Abstract
Enzyme-assisted aqueous extraction of oil from isolated soybean oleosomes was evaluated as an alternative to the conventional organic solvent extraction. Three different processes: hydrolysis of oleosomes, thermal demulsification of the skim or the slurry, and destabilization of the cream by the churning butter process were examined to enhance the release of free oil from isolated oleosomes. The oil extraction involved incubating the oleosomes with either 0, 2.5 or 5% protease (Protex 6L®) at 60 °C, pH 9 for 18 h, destabilizing the slurry by three thermal strategies: freeze/thaw, freeze/thaw and heating, and destabilizing the cream by the churning butter process without and with 5% of phospholipase A2 (Multifect L1 10L®), at 40 °C, pH 8 for 4 h. The best total free oil yield was 83–88% by hydrolyzing oleosomes with 2.5 or 5% Protex 6L®, destabilizing the slurries by heating and destabilizing the resulting cream by the churning butter process. The oleosomes treated with 2.5 and 5% proteases generated hydrolyzed soybean storage proteins at 18–20% degree of hydrolysis, with all the storage proteins hydrolyzed to peptides smaller than 6.5 kDa, compared to the oleosomes disrupted without proteases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The standard practice of oil extraction from seeds in the industry is the organic solvent extraction leading to a cake with residual oil content below 1%, but with inherent disadvantages of high investment and energy requirement [1]. Hexane, the common organic solvent used represents a fire and explosion hazard as well as neurological and respiratory disorders of the workers from prolonged exposure [1, 2]. These economic, safety, environmental and health concerns propelled the need to find an alternative safe and efficient oil extraction process from seeds. Oleosomes or oil bodies are discrete subcellular organelles mainly found in oilseeds, which consist of a lipid core that is surrounded by a phospholipid monolayer in which oleosins are embedded which are proteins that stabilize the oleosome structure and prevent coalescence of the oil in the cell cytoplasm [3, 4]. Oleosome purification from oilseeds and oleosome characterization for different purposes have been reported [5–12]. Recently, a laboratory process for isolating oleosomes from 25 g of soybean flour [13] was evaluated on a mass balance basis. This successful process was scaled up to a pilot plant scale for 75 kg of soybean flour resulting in improvement in the oil yield, as extracted oleosomes, to 93 ± 0.88% (Towa et al. submitted). This research provided a strategy to isolate oleosomes from soybean flour on a large scale and suggested the feasibility of the process for industry. Based on the structure of oleosomes, we hypothesized that free oil could be released from those organelles by disrupting the membrane with specific enzymes during a new process of enzyme-assisted aqueous extraction (EAAE). EAAE has already been studied as an environmentally clean technology to simultaneously extract free oil and protein from oilseeds including soybean [1, 14–19]. Particularly for the soybean, the release of oil into the aqueous medium forms a stable oil-in-water emulsion with soy protein and lecithin acting as surfactants [20]. Lamsal and Johnson [17], Chabrand et al. [18], and Wu et al. [19] used different techniques including freeze/thaw, heating and enzymatic processes to destabilize this emulsion, but with enormous difficulties. Since oleosomes isolated from soybean flour by an EAAE are not a true emulsion like those obtained by these latter authors, but actual subcellular organelles, lipid reservoir surrounded by an intact membrane, we predicted that free oil could be released from those organelles by degrading the membrane. The present study using isolated oleosomes from soybean flour focuses on developing simple and efficient strategies to extract free oil from isolated oleosomes by a new process of EAAE using proteases, phospholipases in combination with mechanical and thermal destabilization processes.
Experimental Procedures
Oleosomes were isolated in the pilot plant of the Center for Crops Utilization Research, Iowa State University, from 75 kg of soybean flour obtained in 2009 from Natural Products Inc. (Grinnell IA, USA). Briefly, the isolation process involved cell wall hydrolyzing enzymes, mechanical disruption of cell walls through the Stephan Microcut® mill (Stephan Machinery Corp., Columbus, OH model MC-10), and flotation centrifugation through the Three Phase Centrifuge (Centrysis, Model 10/4, Kenoshia,, WI) and is reported elsewhere (Towa et al. submitted).
The enzymes used to disrupt oleosome membranes, Protex 6L® (E.C. 3.4.21.62, alkaline serine endopeptidase, 580,000 DU/g, optimal pH 9.5, optimal temperature 60 °C), and Multifect L1 10L® (E.C. 3.1.1.4, phospholipase A2, 400 U/g, optimal pH 8.5, optimal temperature 40 °C) were from Genencor (Genencor, a Danisco company Rochester, NY, USA).
Analytical grade reagents used were bought from Fischer Scientific (Pittsburgh, PA, USA) or Sigma (St Louis, USA).
Initial Procedure
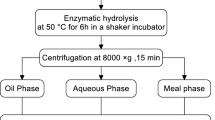
The initial oil extraction procedure from isolated oleosomes started with the hydrolysis of the isolated oleosomes’ protein membrane in a 20-L reaction vessel (Model CG-9253-10, Chemglass Inc., Vineland, NJ, USA) (Fig. 1). A mass of 1.4 kg of isolated oleosomes were dispersed in distilled water (ratio 1/6; dw/v) at 40 rpm, 60 °C. The pH of the dispersion was adjusted to 9 with 2 N NaOH. When optimum conditions for Protex 6L activity were reached (60 °C, pH 9), 5% (v/d w) was added and the mixture incubated for 18 h with constant stirring at 40 rpm. The pH of the slurry was maintained at pH 9 by the pH–stat (702 SM Titrino, Metrohm Ltd., Herisau, Switzerland). After 18 h, free oil and skim were separated from the slurry by centrifugation using a fixed angle rotor (Sorvall RC 5B Plus, L.P., Newtown, USA) at 3,000, 4,000, and 10,000×g for 15 min at 25 °C. The skim represented the aqueous fraction that contains emulsified oil which resided below the free oil phase. The partition was done using a glass separatory funnel at 4 °C for 24 h as described by Lamsal and Johnson [17].
To maximize the free oil recovery, the resulting skim was destabilized using two thermal procedures A (freezing at −20 °C for 24 h and thawing at room temperature for 6 h) and B [freezing, thawing and heating at 96 °C for 3 h in the water bath Isotemp 128 (Fischer Scientific, USA)]. The resulting mixture was centrifuged as described above to separate the free oil from the cream and final skim. The cream represents the small middle phase residing between the free oil and final skim fractions. Aliquots of cream were destabilized by the churning butter process with 0 and 5% Multifect L1 10L (v/wet w), pH 8 at 40 °C for 4 h with, 10 g of the cream in a 25 mL glass beaker, pH 8 using the 1 N HCl, and incubation in the water bath with a constant gentle stirring using a ThermoScientific Variomag Multipoint inductive driver stirrer with external control (ThermoFischer Scientific Walther, MA, USA). When the temperature of the preparation reached 40 °C, 5% (v/wet wt) of Multifect L1 10 L was added and the reaction carried out for 4 h. The preparation without Multifect L1 10L represents the control. Free oil was separated from the residual cream by centrifugation at 25 °C for 15 min as described by Lamsal and Johnson [17]. Free oil, skim and cream fractions were collected for analysis.
Modified Procedure
The first centrifugation step in the initial procedure was eliminated and different concentrations of % Protex 6L were evaluated for the quantity of free oil collected which created a modified procedure (Fig. 2). This procedure involved incubating the oleosomes with either 0, 2.5 or 5% proteases (Protex 6L®) at 60 °C pH 9 for 18 h, destabilizing the slurry by three thermal strategies applied in series: freeze/thaw, freeze/thaw, and heating. Then, the preparation was centrifuged at 10,000×g for 15 min at 25 °C, and the resulting cream was destabilized by the churning butter process without and with 5% of phospholipase A2 (Multifect L1 10L®), at 40 °C, pH 8 for 4 as described above.
Degree of Hydrolysis
The degree of hydrolysis (DH) of proteins in the suspension (oleosomes, water, and proteases) incubated at 60 °C for 18 h, pH 9, was determined by the pH–stat method [21] using the milliequivalents of the base consumed. The procedure consisted of determining the %DH of protein on the basis of the number of free titratable amino groups produced by the hydrolysis of peptides bonds. DH was calculated using the equation: DH = [(V NaOH × N NaOH)/(α × MP × h tot)] × 100%, where α is the degree of dissociation of α-amino groups bonds, MP is the mass of protein (g), and h tot is the number of peptides bonds in the substrate (mequiv/g protein). The NaOH concentration was 2 N, and the α value was 0.98 for the hydrolysis temperature of 60 °C and pH 9.0. The h tot value for soy proteins was 7.8 [21, 22].
Oil and Proteins Determinations
Oil content of different aqueous fractions was determined by the Mojonnier method [23]. Oil recovery was expressed as the percentage of oil in each fraction relative to the initial amount of oil in isolated oleosomes. The total free oil was the combination of free oil released after the hydrolysis of oleosomes and the destabilization of the resulting skim and cream. Protein content was evaluated with the Dumas method using a rapid N III Nitrogen Analyzer (Elementar Americas, Inc. Mt. Laurel, NJ), and was calculated as total nitrogen × 6.25 [24].
Peptides Identification
Urea-SDS–PAGE was performed to determine the effects of hydrolysis on soy protein polypeptides profiles. Soy flour, oleosomes and aqueous samples from the EAAE processes were prepared for the urea-SDS–PAGE according to Beisson et al. [3]. Electrophoresis was run as suggested by Lamsal et al. [25] with a SDS–tris–glycine buffer system, 4% stacking gel and 13% resolving gel (Biorad Mini Protean II Gel). Gels were scanned on an Amersham Pharmacia Biotech Image Scannerflatbed scanner with transparency module and analyzed with Kodak Molecular Imaging (MI) Software version 4.
Statistical Analysis
All the analyses were performed in triplicate. General Linear Model ANOVA, Least Significant Difference and Duncan tests in SAS System (version 9.2, SAS Institute, Inc., Cary, NC) were used to compare data means at p < 0.05.
Results and Discussion
Initial Procedure
This study aims to evaluate strategies to maximize the free oil extracted from isolated oleosomes. Oleosomes isolated from soybean flour by the procedure described by Towa et al. (submitted) consisted of 48.30 ± 0.07% dry matter, 50.07 ± 0.36% oil (d.b.), 21.05 ± 0.88% proteins (d.b.), 22.81 ± 1.23% carbohydrate (d.b.), and 5.54 ± 0.02% ash (d.b.). Electron microscopy shows that these oleosomes isolated (Fig. 3) in the lab- or the pilot plant-scale were not substantively different to the structure or size of seed oleosomes, supporting the fact that isolated oleosomes used in this study were intact organelles, not a typical emulsion like the one obtained by Lamsal and Johnson [17], Chabrand et al. [18], and Wu et al. [19] after application of EAAE process to extract free oil from soybean seed.
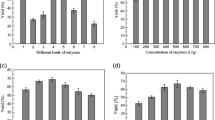
The degree of hydrolysis (DH) for the proteins was 20.07 ± 0.17% obtained after 18 h of incubating isolated oleosomes with 5% Protex 6L in distilled water, pH 9 at 60 °C. The yield of free oil was significantly different (α = 0.05) as a function of the centrifuge speeds, 3,000, 4,000 and 10,000×g (Fig. 4a). The highest amount of the free oil (55.35 ± 1.94%) was isolated using the highest speed of centrifugation (10,000×g). Despite the high degree of protein hydrolysis that occurred for all centrifugation speeds employed 53.51 ± 1.99–46.51 ± 1.37% of the total oil originally contained in the oleosomes was entrapped in the skim fractions. This is due to the presence of hydrolyzed proteins and phospholipids (mainly lecithin) from soy flour in the skim. These proteins are known to be good emulsifiers, and participating to the formation and stabilization of the emulsion [19, 20]. In the food industry, the phospholipids alone are used as emulsifiers or emulsion stabilizer when they are complexed with proteins [26, 27]. The stirring speed of 40 rpm for the slurry during the oleosomes’ protein membrane hydrolysis was also a sufficient mechanical action to cause emulsification. This is in accordance with the study of Sharma et al. [1] which demonstrated that, due to the emulsification, during the EAAE of oil from peanuts, the increase of the shaking speed led to the decrease of oil recovery.
Partition of oil between fractions from the initial procedure of EAAE of isolated from oleosomes. a Effect of centrifugation speed, b Effect of thermal destabilization strategies, c Effect of phospholipase A2 during the churning butter process, d Combination of different strategies, FO1 Free oil collected as function of centrifugation speed of the slurry, SK1 Skim collected as function of the centrifugation speed of the slurry, FO2A Free oil collected by destabilizing the SK1 using the procedure A, FO2B Free oil collected by destabilizing the SK1 using the procedure B, CR1A Cream resulting from the destabilization of the SK1 using the procedure A, CR1B Cream resulting from the destabilization of the SK1 using the procedure B, FSKA Final skim resulting from the destabilization of the SK1 by the procedure A, FSKB Final skim resulting from the destabilization of the SK1 by the procedure B, FO3A Free oil collected by destabilizing the CR1A, FO3B Free oil collected by destabilizing the CR1B, ML Phospholipase A2, TFOA Total free oil collected by combining the hydrolysis of oleosomes, the destabilization of skim by the procedure A and the destabilization cream by churning butter, TFOB Total free oil collected by combining the hydrolysis of oleosomes, the destabilization of skim by the procedure B and the destabilization cream by churning butter, RCRA Residual cream resulting from the extraction of TFOA, RCRB Residual cream resulting from the extraction of TFOB, Means ± SD for a given fraction sharing the same lettered superscript is not significantly different at p < 0.05
To enhance the quantity of free oil released from oleosomes, 2 thermal procedures: A (freezing and thawing), B (freezing, thawing and heating) were applied on the skim fractions. Figure 4b reveals that there is a significant difference at α = 0.05 between effects of these processes on the quantity of free oil released. Independent of the speed of centrifugation, no free oil was released from the skim fractions after its destabilization using freezing and thawing procedure, versus 22.11 ± 0.96–23.43 ± 1.02% for the procedure B. These results are in contrast to those reported by Lamsal and Johnson [17], Chabrand et al. [18], and Wu et al. [19] after application of the EAAE process to extract free oil from full fat flakes soybean. The authors reported that freezing and thawing process leads to the significant release of free oil from the emulsion formed in their studies. The cited work employed oil-in-water emulsions formed from soy flour or flakes. Herein, an aqueous solution of organelles was used. In fact, the approach employed for emulsion destabilization depends on the emulsions properties (molecular and chemicals properties of the emulsifier interfaces), and the environmental and process conditions [28, 29]. The emulsions investigated in the cited work contained proteins and phospholipids as emulsifiers. Herein, the organelles consisted of a fat core surrounded by a fragile membrane (mainly phospholipids) after the hydrolysis of the proteins, but not a true emulsion [26, 27].
The percentage of total oil in isolated oleosomes in the cream and the final skim after the hydrolysis of oleosomes and the thermal demulsification of the skim by procedures: A (freezing and thawing) or B (freezing, thawing and heating) ranged from 17.38 ± 1.69 to 27.07 ± 0.32% and 3.53 ± 0.75–31.82 ± 2.42%, respectively (Fig. 4b). Extraction of oil from the cream fractions using the churning butter process indicated that phospholipase A2 did not enhance the release of the free oil (Fig. 4c). In fact, there was no significant difference between the free oil released with 0 and 5% of Multifect L1 10L. Free oil released from cream fractions (13.83 ± 1.41–15.56 ± 1.51% for the procedure A or 3.47 ± 0.17–5.55 ± 0.43% for the procedure B) was the consequence of agitation, which caused the rupture of the fragile membrane surrounding the fat and thus not a true emulsion as the one formed by Lamsal and Johnson [17], Chabrand et al. [18] and Wu et al. [19]. This is in accordance with Avalli and Contarini [27], who demonstrated that the agitation applied during the churning of milk for butter production, is responsible to the disruption of the milk fat globule membrane, resulting in an important increase of membranous material in the butter milk, and coalescence of fat (butter).
A combination of strategies (hydrolysis of oleosomes, destabilization of skim and cream fractions) to enhance the release of free oil from isolated oleosomes (Fig. 4d) revealed that the best yield of total free oil (84–85% oil recoveries from total oil in isolated oleosomes) (p < 0.05) was obtained using the highest centrifugation speed (10,000×g) of the hydrolyzed oleosomes, the destabilization of the skim by the procedure B (freezing, thawing and heating), and the destabilization of the cream by the churning butter process. These results demonstrate the necessity of demulsification treatments to the skim or cream fraction after hydrolysis of the olesosomes, since the yield of free oil was enhanced from the range of 46.51 ± 1.37–53.51 ± 1.99% to the range of 84–85% after combination of the enzyme treatment of oleosomes, destabilization of skim by the procedure B and destabilization of cream fractions by churning butter process. As expected, the lowest yields of oil entrapped in the residual cream and skim fractions 12.11 ± 0.02 and 3.53 ± 0.75% respectively, were obtained by the procedure B. However, the labor intensive and costly size of this initial procedure (5% Protex 6L) propelled the need to develop a modified procedure.
Modified Procedure
The objective of this modification was to simplify the initial procedure and to evaluate a range of % Protex 6L (0, 2.5, and 5%) concentrations on the total free oil extracted from isolated oleosomes. Separation of oil from slurries or creams was accomplished by centrifugation at 10,000×g as discovered in the initial procedure.
Effect of % Protex 6L on Total Free Oil
Table 1 presents oil distribution in different EAAE fractions obtained from isolated oleosomes using the modified procedure as function of % Protex 6L. The total free oil is the combination of free oil extracted after hydrolysis of oleosomes and destabilization of the slurry using procedures A′, B′ or C′, and the free oil extracted from the cream after the degradation of the fragile membrane around the oil by the churning butter process.
Independent of the percentage of Protex 6L used, the total free oil released using the procedure A′ (freezing and thawing) was not significantly different at α = 0.05 from the control (slurry which does not receive a thermal treatment). The total free oil released using the procedure A′ (freezing and thawing) was lower than those using the procedure B′ (freezing, thawing and heating) or C′ (heating) which were not different from each other. These results suggested that destabilizing the slurry by the procedure B′ or C′ is more effective than by the procedure A′.
Independent of the thermal procedure applied, total free oil yielded using 0% Protex 6L (25.5 ± 1.7–43.4 ± 3.0% of oil from total in oleosomes) is lower than that using 2.5 and 5% Protex 6L (57.1 ± 1.5–87.7 ± 0.8% of oil from total in oleosomes) (p < 0.05) (Table 1). These results indicate the necessity of the protease (Protex 6L) to enhance the oil extractability from oleosomes by the hydrolysis of the protein membrane [16, 30].
Overall, the greatest yield of total free oil (83–88% of oil from total in oleosomes) released using the modified procedure was obtained by hydrolyzing oleosomes with 2.5 and 5% Protex 6L, destabilizing the resulting slurries by the procedure B′ or C′ and destabilizing the resulting cream by the churning butter process. As expected, the lowest yields of oil entrapped in the residual cream (9.9 ± 0.6–17.6 ± 1.2%) or skim fractions (3.4 ± 0.3–5.6 ± 0.0%) were obtained using strategies B′ and C′ (Table 1).
Effect of % Protex 6L on Protein
Figure 5 shows the peptides profiles of oleosomes hydrolysate prepared by incubating isolated oleosomes and 0, 2.5, and 5% Protex 6L in distilled water, pH 9 at 60 °C for 18 h, and the resulting cream and skim fractions. Soy flour and isolated oleosomes represent the controls. All the major soybean storage proteins subunits α′, α, and β subunits of the β-conglycinin and acidic and basic subunits of glycinin, as well as lipoxygenase, were present in the soy flour (Lane 2). The three high molecular weight bands which migrate at higher apparent molecular weights ranging between 66 and 44 kDa were identified as the α′, α, and β-subunits of β-conglycinin since they are glycopeptides. Several band with molecular weights ranging from 40 to 27 and 18 kDa were identified as the acidic and basic polypeptides, respectively of glycinin. These results are consistent with previous reports on structural characteristics of soybean glycinin and β-conglycinin [31]. Surprisingly proteins from the hydrolysate prepared with 0% Protex 6L were hydrolyzed at 4.6 ± 0.15%. The peptides profiles of this hydrolysate (Lane 4), its resulting cream and skim fractions (Lanes 7, 10) present hydrolyzed α′, α, and β subunits of the β-conglycinin and acidic and basic subunits of glycinin. These results were due to the activity of the side protease in the Multifect pectinase preparation used to fractionate oleosomes from soy flour [32, 33]. This explains the disappearance of the lipoxygenase and, the partial hydrolysis of α′ subunits in the oleosome fraction (Lane 3). Isolated oleosomes treated with either 2.5 or 5% Protex 6L generated hydrolyzed soybean storage proteins at 18.2 ± 0.0.2–20.6 ± 0.03% degree of hydrolysis (Lanes 5, 6, 8, 9), with all the storage proteins hydrolyzed to peptides smaller than 6.5 kDa, compared to the oleosomes disrupted without proteases. These results support the close yields of free oil collected from these hydrolysates (slurries prepared with 2.5 and 5% Protex 6L) (Table 1).
Urea SDS-PAGE profiles of hydrolysates of isolated oleosomes of soybean flour. Lane 1 Low molecular markers (6.5–66 kDa) and calculated location of molecular weight of 23 kDa (oleosins molecular weight), Lane 2 Soy flour, Lane 3 Isolated oleosomes, Lane 4 Slurry 1 (isolated oleosomes hydrolyzed with 0% Protex 6L) Lane 5 Slurry 2 (isolated oleosomes hydrolyzed with 2.5% Protex 6L) Lane 6 Slurry 3 (isolated oleosomes hydrolyzed with 5% Protex 6L) Lane 7 Cream from slurry1, Lane 8 Cream from slurry 2, Lane 9 Cream from slurry 3, Lane 10 Skim from slurry 1, 80–85 μg protein/lane. L Lipoxygenase, α′, α, β Subunits of β-conglycinin, A Acidic polypeptides of glycinin, B Basic polypeptides of glycinin
Conclusion
Enzyme-assisted aqueous extraction of oil from isolated oleosomes was an efficient alternative to the conventional organic solvent extraction. The simplest process to extract free oil from isolated oleosomes consisted in the hydrolysis of oleosomes with 2.5 or 5% Protex 6L in distilled water (ratio 1/6; dw/v) pH 9 at 60 °C for 18 h, destabilization of the resulting slurry by heating at 96 °C for 3 h, destabilization of the resulting cream by the churning butter process at 40 °C for 4 h. This strategy yielded 83–88% of free oil, and hydrolyzed proteins.
References
Sharma A, Khare SK, Gupta MN (2002) Enzyme assisted aqueous extraction of peanut oil. J Am Oil Chem Soc 79:215–218
Owusu-Ansah JY (1997) Enzymes assisted extractions. In: Wan PJ, Wakeleyn PJ (eds) Technology and solvents for extracting oil seeds and nonpetroleum oils. AOCS Press, Champain, pp 323–332
Huang AHC (1992) Oil bodies and oleosins in seeds. Ann Rev Plant Physiol Plant Mol Biol 43:177–200
Beisson F, Ferte N, Bruley S, Voultoury R, Verger R, Arondel V (2001) Oil-bodies as substrates for lipolytic enzymes. Biochim Biophys Acta 1531:47–58
Jacks TJ, Yatsu LY, Altschul AM (1967) Isolation and characterization of peanut spherosomes. Plant Physiol 42:585–597
Tzen JTC, Peng CC, Cheng DJ, Chen ECF, Chui JMH (1997) A new method for seed oil body purification and examination of oil body integrity following germination. J Biochem 121:762–768
Millichip M, Tatham SA, Jackson F, Griffiths GS, Shewry PR, Stobart AK (1996) Purification and characterization of oil bodies (oleosomes) and oil–body boundary proteins (oleosins) from the developing cotyledons of sunflower (Helianthus annus L.). Biochem J 314:333–337
Marcoux D, Gorkiewicz-Petkow A, Hirsch R, Korting HC (2004) Dry skin improvement by an oleosome emulsion as carrier for sphingolipid. J Am Acad Dermatol 2004:293
Iwanaga D, Gray DA, Fisk ID, Decker EA, Weiss J, McClements DJ (2007) Extraction and characterization of oil bodies from soybeans: a natural source of pre-emulsified soybean oil. J Agric Food Chem 55:8711–8716
Iwanaga D, Gray DA, Decker EA, Weiss J, McClements DJ (2008) Stabilization of soy oil bodies using protective pectin coating formed by electrostatic deposition. J Agric Food Chem 56:2240–2245
Nikifordis C, Kiosseoglou V (2009) Aqueous extraction of oil bodies from Maize Ger (Zea mays) and characterization of the resulting natural oil in water emulsion. J Agric Food Chem 57:5591–5596
White DA, Fisk ID, Makkhun S, Gray DA (2009) In vitro assessment of the bioaccessibility of tocopherol and fatty acids from sunflower seed oil bodies. J Agric Food Chem 57:5720–5726
Kapchie VN, Wei D, Hauck C, Murphy PA (2008) Enzyme-assisted aqueous extraction of oleosomes from soybeans (Glycine max). J Agric Food Chem 56:1766–1771
McGlone OC, Octavia MC, Canales ALM, Carter JV (1986) Coconut oil extraction by a new enzymatic press. J Food Sci 51:695–697
Freitas SP, Hartman L, Couri S, Jablonka FH, De Carvalho CWP (1997) The combined application of extrusion and enzymatic technology for extraction of soybean oil. Lipid/Fett 99:333–337
Rosenthal A, Pyle DL, Niranjan K (2001) Aqueous enzymatic processes for edible oil extraction. Enzyme Microb Technol 19:402–420
Lamsal BP, Johnson LA (2007) Separating oil from aqueous extraction fractions of soybean. J Am Oil Chem Soc 84:785–792
Chabrand RM, Kim HJ, Zhang C, Glatz CE, Jung S (2008) Destabilization of the emulsion formed during aqueous extraction of soybean oil. J Am Oil Chem Soc 85:383–390
Wu J, Johnson LA, Jung S (2009) Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soy bean flakes. Bioresour Technol 100:527–533
Aoki H, Taneyama O, Inami M (1980) Emulsifying properties of soy proteins: characteristics of 7S and 11S proteins. J Food Sci 45:534–546
Panyam D, Kilara A (1996) Enhancing the functionality of food proteins by enzymatic modification. Trends in Food Sci Technol 7:120–125
Alder-Nissen J (1986) Enzymatic hydrolysis of food proteins. Elsevier, New York, pp 116–125
Association of Official Analytical Chemists [AOAC] (1983) Official Methods of Analysis, 15th edn. AOAC, Washington, DC
Jung S, Rickert DA, Deak NA, Aldin ED, Recknor J, Johnson LA, Murphy PA (2003) Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J Am Oil Chem Soc 80:1169–1173
Lamsal BP, Reitmeier C, Murphy PA, Johnson LA (2006) Enzymatic hydrolysis of extruded-expelled soy flour and resulting functional properties. J Am Oil Chem Soc 83:731–737
Rombaut R, Dewettinck K (2006) Properties, analysis and purification of milk polar lipids. Int Dairy J 16:1362–1373
Avalli A, Contarini G (2005) Determination of phospholipids in dairy products by SPE/HPLC/ELSD. J Chromatogr A 1071:185–190
Damodanan S, Anand K (1997) Sulfhydryl-disulfide interchange-induced interparticle protein polymerization in whey protein-stabilized emulsion and its relation to emulsion stability. J Agric Food Chem 45:3813–3820
McClements DJ (2005) Food emulsion: Principle, practice, and techniques, 2nd edn. CRC Press, Boca Raton, pp 161–232
Lanzani A, Petrini MC, Cozzoli O, Grallavresi P, Carola C, Jacini G (1975) Use of enzymes for vegetable oil extraction: preliminary report. La rivista Italiano delle Sostanze Grasse 52:226–229
Wu S, Murphy PA, Johnson LA, Reuber MA, Fratzke AR (2000) Simplified process for soybean glycinin and β-conglycinin fractionation. J Agric Food Chem 48:2702–2708
Jung S, Lamsal BP, Stepien V, Johnson LA, Murphy PA (2006) Functionality of soy proteins produced by enzyme-assisted extraction. J Am Oil Chem Soc 83:71–78
Kapchie VN, Towa TL, Hauck C, Murphy PA (2009) Recycling of aqueous supernatants in soybean oleosomes isolation. J Am Oil Chem Soc (in press). doi:10.1007/s11746-009-1485
Acknowledgments
This work was supported by USDA Special Grant (20063443217128). Genencor, a Danisco company, is gratefully acknowledged for providing enzymes.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Towa, L.T., Kapchie, V.N., Hauck, C. et al. Enzyme-Assisted Aqueous Extraction of Oil from Isolated Oleosomes of Soybean Flour. J Am Oil Chem Soc 87, 347–354 (2010). https://doi.org/10.1007/s11746-009-1503-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1503-3