oil–water interface is generally regarded as irreversible, so it is unlikely this desorption would have occurred in the absence of some phospholipid-protein interaction.
Droplet size is one measure of emulsion stability. Physical pretreatment (i.e., grinding, flaking, wet homogenization, and extrusion) as well as extraction conditions affect the cream oil droplet size distribution. The surface-weighted mean particle size of the cream fraction (D 3,2) is 0.3 μm for extraction by homogenizing whole, imbibed seeds for 2 min in order to extract intact oil bodies [56], and 2.1 μm after 15 min extraction from soy flour, 4000 × g centrifugation, screen separation, and resuspension by agitation in de-ionized water [60]. Cream droplet size from extruded soybean flakes is much larger than from flour extracts, with volume-weighted mean diameter (D 4,3) of 45 μm compared to 20 μm for cream unextruded soybean flour, respectively [67], although others have reported D 4,3 values for cream from flour of 5 μm [60].
Recovering Value from the Skim Fraction
Applications of Soy Protein Products
Conventional soybean extraction processes depend on revenues from both oil and meal. The meal consists of protein, fiber, sugars (sucrose, raffinose, and stachyose) and ash, and is sold primarily as swine and poultry feed [29]. Some of the soy protein from defatted soybean flakes is also made into food-grade soy protein ingredients, however this market consists of only 2–3% of total soy protein produced in the United States [70]. In soybean AEP and EAEP, the soluble proteins, sugars and ash (more than 40% of total soybean mass) end up in the skim, decreasing the protein content and mass of the residual insoluble fraction and creating a new fraction with composition and properties unlike any process stream produced in conventional soybean extraction processes. Process economic analyses show that creating value from the skim fraction is critical to economic viability of AEP/EAEP of soybeans [71].
There are many possible strategies that could lead to valuation of the skim, the most obvious being food and feed applications. The important qualities for such a product include amino acid composition and the content of antinutritional factors, such as oligosaccharides and, specifically for infant animals, trypsin inhibitor activity. For food applications, functional properties such as emulsification capacity, foaming capacity and gel strength are important. Clinical applications for soy protein hydrolyzates have also received recent attention. Soybean proteins below 3,000 kDa have low allergenicity, and would be suitable for clinical parenteral and enteral diets [72]. In addition, bioactive soybean peptides have been identified which have hypertension reducing [73] and cholesterol reducing [74] activities. Protein purification and recovery from conventional soy extraction utilizes either isoelectric precipitation or ethanol extraction, two technologies that may be applicable for purifying skim proteins. Researchers have so far investigated isoelectric precipitation, ultrafiltration and ion-exchange chromatography, as will be discussed below. The cost of purifying and drying skim proteins, however, may be avoided if an AEP/EAEP process were incorporated into a fermentation process. The skim proteins may make a good nutrient source for fermentation or other on-site biological processes, with the fiber fraction providing an additional source of fermentable sugars. Therefore, AEP and EAEP technologies may be especially suitable as part of an integrated biorefinery, especially a dry-grind ethanol production plant.
Recovering Soy Proteins from the Skim
The skim composition from the extraction of extruded soybean flakes with protease is typically (on a dry basis) 55% protein, 5% oil, 6% stachyose, and the remainder being ash and other soluble carbohydrates [75]. Moisture content depends on the S/L condition used, but is between 95 and 88% [9, 42, 75]. To date, there are only a few studies on the properties of the skim fraction. de Moura et al. [8] characterized the skim proteins and antinutritional factors from protease-assisted EAEP using extruded soybean flakes and suggested the skim fraction might be a suitable feed for early weaned pigs. Using Protex 6L during EAEP reduced most of the skim proteins to molecular weights between 3 and 10 kDa and reduced trypsin inhibitor activity by 83% without altering the soy protein amino acid profile. Indigestible oligosaccharides were also eliminated with the addition of galactosidase.
Campbell and Glatz [75] investigated isoelectric precipitation, ultrafiltration, and ion-exchange chromatography to recover protein values from the same skim fraction as de Moura et al. [8] The best one-step method to recover and purify hydrolyzed skim proteins was with a 3-kDa molecular weight cut-off (MWCO) membrane, which was able to reduce the stachyose content by a factor of three while achieving overall protein recovery yields of 60–63% at a protein purity of 70%, comparable to yields and purity of soy protein concentrates (SPCs) from conventional processes, although oil, sugar, and fiber contents differ from conventional SPCs. Protein retention on a 1-kDa membrane was greater, but stachyose was also retained, reducing final purity. Protein purity could be improved by adding a galactosidase to hydrolyze the oligosaccharides to monosaccharides having lower rejection.
Hydrolysis reduced recovery by isoelectric precipitation by increasing protein solubility at isoelectric pH. Jung and Mahfuz [36] achieved overall yields of 62 and 51% by isoelectric precipitation of skim proteins from AEP of soy flakes and EAEP of extruded soy flakes, respectively with purity near 80% and 90% for AEP/EAEP of soybean flakes and extruded soy flakes, respectively. While protease enzymes reduced overall protein yields in the precipitate, they had a marginal effect on the precipitate purity. The presence of oil in the skim reduced protein purity in both the isoelectric precipitate [36, 75] and the ultrafiltration retentate [75]. While Campbell and Glatz [75] were able to separate protein from emulsified oil using expanded-bed ion-exchange chromatography, protein yields were <20%, and recovery was limited to molecular weights between 12 and 30 kDa.
The extensive hydrolysis required to extract oil and protein from extruded soybean flakes complicates protein recovery. To address this, de Moura et al. [76, 77] investigated different enzyme strategies for use in countercurrent two-stage EAEP (Fig. 4) to produce soy protein products with different degrees of hydrolysis and protein functionalities. In the two-stage EAEP process, the skim from the second extraction stage is either recycled as is to the first extraction stage so that the enzyme is active in both extraction stages or it the enzyme is heat-inactivated before being used in the first extraction stage reducing proteolysis. Inactivating the enzyme between extraction stages enables producing part of the protein that could be precipitated at isoelectric pH (first extraction stage) while the remaining soluble hydrolyzed protein (second stage) would need to be recovered by membrane filtration and/or spray-drying. Although, as discussed above, deactivating enzyme slightly reduces oil and protein extraction yields, small reductions in oil and protein extraction might be justified to produce soy protein products with different degrees of hydrolysis and functional properties [76–78]. To that effect, it should be pointed out that although AEP of soybean flours also achieves lower oil yields than EAEP of extruded soybean flakes, the fact that soybean flours consist of native proteins means that high yields from low-cost processes, such as isoelectric precipitation, should be possible.
Some functional properties of the isoelectric precipitate from skim in AEP and EAEP of soybean flakes and extruded flakes have been characterized. Extrusion reduces water-holding capacity, but not oil-holding capacity, compared to proteins from skim from flakes [36]. Hydrolysis reduces oil-holding capacity for extruded flakes and reduces water-holding capacity for flakes. Soy protein solutions from flakes exhibit shear-thinning behavior [36]. Both hydrolysis and extrusion reduce viscosity and result in Newtonian flow behavior [36]. Hydrolysis during EAEP of extruded flake reduces emulsion capacity and stability as well as foaming capacity and gel strength, but foaming stability increases upon hydrolysis [78].
Oil Remaining in the Skim Fraction
For most AEP/EAEP processes (with the exception of oil body EAEP, which will be discussed below), oil remaining in the skim ranges from 10 to 20% of total oil. This is a substantial loss of oil product as well as a difficult impurity to remove from a skim protein product. Unless applications for skim containing oil are found, the quantity of oil remaining in the skim fraction after centrifugal separation remains a major obstacle for AEP/EAEP of extruded soybean and soybean flour and, therefore, further investigation is needed to recover this oil.
Factors contributing to the stability of the oil remaining in the skim fraction after centrifugation are not well understood for any AEP or EAEP process. Protein binding to oil droplets will alter the density of individual oil droplets, especially for the smallest oil droplets, causing neutral or negative buoyancy. For creams from full-fat flour-based extractions, protein coverage ranged from 10 mg/m2 for the oil body extraction process of Fig. 6A [56] to 14.7 mg/m2 for 2 h extraction from full-fat flour [60], although the cream in the flour process was not rinsed of serum proteins prior to characterization. Assuming a protein density of 1.3 g/cm3, protein coverage of 10 mg/m2 would give droplets smaller than 0.1-μm negative buoyancy in water at room temperature. By comparison, isolated oil bodies have protein surface coverage of 3.2 mg/m2 [25], indicating the extent of protein adsorption to the oil body membrane surface. Therefore protein-oil interactions are an important area for researchers to investigate to reduce the quantity of oil in the skim.
Oil body EAEP, however, has a very low oil yield in the skim fraction [49], which appears to be attributable to the cell-wall degrading enzymes used which allow oleosomes to remain intact. For Kapchie et al. [49] oil in the skim ranged from 15 to 30% of total oil when extracting without enzymes (Table 2), presumably because oil bodies extracted in the absence of the enzyme cocktail were disrupted by the shearing action of the blender used during extraction. When extracted in the presence of an enzyme cocktail consisting of pectinase and cellulase enzymes, oil yield in the skim fraction decreased with increasing enzyme concentration, becoming negligible at 3% total enzyme concentration (Table 2).
General Conclusions
Performance of AEP and EAEP of soybeans has advanced over the last ten years with extraction yields of oil and protein from the solids fraction now exceeding 95%; however, considerable challenges facing commercial adoption of this process remain. Yields of free oil or oil bodies are still >10% lower than hexane extraction yields because of losses to the skim fraction. The purification of the skim protein is complicated both by the presence of oil and by the nature of the hydrolysis needed to maximize oil extraction yields. Still, improvements in the fundamental understanding of these problems represent substantial progress toward the goal of a greener, economical, high-yielding extraction process.
The nature of the oil-confining matrix and its geometry are important factors, which must be considered in process design. Extrusion achieves complete cellular disruption, but creates a matrix of insoluble proteins that adsorb and entrap oil creating different complications for oil recovery than are observed in extraction from soybean flours. To maximize extraction yields from full-fat soybean flour, it is necessary to maintain oil droplets small enough to pass out of the matrix of disrupted cells, but droplets need to be large enough to be recovered by centrifugal separation. Understanding the nature of the species at the oil–water interface that stabilize these emulsions is critical to our ability to control the properties of AEP emulsions and maximize oil recovery from the skim as well as from the residual fraction.
Creating value from the skim fraction is vital to the economic viability of AEP/EAEP. If value is to be created by purification of the skim proteins, then this must be taken into account when considering oil extraction strategies. Isoelectric precipitation is the simplest and most economical method to purify soy proteins, but because of the high oil-binding capacity of soybean protein, the presence of emulsified oil in the skim limits the purity that can be achieved by this method. Likewise, the extensive hydrolysis needed to free oil from extruded soybean flakes dramatically reduces the yields of isoelectric precipitation. Ultrafiltration using membranes with nominal MWCOs of no smaller than 3,000 Da achieved the highest protein yields while still improving protein purity from extruded soy hydrolyzate of a high extent of hydrolysis. Losses of the smallest hydrolyzed polypeptides represent an important lost value from the skim fraction. Size-selective methods will not be suitable for purifying these smallest polypeptides. The development of additional applications for the skim fraction, in particular applications requiring minimal additional processing such as fermentation media, would be very beneficial.
References
Johnson LA (1998) Theoretical, comparative and historical analyses of alternative technologies for oilseeds extraction. In: Wan PJ, Wakelyn PJ (eds) Technology and solvents for extraction oilseeds and nonpetroleum oils. AOCS Press, Champaign, IL, pp 4–47
Johnson LA, Lusas EW (1983) Comparison of alternative solvents for oils extraction. J Am Oil Chem Soc 60(2):229–242
Wan PJ, Hron RJ (1998) Extraction solvents for oilseeds. Inform 9(7):707–709
Anonymous (2002) Isohexane: likely choice for crushers seeking to replace n-hexane. Inform 13(4):282–286
Hagenmaier RD (1997) Aqueous processing. In: Wan PJ, Wakelyn PJ (eds) Technology and solvents for extraction oilseeds and nonpetroleum oils. AOCS Press, Champaign, IL, pp 311–322
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme and Microb Technol 19:403–429
Lamsal BP, Murphy PA, Johnson LA (2006) Flaking and extrusion as mechanical treatments for enzyme-assisted aqueous extraction of oil from soybeans. J Am Oil Chem Soc 83(11):973–979
de Moura JMLN, Campbell KA, Mahfuz A, Jung S, Glatz CE, Johnson L (2008) Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification. J Am Oil Chem Soc 85(10):985–995
de Moura JMLN, Johnson LA (2009) Two-stage countercurrent enzyme-assisted aqueous extraction processing of oil and protein from soybeans. J Am Oil Chem 86(3):283–289
Johnson LA (2000) Recovery of fats and oils from plant and animal sources. In: O’brian RD, Wan PJ, Farr W (eds) Introduction to fats and oils. AOCS Press, Champaign, Illinois., pp 108–135
Johnson LA (2008) Oil recovery from soybeans. In: Johnson L, White PJ, Galloway R (eds) Soybeans: chemistry, production, processing, and utilization. AOCS Press, Champaign, Illinois., pp 331–375
Lusas EW, Lawhon JT, Rhee KC (1982) Producing edible oil and protein from oilseeds by aqueous processing. Oil Mill Gaz 4:28–34
Perkins EG (1995) Composition of soybeans and soybean products. In: Erickson DR (ed) Practical handbook of soybean processing and utilization. AOCS Press and United Soybean Board, St. Louis, pp 9–28
Bair CW (1979) Microscopy of soybean seeds: cellular and subcellular structure during germination, development, and processing with emphasis on lipid bodies. PhD Dissertation, Iowa State University, Ames
Campbell KA, Glatz CE (2009) Mechanisms of aqueous extraction of soybean oil. J Ag Food Chem 57(22):10904–10912
Cater CM, Hagenmaier RD, Mattil KF (1974) Aqueous extraction—an alternative oilseed milling process. J Am Oil Chem Soc 54(4):137–141
Rosenthal A, Pyle DL, Niranjan K (1998) Simultaneous aqueous extraction of oil and protein from soybean: mechanisms for process and design. Trans Food Bioprod Proc 76:224–230
Dominguez H, Nunez MJ, Lema JM (1994) Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds—a review. Food Chem 49(3):271–286
Kasai N, Imashiro Y, Morita N (2003) Extraction of soybean oil from single cells. J Ag Food Chem 51:6217–6222
Aguilera JM, Stanley DW (1999) Microstructural principles of food processing and engineering, 2nd edn. Aspen Publishers, Gaithersburg, MD
Wolf WJ (1970) Scanning electron microscopy of soybean protein bodies. J Am Oil Chem Soc 47(3):107–108
Lee CH, Kim CS, Yang HC (1983) Microstructure and hydrodynamic properties of soybean protein bodies in solutions. J Food Sci 48(3):695–702
Wolf WJ, Baker FL (1972) Scanning electron-microscopy of soybeans. Cereal Sci Today 17(5):124–127
Tzen JTC, Huang AHC (1992) Surface-structure and properties of plant seed oil bodies. J Cell Biol 117(2):327–335
Tzen JTC, Cao Y, Laurant P, Ratnayake C, Hagenmaier RD (1993) Lipids, proteins, and structure of seed oil bodies from diverse species. Plant Phys 101:267–276
Bair CW, Snyder HE (1980) Electron-microscopy of soybean lipid bodies. J Am Oil Chem Soc 57(9):279–282
Tzen JTC, Peng CC, Cheng DJ, Chen ECF, Chiu JMH (1997) A new method for seed oil body purification and examination of oil body integrity following germination. J Biochem 121(4):762–768
Wang L (2004) Properties of Soybean Oil Bodies and Oleosin Proteins as Edible Films and Coatings. PhD Dissertation, Purdue University, West Lafayette, IN
Woerfel JB (1995) Extraction. In: Erickson DR (ed) Practical Handbook of Soybean Processing and Utilization. AOCS Press, Champaign, IL, pp 72–75
Bair CW, Snyder HE (1980) Ultrastructural-changes in soybeans during processing to yield desolventized-toasted meal. J Food Sci 45(3):529–533
Yiu SH, Altosaar I, Fulcher RG (1983) The effects of commercial processing on the structure and microchemical organization of rapeseed. Food microstruct 2(2):165
Aguilera JM, Kosikowski FV (1976) Ultrastructural changes occurring during thermoplastic extrusion of soybean grits. J Food Sci 41(5):1209–1213
Ko JY, Ha JK, Kim HD (1992) Effect of extrusion on the physical property of soybean in sacco nitrogen disappearance and in vivo digestibility in the sheep. Korean J Animal Sci 34(2):101–107
Jung S, Mahfuz A, Maurer D (2009) Structure, protein interactions and in vitro protease accessibility of extruded and pressurized full-fat soybean flakes. J Am Oil Chem Soc 86(5):475–483
Kim IH, Friesen KG, Kim CS (1995) Use of soy protein for early weaned pigs. Korean J Animal Nutr Feed 19(5):352–370
Jung S, Mahfuz AA (2009) Low temperature dry extrusion and high-pressure processing prior to enzyme-assisted aqueous extraction of full fat soybean flakes. Food Chem 114(3):947–954
Schaich KM (1980) Free radical initiation in proteins and amino acids by ionizing and ultraviolet radiations and lipid oxidation. Part III. Free radical transfer from oxidizing lipids. Crit Rev Food Sci Nutr 13(3):188–244
Camire ME (2000) Chemical and nutritional changes in food during extrusion. In: Riaz MN (ed) Extruders in food applications. Technomic Publishing Company, Lancaster, pp 127–147
Rosenthal A, Pyle DL, Niranjan K, Gilmore S, Trinca L (2001) Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzyme Microb Technol 28:499–509
Nielsen NC (1985) Structure of soy proteins. In: Altschul AA, Wilcke HL (eds) New protein foods, vol 5: seed storage proteins. Academic Press, Orlando, pp 27–64
Seguchi M (1986) Lipid binding by protein films heated on glass beads and wheat starch. Cereal Chem 63(4):311–315
de Moura JMLN, NMd Almeida, Johnson LA (2009) Scale-up of enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc 86(8):809–815
Freitas PS, Couri L, Janklonka F, Carvalho C (1997) The combined application of extrusion and enzymatic technology for extraction of soybean oil. Fett/Lipid 9:333–337
de Moura JMLN, Almeida NM, Jung S, Johnson L (2010) Flaking as a pretreatment for enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc, DOI 10.1007/s11746-010-1626-6
de Moura JMLN, Maurer D, Jung S, Johnson L (2010) Integration of extraction and cream de-emulsification in the two-stage enzyme assisted aqueous extraction of soybeans. J Am Oil Chem Soc (submitted)
de Moura JMLN, Maurer D, Jung S, Johnson L (2010) Proof-of-concept of countercurrent two-stage enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc (submitted)
Jung S (2009) Aqueous extraction of oil and protein from soybean and lupin: A comparative study. J Food Proc Preserv 33(4):547–559
Yoon SH, Kim I-H, Kim S-H, Kwon T-W (1991) Effects of enzyme treatments and ultrasonification on extraction yields of lipids and proteins from soybean by aqueous process. Korean J Food Sci Technol 23(6):673–676
Kapchie VN, Wei D, Hauck C, Murphy PA (2008) Enzyme-assisted aqueous extraction of oleosomes from soybeans (Glycine max). J Ag Food Chem 56(5):1766–1771
Kapchie VN (2010) Personal communication
Murphy DJ (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40(5):325–438
Nikiforidis CV, Kiosseoglou V (2010) Physicochemical stability of maize germ oil body emulsions as influenced by oil body surface–xanthan gum interactions. J Ag Food Chem 58(1):527–532
Nikiforidis CV, Kiosseoglou V (2009) Aqueous extraction of oil bodies from maize germ (Zea mays) and characterization of the resulting natural oil-in-water emulsion. J Ag Food Chem 57(12):5591–5596
Kapchie VN, Towa LT, Hauck C, Murphy PA (2010) Recycling of aqueous supernatants in soybean oleosome isolation. J Am Oil Chem Soc 87(2):223–231
Kapchie VN, Towa LT, Hauck C, Murphy PA (2010) Evaluation of enzyme efficiency for soy oleosome isolation and ultrastructural aspects. Food Res Int 43(1):241–247
Iwanaga D, Gray DA, Fisk ID, Decker EA, Weiss J, McClements DJ (2007) Extraction and characterization of oil bodies from soy beans: A natural source of pre-emulsified soybean oil. J Agric Food Chem 55(21):8711–8716
Towa LT, Kapchie VN, Hauck C, Murphy PA (2009) Enzyme-assisted aqueous extraction of oil from isolated oleosomes of soybean flour. J Am Oil Chem Soc 87:347–354
Huang AHC (1994) Structure of plant seed oil bodies. Curr Opin Struct Biol 4:493–498
Tzen JTC, Lai YK, Chan KL, Huang AHC (1990) Oleosin isoforms of high and low-molecular weights are present in the oil bodies of diverse seed species. Plant Phys 94(3):1282–1289
Chabrand RM, Kim HJ, Zhang C, Glatz CE, Jung S (2008) Destabilization of the emulsion formed during aqueous extraction of soybean oil. J Am Oil Chem Soc 85(4):383–390
Chabrand RM, Glatz CE (2009) Destabilization of the emulsion formed during the enzyme-assisted aqueous extraction of oil from soybean flour. Enzyme Microb Technol 45(1):28–35
Herman EM, Melroy DL, Buckhout TJ (1990) Apparent processing of a soybean oil body protein accompanies the onset of oil mobilization. Plant Physiol 94(1):341–349
Ogawa T, Tsuji H, Bando N, Kitamura K, Zhu YL, Hirano H, Nishikawa K (1993) Identification of the soybean allergenic protein, gly-m Bd 30K, with the soybean seed 34-kDa oil-body-associated protein. Biosci Biotechnol Biochem 57(6):1030–1033
Kalinski A, Melroy DL, Dwivedi RS, Herman EM (1992) A soybean vacuolar protein (P34) related to thiol proteases is synthesized as a glycoprotein precursor during seed maturation. J Biol Chem 267(17):12068–12076
Jung S, Maurer D, Johnson LA (2009) Factors affecting emulsion stability and quality of oil recovered from enzyme-assisted aqueous extraction of soybeans. Bioresource Technol 100(21):5340–5347
Wu J, Johnson LA, Jung S (2009) Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soybean flakes. Bioresource Technol 100(2):527–533
Lamsal BP, Johnson LA (2007) Separating oil from aqueous extraction fractions of soybean. J Am Oil Chem Soc 84:785–792
List GR, Mounts TL, Lanser AC (1992) Factors promoting the formation of the nonhydratable soybean phosphatide. J Am Oil Chem Soc 69(5):443–446
Yao LX, Jung S (2010) P-31 NMR phospholipid profiling of soybean emulsion recovered from aqueous extraction. J Agric Food Chem 58(8):4866–4872
Goldsmith PD (2008) Economics of soybean production, marketing, and utilization. In: Johnson LA, White PJ, Galloway R (eds) Soybeans: Chemistry, Production Processing and Utilization. AOCS Press, Urbana, pp 117–150
Campbell K (2010) Protein and oil recoveries from enzyme-assisted aqueous extraction of soybeans and sunflower seed. PhD dissertation, Iowa State University, Ames, IA, p 178
de la Barca AMC, Ruiz-Salazar RA, Jara-Marini ME (2000) Enzymatic hydrolysis and synthesis of soy protein to improve its amino acid composition and functional properties. J Food Sci 65(2):246–253
Chen JR, Okada T, Muramoto K, Suetsuna K, Yang SC (2002) Identification of angiotensin I-converting enzyme inhibitory peptides derived from the peptic digest of soybean protein. J Food Biochem 26(6):543–554
Wang WY, de Mejia EG (2005) A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Comp Rev Food Sci Food Safety 4(4):63–78
Campbell KA, Glatz CE (2009) Protein recovery from enzyme-assisted aqueous extraction of soybean. Biotechnol Prog 26(2):488–495
de Moura JMLN, Campbell KA, de Almeida NM, Glatz CE, Johnson LA (2010) Protein extraction and recovery in enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc (accepted)
Moura JMLN, Campbell KA, de Almeida NM, Glatz CE, Johnson LA (2010) Protein recovery in aqueous extraction processing of soybeans using isoelectric precipitation and nanofiltration. J Am Oil Chem Soc (submitted)
de Almeida NM, de Moura JLMN, Johnson LA (2010) Functional properties of protein produced by two-state aqueous countercurrent enzyme-assisted aqueous extraction. In: American Oil Chemists’ Society Annual Meeting 2010. Phoenix, AZ
Acknowledgments
The authors would like to thank the U.S. Department of Agriculture for funding this work under USDA research grant number 2009-34432-20057. We would also like to thank Devin Maurer and Bill Colonna for their assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Campbell, K.A., Glatz, C.E., Johnson, L.A. et al. Advances in Aqueous Extraction Processing of Soybeans. J Am Oil Chem Soc 88, 449–465 (2011). https://doi.org/10.1007/s11746-010-1724-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1724-5