Abstract
Previous research has shown that enzyme-assisted aqueous extraction processing (EAEP) extracts 88–90% of the total soybean oil from extruded full-fat soy flakes into the aqueous media, which is distributed as cream (oil-in-water emulsion), skim, and free oil. In the present work, a simple separatory funnel procedure was effective in separating aqueous skim, cream and free oil fractions allowing mass balances and extraction and recovery efficiencies to be determined. The procedure was used to separate and compare liquid fractions extracted from full-fat soy flour and extruded full-fat soy flakes. EAEP extracted more oil from the extruded full-fat soy flakes, and yielded more free oil from the resulting cream compared to unextruded full-fat soy flour. Dry matter partitioning between fractions was similar for the two procedures. Mean oil droplet sizes in the cream and skim fractions were larger for EAEP of extruded flakes compared to non-enzymatic AEP of unextruded flour (45 vs. 20 μm for cream; 13 vs. 5 μm for skim) making the emulsions from EAEP of extruded flakes less stable. All major soy protein subunits were present in the cream fractions, as well as other fractions, from both processes. The cream could be broken using phospholipase treatments and 70–80% of total oil in the extruded full-fat flakes was recovered using EAEP and a phospholipase de-emulsification procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stringent environmental and safety restrictions imposed on the vegetable oil extraction industry have spurred interest in alternatives to hexane extraction [1]. Today, the only viable alternatives to hexane-based extraction of soybeans are much smaller scale processes such as screw pressing and extruding-expelling. These processes leave 7–8% residual oil contents in the soybean meal [2] and denature proteins due to high heat exposure rendering the meal deficient in functional properties for food use or further fractionation [3, 4].
Although aqueous extraction processing (AEP) has been considered as an alternative to solvent extraction for nearly a half century, this process has received intense scrutiny during the last 10–20 years. AEP, which uses water as an extraction and separation medium, has been developed using soybeans, canola, corn germ, peanuts, sunflower seed, cereal brans, mustard seed, avocados, coconuts, palm kernels and olives [5, 6]. AEP has significant challenges, however, that must be overcome before becoming practical: the low extraction recovery of oil, and formation of cream with no free oil. In laboratory experiments, oil recovery from soybeans via AEP has been reported to be in the range of 65 to 75% of the total oil in the form of a stable cream. This has discouraged commercial adoption of AEP.
To improve oil recovery via AEP, considerable mechanical treatment is required to rupture cell walls and facilitate flushing action of water; oleosin protein molecules that surround the oil bodies and stabilize them in seed matrices must be destabilized; and efficient de-emulsification and separation of free oil from the aqueous fractions must be developed. Most of the oil released into the aqueous medium forms a stable oil-in-water emulsion with soy proteins and lecithin acting as surfactants.
Most AEP work using soybeans has been with full-fat soy flour without enzymes to assist extraction and achieves extraction yields in the range of 65–70% [5, 7], although up to 90% AEP oil yields from other high oil content oilseeds have been reported [5]. We have shown that enzyme-assisted AEP of extruded full-fat soy flakes achieves nearly 90% oil extraction from insoluble solids [6]. Other reports on EAEP of extruded substrates include whole soybeans [8] and sunflower kernels [9]. These reports and those of our own indicate that extrusion is key to increasing oil recovery by rupturing cell walls, denaturing protein, and facilitating protease action on denatured protein including oleosin. Incorporating extrusion into EAEP of full-fat flakes represents a significant improvement over conventional AEP methods using full-fat flour and improves the efficiency of enzyme treatments.
The fractions recovered by AEP and EAEP are a fiber-rich insoluble fraction, a protein- and sugar-rich soluble aqueous fraction (skim), an oil-rich emulsified cream fraction (oil-in-water emulsion), and a free oil fraction. These fractions can be further processed into various food, feed and industrial products (biomaterials and bioenergy), thus making EAEP a key process to potential soybean biorefineries. Additionally, EAEP may offer unique functional properties for use in feed, food and biobased products. Enzyme hydrolysates of proteins have been reported to enhance various functional properties [10, 11]. Such benefits of EAEP can only be realized if fraction separation is efficient, especially the oil from the aqueous phase. Characterizing EAEP fractions will provide us with valuable information leading to strategies to efficiently utilize them.
Past AEP work has focused on improving oil and protein recovery from full-fat soy flour, but little is known about fraction separation, and compositions and properties of the fractions. Lawhon et al. [12] processed the aqueous extracts from full-fat soybean flour using membranes, with or without separating the oil, to produce new soy ingredients including full-fat, intermediate-fat and low-fat protein products. Recovering the oil in the cream as well as free oil during AEP and EAEP is an important step since oil is the high-value product, but separation and recovery are not simple. Cream separation from aqueous media is relatively difficult, compared to centrifuging to recover the insoluble fraction. Cream and free oil need to be separated from the skim fraction by using de-emulsification strategies to maximize free oil recovery. Pre-extraction mechanical processing steps, such as grinding, flaking and/or extruding, also impact emulsion formation and stability. Emulsions from full-fat flour are much more viscous and thicker in consistency, similar to mayonnaise, compared to the emulsion from extruded full-fat flakes. The cream fraction emulsion is stabilized by soy lecithin [13], and proteins and de-emulsification strategies depend on the characteristics of the cream fraction. Potential means of de-emulsifying the AEP cream fraction include centrifuging [14], freezing and thawing [15], phase inverting [12], and heating [16].
Devising an effective separation method and characterizing the various EAEP fractions may lead to improved AEP processing strategies. The objectives of the present work were to: (1) devise a simple and efficient procedure to isolate oil-rich fractions from EAEP of extruded full-fat soy flakes, (2) characterize the EAEP fractions in terms of oil and protein contents, and (3) develop efficient methods to de-emulsify the cream fraction to recover free oil.
Materials and Methods
Materials
Full-fat soy flakes and flour were prepared from variety IA1008 soybeans harvested in 2002. Enzymes Multifect Neutral® (1,738 AZO/g), Lysomax (phospholipase A2) and G-zyme G999 (phospholipase A1) (1,000 U/g), were all from Genencor Int., Inc. Rochester, NY, USA. Enzyme phospholipase C (product no. P7633–500UN, 10–50 units/mg) was bought from Sigma, St Louis, MO, USA. The reagents for gel electrophoresis, sodium dodecyl sulfate, Tham, glycine, acetic acid, methanol, Coomassie blue dye, glycerol, urea, bromophenol blue, and mercaptoethanol were from Sigma (Sigma, St Louis, MO, USA), or Fisher Scientific (Pittsburgh, PA, USA). Globular protein molecular weight markers M3913 (66–6.5 kDa) were from Sigma. Petroleum ether “A”, boiling point 40–45 °C, was bought from Fisher Scientific. Other general chemicals were analytical grade. Deionized water was used in all experiments.
Full-Fat Soy Flakes and Flour Preparation
The soybeans were cracked with a corrugated roller mill (model 10X12SGL, Ferrell-Ross, Oklahoma City, OK, USA) and aspirated in a multi-aspirator (Kice, Wichita, KS, USA) to separate into meats and hulls fractions. The meats were conditioned to 60 °C using a triple-deck seed conditioner (French Oil Mill Machinery Co., Piqua, OH, USA). The conditioned meats were flaked by using a smooth-surfaced roller mill (Rosskamp Mfg, Inc., Waterloo, IA) to approximately 0.30 mm thickness and 3–5 mm in width. The flakes contained 23.8% oil (dry basis) and 9% moisture (as is basis). About 2 kg of flakes were ground to full-fat flour to a size less than approximately 100 mesh by passing once through a pin mill (model UT-03, Bauermeister, Inc., Memphis, TN, USA). Preliminary extrusion runs showed that full-fat soy flakes needed to contain 15% moisture or more for satisfactory extrusion, so water was added to increase the moisture content to 15% by using a Gilson mixer (model # 59016A, St Joseph, MO, USA). The moisture-adjusted flakes were then placed into double polyethylene bags and kept at 4 °C until used.
Extrusion- and Enzyme-Assisted AEP
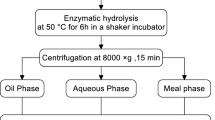
A twin-screw extruder (18-mm screw diameter, Micro 18, American Leistritz Extruders, Somerville, NJ, USA) was used for all extrusion trials. The best extrusion conditions for full-fat soy flakes with this extruder in the EAEP process have been reported earlier [6]. Briefly, full-fat soy flakes were extruded at 15% feed moisture, 100 °C barrel temperature, and 100 rpm rotational speed with a high-shear geometry screw. About 200 g of extrudate was collected directly into water in a beaker. Additional water was added to achieve 1:10 flakes:water ratio. The pH of the slurry was then adjusted to 7.0 with 2 N NaOH and the temperature was raised to 50 °C using a water bath. A protease, Multifect Neutral® was then added to the slurry at 0.5% w/w dry solids and the mixture was incubated for 1 h with stirring using a magnetic stir bar. The slurry pH dropped with enzymatic hydrolysis, but was kept nearly constant (pH 7.0) by periodically adding NaOH. After 1 h, the pH was adjusted to 8.0 and the slurry was stirred for 15 min to conform to conventional AEP. The treated slurry was then centrifuged at 3,000g for 15 min to separate the insoluble fraction from the other liquid fractions. The various AEP fractions (skim, cream, and free oil) were separated as described below. The fractions were sampled in triplicate for solids content. Once the separation procedure was established, AEP of unextruded full-fat flour without protease addition was also carried out for comparison. Because past AEP research has mostly used full-fat flour, we felt it was necessary to include this treatment as a control in our study for comparison.
Separatory Funnel Procedure for Separating Liquid Fractions
Both glass and Nalgene FEP chemical-resistant plastic separatory funnels were used to determine if the free oil and cream fractions adsorbed to the funnel surfaces differently and affected oil recovery. After centrifugation, the AEP and EAEP aqueous mixture (skim, cream, and free oil) was poured into individual separatory funnels and allowed to settle overnight (about 18 h) in a cold room (4 °C). For quantification purposes only, petroleum ether “A” (boiling point 40 °C) was added to solubilize and separate the free oil fraction; we do not envision hexane being employed to separate free oil in commercial practice. Solvent addition was about 150 mL for 700 mL of aqueous mixture. To test for possible effects of adding petroleum ether “A” to the cream, two procedures were used: (1) allowing a layer of petroleum ether “A” to remain on top of the aqueous mixture overnight, and (2) allowing only momentary contact (<5 min) between the solvent and aqueous mixture. After settling, the heavier aqueous skim fraction was collected first in a beaker. The lighter cream fraction was insoluble in petroleum ether “A” and was collected next, and the solvent-free oil mixture was collected last. The funnel was rinsed with petroleum ether “A” to remove any free oil adhering to the funnel walls. The solvent was then evaporated using a hot plate in a fume hood at about 80 °C for several hours. Visual inspection of the beaker and oil ascertained that it was free of precipitating contaminants and the free oil was determined gravimetrically. The oil contents of the cream and skim fractions were determined as described below. The total oil masses of the various fractions were calculated based on fraction weights and their oil contents.
Oil Droplet Size Determination
A Mastersizer-2000 particle size analyzer (Malvern Instruments Ltd, Worcestershire, UK) with a wet module (Hydro 2000) was used to determine the size distribution and mean oil droplet size in the skim and cream fractions. The cream fraction was diluted with deionized water to be in the obscuration range of 11–14% as recommended by the manufacturer.
Peptide Identification by Sodium Dodecyl Sulfate Polyacryalamide Gel Electrophoresis (SDS-PAGE)
Identification and comparison of peptides in fraction samples from AEP and EAEP was done with SDS-PAGE. The procedure was carried out with a SDS-Tris-glycine buffer system and 4% stacking gels and 13% resolving gels (Biorad Mini Protean II Gel) [17]. The low-range globular proteins MW markers and soy protein standards prepared in our laboratory were used for protein identification. Glycinin and β-conglycinin standards were prepared from defatted soy flakes according to methods of O’Keefe et al. [18]. The soy protein standards were pure, as indicated by the presence of single band for each on SDS-PAGE gels, and kept at −20 °C in the same manner as samples were prepared for the SDS-PAGE procedure.
De-Emulsification of Cream Fractions
In one set of EAEP experiments using extruded full-fat soy flakes, the separated cream fraction was treated by physical or enzymatic methods to de-emulsify and recover free oil. The physical methods evaluated included: (1) heating at 95 °C for 3–4 h in a water bath, and (2) freezing overnight at −20 °C and thawing at room temperature. Enzymatic treatments included: (1) a 1:1 v/v cocktail of Lysomax (phospholipase A2) and G-zyme G999 (phospholipase A1), and (2) phospholipase C. Enzyme incubations were 90 min at pH 4.5 and 60 °C for Lysomax/G-zyme cocktail, and at pH 7 and 37 °C for phospholipase C. Both enzymatic and physical treatments were carried out in 50-mL plastic centrifuge tubes followed by centrifuging at 100g for 5 min. The free oil was then collected in tared beakers by decanting. Petroleum ether “A” was added to the centrifuge tubes to solubilize any oil adhering to walls. The solvent was evaporated as previously described and the weight of oil was determined. The residual cream in the tubes was also weighed. Oil in the residual cream and other EAEP fractions was determined as described below and compared to the total oil in the extruded flakes.
Oil, Protein and Moisture Determinations
Total oil content of full-fat soy flour, full-fat soy flakes, extruded full-fat flakes and AEP fractions were determined in duplicate by using the Mojonnier method (AOAC Method 922.06 for solid samples, and AOAC Methods 995.19 and 989.05 for cream and skim fractions, respectively). The Mojonnier method was much more accurate for quantifying oil contents of EAEP fractions compared to the Goldfisch procedure because denatured protein can bind some oil, which is not extracted by using the Goldfisch procedure [6].
Total nitrogen content was determined in duplicate by using the Dumas method (AOAC Method 993.13), in a combustion-type nitrogen analyzer (Elementar Americas, Mt Laurel, NJ, USA). Protein contents were calculated as total nitrogen x 6.25.
Moisture content was determined by heating samples in a forced-convection oven at 130 °C for 3 h. Triplicate samples of 5–10 g were dried in aluminum dishes.
Statistical Analysis
The General Linear Model, PROC GLM, in SAS system (version 8.2, SAS Institute, Inc., Cary, NC, USA) was used to compare means (n = 2 or 3) at P < 0.05.
Results and Discussion
Separatory Funnel Procedure for Quantifying Oil in AEP Fractions
Quantifying how oil partitions among the insolubles (spent solids) and various liquid fractions (i.e., skim, cream and free oil) has been a challenge in evaluating different treatments employed in AEP or EAEP processes. Previously, we were able to separate the cream fraction from other fractions by using a 240-mesh wire sieve for AEP of unextruded full-fat soy flour. The cream layer after centrifugation was homogeneous and thick with no adhering free oil and remained on wire sieves when decanted (unpublished work). In the present work, our attempts to separate liquid fractions with the same wire sieve were not successful when using EAEP with extruded full-fat flakes. The EAEP cream from extruded full-fat flakes was much lower in viscosity with large amounts of free oil, and thus, passed through the wire sieve with the skim fraction. Denaturation of proteins due to extrusion and the subsequent enzymatic hydrolysis produced short-chain peptides that perhaps prevented formation of a strong and firm cream layer.
A separatory funnel was a convenient and simple tool to separate EAEP liquid fractions. Table 1 shows the mean distribution, as percentage of total, for oil, protein, and dry matter in EAEP fractions from extruded full-fat flakes using the two procedures to recover free oil. Experiments in which the petroleum ether addition was eliminated were not conducted, because doing so did not yield any free oil fraction. There were no significant differences (P < 0.05) among type of funnel used for separation (plastic or glass surfaces) as well as contact duration with petroleum ether “A” (overnight or momentary) for oil, protein or dry matter partitioning, confirming that petroleum ether “A” did not affect the cream system and merely dissolved the free oil. This procedure was subsequently utilized for separating the liquid fractions from AEP of unextruded full-fat soy flour and EAEP of extruded full-fat soy flakes. Momentary hexane contact in glass separatory funnels was used for all subsequent experiments.
EAEP Fractions from Full-fat Soy Flour and Extruded Full-fat Soy Flakes
Table 2 summarizes the oil, protein, and dry matter distribution in the various fractions from EAEP of extruded full-fat soy flakes and AEP of full-fat soy flours when using our separatory funnel procedure. AEP of full-fat soy flour without enzyme treatment served as the baseline control for evaluating EAEP of extruded full-fat soy flakes. From Table 2, it is clear that EAEP of extruded full-fat flakes was superior to AEP of full-fat flour in terms of total oil extracted (12.8 vs. 34.6% oil remaining in the insoluble fraction, respectively), more oil in the cream fraction (59.6 vs. 45.3%), and more free oil (15.9 vs. 2.5%). In our earlier work [2], we showed that flaking and extruding soybeans yielded significantly more free oil when using EAEP. Ideally, the skim would contain no oil and no cream would form; all oil would be directly recovered as free oil. The cream fractions contained the largest portions of extracted oil by either process, but the consistencies of the cream fractions were very different, with that derived from EAEP being less viscous.
The amounts of protein obtained in the skim fractions were similar for both processes. This was attributed to the pH being raised to 8.5 for EAEP, which also favors soy protein extraction due to enhanced protein solubility in the basic pH range. The cream fraction from EAEP using extruded full-fat flakes contained only 0.7% of the total protein, whereas cream from flour AEP contained 1.8% proteins. The difference in protein contents, and arguably the presence of short-chain peptides in EAEP cream due to extrusion and hydrolysis, resulted in thinner cream for EAEP process compared to AEP of flour. Total dry matter contents of the skim fractions were not significantly different for both processes, but significantly differed for the insolubles, cream and free oil fractions from both processes.
Oil Droplet Size Distribution in Skim and Cream Fractions
Figure 1 shows the oil droplet size distributions (top) and mean droplet sizes (bottom) in the cream and skim fractions produced by EAEP using extruded full-fat soy flakes and AEP using full-fat soy flour. Oil droplet size distributions in the cream fractions from both processes were mostly unimodular with normal distributions. The oil droplet distribution for cream fractions from AEP of flour and EAEP of extruded flakes showed highest volume fraction at 8 and 30 μm, respectively. The oil droplet size distributions of the skim fractions, on the other hand, were bimodal (AEP using flour) and trimodal (EAEP using flakes). Oil droplet volume fraction for the skim fraction from AEP of flour was high at around 0.25 and 6 μm. The skim fraction from EAEP of flakes, however, had the highest volume fraction for droplets 0.25 μm in size, followed by substantially lesser volume fraction at 2 and 60 μm.
Distributions (top) and mean (n = 3) droplet diameters (bottom) in aqueous cream and skim milk fractions. Top EAEP skim from extruded flakes (filled diamonds), AEP skim from flour (open square), EAEP cream from extruded flakes (multiple), and AEP cream from flour (open triangles). Bottom: Plain bars AEP of full-fat flour, and hatched bars EAEP of extruded full-fat flakes, with error bars representing standard error of means. Fraction means diameters (n = 3) sharing the same letter, a, b, c, d, are not significantly different atp < 0.05. D(4,3) is volume-average diameter of droplets
The mean droplet sizes of the cream emulsions from AEP of flour and EAEP of flakes were 20 and 45 μm, respectively (Fig. 1, bottom). This was an important finding because as per Stokes Law, larger emulsion droplets would have higher terminal velocities in aqueous skim medium during settling, given the similarity of aqueous medium. This would give rise to rapid formation of a cream layer on the top part of EAEP derived mixture. Once the larger droplets are on top, they are more likely to coalesce and result in more free oil, more so in the cream layer from EAEP of extruded flakes, because of its thinner consistency. We believe, the droplet size distribution trend may partly explain why more free oil was observed from EAEP of extruded flakes. Other important factors in coalescence of droplets are interfacial forces and surface-active stabilizers. The overall mean droplet sizes for the skim fractions of AEP of flour and EAEP of flakes were 5 and 13 μm, respectively.
Peptide Identification by SDS-PAGE
Peptide profiles of the proteins present in the fractions obtained by AEP of flour and EAEP of extruded flakes, as well as for defatted soy flakes, are shown in Fig. 2. All major soy protein subunits, namely α′, α, and β subunits of β-conglycinin and acidic and basic subunits of glycinin, were present in defatted soy flakes (lane 10) and were confirmed by corresponding standards (lanes 2 and 3). The insoluble fraction of EAEP of extruded flakes (lane 5), which was treated with the protease Multifect Neutral, contained extensively hydrolyzed β-conglycinin subunits. The acidic subunits of glycinin were partially hydrolyzed, whereas the basic subunits had little hydrolysis. Peptides also accumulated at the bottom of the gel, indicating the formation of peptides smaller than 6.5 kDa. The insoluble fraction from AEP of flour (lane 4) showed most major subunits to be present, although at low concentrations. The skim fraction from EAEP of extruded flakes was missing the α‘subunit of β-conglycinin (lane 7), most likely being hydrolyzed by the enzyme, unlike the skim fraction from AEP of flour (lane 6). New peptide bands for the skim fraction of EAEP extruded flakes were observed at a molecular weight slightly higher than the basic subunit of glycinin (at about 22 kDa). This new peptide, a hydrolytic product, was also present in the cream fraction of EAEP extruded flakes (lane 9). All major soy protein subunits were present in the cream fractions from both procedures, except the α subunit of β-conglycinin in the cream of EAEP of extruded flakes. This indicated that protein may also play a role in stabilizing the cream. Both procedures produced stable creams, which required further treatments to recover free oil.
Peptide identification in fractions of AEP using flour and EAEP using extruded dodecyl sulfate polyacryalaniide gel electrophoresis. Lane 1, globular protein molecular markers; 2 β-conglycinin; 3 glycinin; 4 insolubles for AEP of flour; 5, insolubles for EAEP of flakes; 6 skim for AEP of flour; 7 skim for EAEP of flakes; 8 cream for AEP flour; 9 cream for EAEP of flakes; 10 defatted soy flakes
Cream De-Emulsification
Table 3 shows the oil distributions, as percentage of total available oil, in various fractions from EAEP of extruded flakes. The total free oil was the result of the combination of flaking, extrusion, enzyme hydrolysis and de-emulsification. There was a large and significant difference in the residual cream oil content between the control and freezing-thawing, and both the enzyme treatments, but not between the control and heating. Heating to 95 °C for up to 4 h was not effective in breaking the emulsion. The other treatments were equally effective in breaking the emulsion. While freezing-thawing denatures soy protein at the emulsion interface, thus destabilizing the emulsion, enzymatic cream destabilization indicated that soy phospholipids were also important as stabilizers. Lysomax, a phospholipase A2, cleaves the ester bond between fatty acid groups at the sn-2 position in the acylglycerophosphatide backbone. When position 2 is free, G-zyme 999, phospholipase A1, is said to cleave ester bond at the sn-1 position in the backbone. This destabilized the cream as effectively as did phospholipase C. Treating with phospholipase C cleaves the ester bond at the sn-3 position of glycerolphosphatide releasing PO− 4 moiety, which was hypothesized as the chief reason for cream stabilization. Our results also showed that removing fatty acids from the sn-1 and sn-2 positions was effective in breaking the emulsion, despite the probability that these fatty acids would be sequestered within the oil droplet. De-emulsification was reflected by a large increase of free oil. The phospholipase treatment of the cream fraction obtained from EAEP of extruded full-fat soy flakes is one approach to recovering 70–80% of the total oil as free oil.
About 10–12% of the total oil in extruded full-fat soy flakes was in the skim fraction, which needs to be recovered to improve the overall effectiveness of enzymatic aqueous extraction process. Another 10–12% of total oil was not extracted by EAEP process from extruded full-fat flakes during the current single-stage extraction procedure and ended up in the insoluble fraction.
References
Anonymous (2001) National emissions standards for hazardous air pollutants: solvent extraction for vegetable oil production, US EPA Final Rule. 40 CFR Part 63. Fed Reg 66:19006–19026, April 12
Nelson LL, Wijeratne WB, Yeh SW, Wei TM, Wei LS (1987) Dry extrusion as an aid to mechanical expelling of oil from soybeans. J Am Oil Chem Soc 64:1341–1347
Heywood AA, Myers DJ, Bailey TB, Johnson LA (2002) Functional properties of extruded-expelled soybean flours from value-enhanced soybeans. Ibid 79:699–702
Heywood AA, Myers DJ, Bailey TB, Johnson LA (2002) Functional properties of low-fat soy flour produced by and extrusion-expelling system. Ibid 79:1249–1253
Rosenthal A, Pyle DL, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enz Microb Technol 19:402–420
Lamsal BP, Murphy PA, Johnson LA (2006) Flaking and extrusion as mechanical treatments for enzyme-assisted aqueous extraction of oil from soybeans. J Am Oil Chem Soc 83:973–979
Rosenthal A, Pyle DL, Niranjan K, Gilmour S, Trinca L (2001) Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enz Microb Technol 28:499–509
Freitas SP, Hartman L, Couri S, Jablonka FH, de Carvalho CWP (1997) The combined application of extrusion and enzymatic technology for extraction of soybean oil. Fett/Lipid 99:333–337
Caetano MF, Couri S, Freitas SP (2002) Enzymatic aqueous extraction of sunflower oil from extruded kernels. La Rivista Italiana Delle Sostanze Grasse 79:165–169
Jung S, Murphy PA, Johnson LA (2005) Physicochemical and functional properties of soy protein substrates modified by low levels of protease hydrolysis. J Food Sci 70:C180–187
Jung S, Lamsal BP, Stepien V, Johnson LA, Murphy PA (2006) Functionality of soy proteins produced by enzyme-assisted extraction. J Am Oil Chem Soc 83:71–78
Lawhon JT, Rhee KC, Lusas EW (1981) Soy protein ingredients prepared by new processes - Aqueous processing and industrial membrane isolation. J Am Oil Chem Soc 58:377–384
Weete JD, Betageri S, Griffith GL (1994) Improvement of lecithin as an emulsifier for water-in-oil emulsions by thermalization. Ibid 71:731–737
Cater CM, Rhee KC, Hagenmaier RD, Mattil KF (1974) Aqueous extraction—an alternative oilseed milling. Ibid 51:137–141
Gunetileke KG, Laurentius SF (1974) Conditions for the separation of oil and protein from coconut milk emulsion. J Food Sci 39:230–233
Rajsekharan N, Sreenivasan A (1967) The use of coconut preparations as a protein supplement in child feedings. J Food Sci Technol (India) 4:59–61
Lamsal BP, Reitmeier C, Murphy PA, Johnson LA (2006) Enzymatic hydrolysis of extruded-expelled soy flour and resulting functional properties. J Am Oil Chem Soc 83:731–737
O’Keefe SF, Wilson L, Resurreccion A, Murphy PA (1991) Determination of the binding of hexanal to soy glycinin and beta-conglycinin in an aqueous model system using a headspace technique. J Agric Food Chem 39:1022–1028
Acknowledgments
This work was supported by USDA Special Grants 2005–34432–64 and the Iowa Agricultural and Home Economics Experiment Station project number 3702.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lamsal, B.P., Johnson, L.A. Separating Oil from Aqueous Extraction Fractions of Soybean. J Amer Oil Chem Soc 84, 785–792 (2007). https://doi.org/10.1007/s11746-007-1090-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-007-1090-0