Abstract
Phase-equilibrium diagrams of biodiesel synthesis substrate (oil/alcohol/alkyl esters) and product (alkyl esters/glycerol/alcohol) mixtures containing methanol, ethanol and propanol, respectively, were used for the identification of those composition ratios leading to mixture homogeneity. Based on the diagrams, complete conversion of the substrate oil contained in a sunflower oil/ethanol/ethyl ester mixture at a weight ratio of 7.6:11.4:81.0 resulted in the generation of an amount of glycerol capable of remaining in solution in the product mixture. In contrast, complete conversion using a mixture of a lower alcohol and ethyl ester content (19.0:6.0:75.0) leads to the separation of phases. A continuous reactor charged with Lypozyme TL-IM and fed with the former mixture showed constant conversion and productivity with time, whereas process efficiency was found to decrease drastically when the latter mixture was fed. When a sunflower oil/isopropanol/isopropyl ester mixture at a weight ratio of 35:35:30 was fed in the reactor charged with Novozym 435, the output glycerol concentration was equivalent to that theoretically expected. In contrast, feeding a sunflower oil/ethanol/ethyl ester mixture of equal proportions as above, the glycerol concentration was lower than expected, suggesting the occurrence of glycerol adsorption on the enzyme support.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel, the product of transesterifying triglycerides with alcohol, i.e., alcoholysis, has gained in importance due to diminishing petroleum reserves and the environmental consequences of exhaust gas emissions from petroleum-fueled engines [1, 2]. The combustion of biodiesel, a plant-derived rather than a fossil fuel, does not increase current net atmospheric levels of carbon and sulfur oxides. In view of the similarity in physicochemical properties and high compatibility between traditional diesel fuel and biodiesel [3], the latter has been identified as a major alternative to petroleum-based fuel.
Currently, industrial-scale biodiesel synthesis relies on the chemically-catalyzed transesterification of vegetable oils with short-chain alcohols, usually methanol. Chemically-catalyzed transesterification of triglycerides to their corresponding alkyl esters provides high conversion yields with short reaction times [1]. When acid catalysts are used, careful removal of catalyst residues from the final product, entailing large of volumes of wash-effluent, is required to avoid damage to engine parts [4]. The purification of glycerol, which is obtained as a secondary product of chemical alcoholysis, depends critically on the removal of inorganic material and, occasionally, on the removal of undesirable by-products requiring multiple-stage separation procedures [1, 4].
The above problems are not encountered in enzyme-catalyzed transesterification methods, which do not require complex glycerol recovery and catalyst and salt removal operations, and allow milder reaction conditions which in turn lead to increased product integrity [4–8]. However, in view of the high cost of enzymes, enzyme-catalyzed biodiesel production processes have been the subject of little dissemination and scale-up research [6, 9]. The negative effects of the progress of alcoholysis on catalytic activity and the eventual inactivation of the catalyst depend strongly on reaction conditions. Where no solvents and an excess amount of a short-chain alcohol, such as methanol or ethanol, are used, complete enzyme denaturation may be associated with the heterogeneity of the reaction media and the separation of two phases due to the low miscibility between alcohol and oil [10].
Furthermore, the low solubility of glycerol, in the product mixture has a strong negative effect, expressed as a blocking effect, on enzyme activity. This effect has been attributed to the deposition of glycerol on the enzyme surface, leading to hydrophilic hindrance of the enzyme, thus causing diffusion constraints for the transfer of the hydrophobic substrate from the organic phase to the enzyme’s active sites [8]. While this effect may be amplified in continuous reactors, it may be lessened by washing the enzymes with isopropanol between operating cycles [11].
Problems involving catalyst deactivation and the high cost of immobilized enzymes have been major limitations for the industrialization of enzymatic biodiesel production [9]. In order to improve production economics, research has been focused on alternative methods of preserving enzyme activity over extended operation periods. Other research has been focused on the use of organic solvents to increase the solubility of alcohol and glycerol in the reaction medium [6]. However, solutions found to date have proven to be ineffective insofar as continuous biodiesel production is concerned [8]. In view of the toxicity and flammability of organic solvents and the cost of their recovery from the product mixture, solvent-free lipase-catalyzed alcoholysis has received particular attention [12].
An attractive alternative to the use of solvents has been proposed by Watanabe and coworkers [13, 14]. This alternative relies on multiple-step methanol additions to the reaction medium, leading to higher conversion yields in both batch and continuous transesterification of vegetable oil to methyl esters.
Du and coworkers [15] proposed the use of methyl acetate as a methanol substitute with a view toward preventing enzyme denaturation associated with the use of excess methanol as well as problems related with the deposition of glycerol on the catalyst surface. Despite fairly high yields, difficulty separating triacetin from the final product mixture is among the method’s drawbacks.
State-of-the-art process design methodologies, which are aimed at the optimization of enzyme performance and productivity, rely on the use of solubility data of the reaction species occurring during the transesterification process. Biodiesel/methanol/glycerol ternary phase-equilibrium diagrams for methanolysis found in the literature demonstrate the difficulty of separating glycerol from the residue when no solvents are used, due to the low solubility of glycerol in methanol [16]. Zhou and coworkers [17] reported on the chemically-catalyzed conversion of Jatropha curcas oils to methyl esters, whereby the homogeneity of the oil/methanol/FAME mixture resulted in improved reaction kinetics and an increase in FAME content to 70%, while glycerol remained insoluble in both oil and FAME consistently at all conditions and was easily separated from the final biodiesel product. Intersolubility phenomena are also a key to defining the compatibility of blends comprised of diesel fuel and biodiesel/alcohol mixtures [18].
Ternary mixtures are generally used in studies of product compositions such as biodiesel, glycerol and alcohol, whereas the substrate is considered to be a binary mixture of oil and alcohol. Considering biodiesel as a third substrate component, the addition of fatty acid alkyl esters at the start of the reaction may prove useful in modifying the intersolubility between the oil and the alcohol. In turn, a strong influence of composition on mixture characteristics may be conveniently oriented toward the improvement of enzyme performance.
Here, ternary phase-equilibrium diagrams were constructed for the substrate and product mixtures of each of three systems containing methanol, ethanol and isopropanol, respectively. Two commercial enzymes were assayed for catalytic performance and enzyme productivity in continuous processing using substrate feeds of varying composition.
Experimental Procedures
Materials
Refined sunflower oil, which was provided courtesy of COUSA S.A., Montevideo, Uruguay, had a fatty acid composition of: 5.3% of 16:0; 3.8% of 18:0; 29.3% of 18:1; 60.7% of 18:2. Total free fatty acids in the oil were 0.04% (as oleic acid) and the moisture content was less than 0.05%.
HPLC-grade alcohols (Sigma-Aldrich) were used for ester synthesis and mixture preparation. Other solvents, standards and reagents used in the derivatization step, as required for the analysis, were also purchased from Sigma-Aldrich. Commercial enzymes, Novozym 435 and LipoZyme TL-IM were provided courtesy of Novozymes Latinoamerica, Curitiba, Brasil.
Chemically-Catalyzed Synthesis of Fatty Acid Alkyl Esters
The fatty acid alkyl esters (FAAE) used for phase-equilibrium studies were synthesized using the corresponding sodium alkoxide, prepared by dissolution to 0.5% of metallic Na in the amount of alcohol required to obtain an alcohol to oil molar ratio of 6:1 using 100 g of sunflower oil. The 100 g of sunflower oil was added to the above solution under agitation, the system was heated to boiling temperature and kept under reflux for 60 min. The products were washed five times with 300 ml of distilled water (40 °C), centrifuged at 3,000 rpm (900 g) and dried under anhydrous sodium sulfate overnight. Biodiesel purity (greater than 99.5%) was tested according to UNE-EN 14103 [19], as described below.
Construction of Phase-Equilibrium Diagrams
Ternary phase-equilibrium diagrams were determined for the oil/alcohol/FAAE substrate mixtures and FAAE/glycerol/alcohol product mixtures. Solubility curves were determined by means of turbidimetric analysis under isothermal conditions (50 °C) as described by Komers and coworkers [16]. A binary mixture of two selected components was placed in screw-cap tubes with a septum cap. The third component was added through the septum using a micro syringe, and the weight increase of the system was determined. The mixture was placed in an ultrasonic thermostatic bath (±0.1 °C) and agitated with a magnetic stirrer. The procedure was repeated until the appearance of turbidity and the associated composition was consistent with a point on the solubility line in the phase-equilibrium diagram. The procedure was repeated at least three times, or that required to reduce the relative standard deviation of the concentration value to less than 0.5%. Results obtained for varying starting proportions of two components led to the construction of ternary diagrams.
Continuous Enzyme-Catalyzed Transesterification
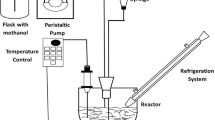
Figure 1 shows a diagram of the equipment used for assays of the continuous enzyme-catalyzed process. The oil/alcohol/FAAE mixture, using the selected concentration ratios, was stirred in a mixer and pumped (Shimadzu LC-6A pump) to the top of the column at a constant flow rate (3 g/min). The reactor comprised a vertical glass column (200 mm × 18 mm ID) in a water-heated jacket (50 °C), equipped with a sintered glass frit at the bottom and packed with the immobilized enzyme (17 g). Samples of the reaction product were collected at 45-min intervals using an automatic fraction collector (LKB Ultrorac 7000).
Ester and Glycerol Content by GC Analysis
Samples were subjected to a gentle nitrogen flow at 40 °C until constant weight, ensuring the elimination of residual alcohol, and different portions were subjected to ester or glycerol content determinations, respectively according to European standard methods for biodiesel analysis UNE-EN 14103 [19] and UNE-EN 14105 [20].
Ester content determinations were performed using a Shimadzu GC-2014 capillary gas chromatographer equipped with a FID and an SGE BPX70 capillary column (25 m × 0.5 mm × 0.25 mm). The temperature was programmed to increase at a heating rate of 4 °C/min from 160 to 230 °C, and was held at this value for 10 min. Nitrogen at 40 kPa at the column head was used as the carrier gas using a split ratio of 1:80.
Samples used for glycerol analysis were conditioned as described above and treated with MSTFA/pyridine to ensure the derivatization of glycerol to the corresponding trimethylsilyl esters. GC analysis was conducted on the above-described equipment, using an OPTIMA-1TG column (Machery-Naguel, 10 m × 0.32 mm × 0.1 mm). Nitrogen at 70 kPa at the column head was the carrier gas. The oven temperature was programmed from 50 to 370 °C using a heating rate of 10 °C/min.
Results and Discussion
Product Composition
Figure 2 shows the phase diagrams of FAAE/glycerol/alcohol mixtures for each of the three assayed alcohols and respective alkyl esters. The usable sector of the phase diagram is limited by a maximum glycerol concentration defined by process stoichiometry (1 mol of glycerol per 3 mol of FAAE). Such concentrations, expressed in terms of the alkyl ester/glycerol weight ratio, are 90.6:9.4 for methyl ester/glycerol, 91.0:9.0 for ethyl ester/glycerol, and 91.3:8.7 for isopropyl ester/glycerol.
An average alkyl ester/glycerol ratio of 91:9 is shown in Fig. 2 by a dotted line; a greater quantity of glycerol than results from complete oil conversion falls in the area toward the right of the above line and has no physical meaning.
All curves shown in Fig. 2 start at an alkyl ester/glycerol/alcohol ratio of 100:0:0, indicating a high mutual solubility of the alkyl ester/alcohol mixture and a low mutual solubility of the alkyl ester/glycerol mixture. Results are consistent with data previously reported on the products of vegetable oil methanolysis [16–18].
According to the curves shown in Fig. 2 for the ethanol and isopropanol systems, an initial increase in alcohol concentration allows the incorporation of small amounts of glycerol in the respective ethyl or isopropyl ester phase. Thus, product mixture homogeneity depends critically on the addition of alcohol at the start of reaction. Excess alcohol should be supplied in an amount adequate to ensure sufficient excess of alcohol upon reaction completion capable of dissolving the glycerol resulting from complete conversion of the substrate oil.
Substrate Composition
Figure 3a–c shows phase-equilibrium diagrams of the oil/alcohol/FAAE substrate mixtures, determined at 50 °C for each of the three alcohols and respective alkyl esters. Regardless of alcohol type, binary mixtures of alkyl ester/alcohol and alkyl ester/oil were completely miscible, whereas great differences in intersolubility were found for the sunflower oil (SFO)/alcohol mixtures depending on the alcohol type.
Phase-equilibrium diagrams at 50 °C for the different SFO/alcohol/alkyl ester substrate mixtures: saturation curve (filled circles); curve corresponding to product saturation curve (filled triangles). The dotted line shows the stoichiometric amount of alcohol. (a) SFO/methanol/methyl esters (points 1–4 and E correspond to compositions as shown in Table 1); (b) SFO/ethanol/ethyl esters; (c) SFO/isopropanol/isopropyl esters
The diagram area was divided into three different regions according to mixture characteristics. The shaded area, identified with the letter “A”, represents a zone of partial solubility among reactants, corresponding to a heterogeneous mixture.
The largest zone A is for methanol (Fig. 3a), on account of a very low miscibility between this alcohol and SFO. The solubility curve limiting this region intercepts the methanol axis at two points (both corresponding to a binary mixture of SFO and methanol): point 94:6 (pure SFO dissolves methanol at a concentration of 6%) and point 0:100 (pure methanol dissolves a negligible percentage of SFO). Figure 3a shows that the addition of methyl esters to a SFO/methanol mixture leads to improved intersolubility of the mixture, attaining complete miscibility at methyl ester concentrations as high as 62%.
As shown in Fig. 3b, the behavior of the SFO/ethanol binary mixture differed widely from that of the mixture containing methanol. Examining the two points where the solubility curve intercepts the alcohol axis (corresponding to 0% of alkyl esters), pure SFO dissolves ethanol at a concentration of 7.5% and pure ethanol dissolves SFO at a concentration of 25%. A higher compatibility between ethanol and SFO is reflected in a smaller area of zone A than for the methanol system. Accordingly, complete miscibility may be attained at ester concentrations as low as 11%.
Finally, Fig. 3c shows the phase-equilibrium diagram of the SFO/isopropanol/isopropyl ester mixture, where the nonoccurrence of zone A indicates a homogeneous mixture regardless of composition ratio.
As shown in Fig. 3, an increase in alcohol molecular weight leads to improved compatibility of a SFO/alcohol/alkyl ester mixture; a wider range of concentration ratios thus rendering a homogeneous substrate mixture.
The substrate composition should be limited by the minimum amount of alcohol required for complete oil conversion according to reaction stoichiometry. The minimum oil/alcohol weight ratio will depend on alcohol molecular weight: 90.1:9.9 for SFO/methanol; 86.4:13.6 for SFO/ethanol; and 82.9:17.1 for SFO/isopropanol (considering a molecular weight of SFO of 876.8 g/mol). In Fig. 3a–c the above ratios, shown by dotted lines, enable the identification of zone B areas, defined by a stoichiometric deficit of alcohol.
Further, stoichiometric back-calculation from each individual point on the solubility curves shown in Fig. 2 (saturation of FAAE/glycerol/alcohol) enables one to define the substrate mixture compositions (SFO/alcohol/alkyl ester) leading to product homogeneity at complete conversion yields, considering the associated generation of glycerol. The above procedure leads to the definition of a product-saturation curve (shown in Fig. 3a–c for each alcohol) and to the identification of zone C areas, wherein complete conversion of the oil in the substrate mixture results in a heterogeneous product with the associated separation of glycerol. The generation of glycerol in larger amounts than may be dissolved in the product mixture is a consequence of the addition of excess oil to the substrate mixture, over a maximum allowable amount in terms of product homogeneity.
Therefore, A, B and C define restrictions on substrate composition associated with substrate homogeneity, alcohol requirement and product homogeneity, respectively. Using a substrate composition falling in any area not included in the above zones A, B or C, defined as zone D, no adverse effect on enzyme performance should be expected during the continuous process, thus maximizing catalyst productivity. Worth noting is the large zone D in both ethanol and isopropanol diagrams compared with the extremely narrow zone D found for the methanol system—ascribed to the extremely low solubility of oil in methanol and of glycerol in methyl esters.
Watanabe and co-workers [13, 14] reported on the continuous methanolysis of a mixture of soybean and rapeseed oils catalyzed by Candida antartica (Novozym 435), relying on three-step (1/3 mol equivalents per step) and two-step (2/3 + 1/3 mol equivalents) methanol additions to ensure that the alcohol was kept in solution at all times during the reaction, thus preventing the separation of a methanolic phase. The mixture composition at each step is shown in Table 1 and Fig. 3b. Unlike the addition of a stoichiometric amount of methanol, clearly defining a point in zone A (point E), three successive additions of 1/3 mol equivalent of methanol (points 1–3, respectively) result in a condition such that methanol is completely soluble at all times. A consistently high level of performance has been reported for the enzyme operating in conditions defined by three-step methanol additions. In contrast, as shown in Fig. 3, the addition of methanol in two steps (points 4 and 3) leads to unfavorable conditions, as point 4 (2/3 methanol/oil mol equivalents) is slightly inside zone A, with a methanolic phase separating from the mixture. Consistently, a lower performance has been reported for the latter operation modality in continuous processes [13, 14].
While three-step methanol additions may be used to avoid alcohol solubility problems, it does not provide a solution to the problem of glycerol deposition. Figure 3 shows that all three points (1–3) are located to the right of the product saturation curve (inside zone C), indicating that complete conversion of the oil to FAAE will result in glycerol separation from the product mixture. Removal of the glycerol produced between steps has often been employed as a partial solution to this problem.
In view of all the above, the analysis of phase-equilibrium diagrams of substrate mixtures used in transesterification processes sheds some light on the performance of lipase-catalyzed processes.
Continuous Transesterification
In order to verify the effect of substrate composition on enzyme performance in continuous processes, a column packed with lipase was fed with different mixtures, and reaction product samples were collected at time intervals and analyzed. In view of previous work reported on continuous lipase-catalyzed methanolysis and in the light of the results shown above, the methanol system does not allow operation in a convenient homogeneous region. Therefore, only those substrate mixtures corresponding to the ethanol and isopropanol systems were assayed.
Figure 4a shows the ester content in the product mixture obtained by SFO ethanolysis catalyzed by Lipozyme TL-IM, using substrate mixtures at different SFO/ethanol/ethyl ester composition ratios:
-
Mixture 1: SFO/alcohol/ethyl ester (%) = 7.6: 11.4: 81.0
-
Mixture 2: SFO/alcohol/ethyl ester (%) = 19.0: 6.0: 75.0
The points corresponding to both mixtures are shown in Fig. 3b (points 1 and 2, respectively); with mixture 1 falling slightly inside zone D (totally homogeneous) and mixture 2 in zone C, where glycerol will not dissolve in the product. As shown in Fig. 4a, although high initial conversions (ester content from 96 to 100%) were attained for both systems, a strong reduction in process performance was observed for mixture 2, whereas using mixture 1 as substrate, the ester content was found to increase slightly over the progress of reaction. These results suggest that the solubility of glycerol in the product may be a critical factor in adjusting the substrate composition so that it will not lead to glycerol deposition on the enzyme surface and the consequent reduction in catalyst activity. The substrate composition also clearly affects enzyme productivity in terms of the mass of ethyl esters produced per gram of enzyme and per hour. Figure 4b shows the productivity in the continuous process using either substrate mixture. As expected, the highest concentration of oil (mixture 2) showed the highest initial productivity, which rapidly decreased to values similar to those obtained for mixture 1. The glycerol concentration in the product was also analyzed; results showed strong variation (data not shown), probably due to strong interactions with the solid enzyme support (porous silica granulates).
In order to test the continuous process using a higher concentration of oil and ethanol, the column was charged with lipase Novozym 435 (immobilized Candida antarctica lipase B), a high-performance catalyst in transesterification [10, 13, 14]. As shown in Fig. 5a, when the column was fed with a SFO/ethanol/ethyl ester mixture at a 35:35:30 ratio, a composition falling in zone D (point 3, Fig. 3b), a product of constant ester content (ca. 80%) was obtained. Results did not show any negative effect on catalyst activity during the first 80 h of reaction, although the glycerol concentration in the product was lower than expected, assuming complete oil conversion.
According to the above results, even when substrate composition falls within a “safe” zone in terms of product homogeneity, some degree of glycerol retention within the catalyst bed may occur. Considering that the glycerol produced in the process is expected to be completely soluble in the product mixture, glycerol retention may be associated with phenomena other than insolubility, such as adsorption on the catalyst surface. Figure 5b shows the catalyst productivity of the process to be at a nearly constant value of 315 mg ester g enzyme−1 h−1.
The use of a SFO/isopropanol/isopropyl ester substrate mixture at the same concentration ratio as above (point 3, Fig. 3c) led to a higher ester content (greater than 90%, Fig. 6a), and a glycerol concentration close to stoichiometric conversion. Accordingly, the enzyme productivity (330–400 mg ester. g enzyme−1 h−1) was higher than for the ethanol system, increasing slightly with the progress of the reaction (Fig. 6b).
On comparing the results obtained for the ethanol and isopropanol systems, using the same enzyme and concentration ratios, the nature of the mixture appears to be a critical factor insofar as glycerol elution is concerned, with the isopropanol system leading to improved efficiency. As both systems were operated under total solubility conditions, glycerol retention may be associated with adsorption phenomena.
Conclusion
Substrate composition has been deemed critical for improvement of enzyme productivity in continuous processing. The most convenient substrate mixture design may be that which prevents the separation of alcohol and/or glycerol from the substrate and product mixtures, respectively, leading to optimal enzyme performance and productivity. The addition of alkyl esters appears to be a useful tool to ensure homogeneous conditions in both substrate and product mixtures, due to the combined favorable effects resulting from an increase in intersolubility among reactants (oil/alcohol) and the dilution of substrate oil, leading to a reduction in glycerol concentration in the product mixture when complete oil conversions are achieved.
Although substrate and product mixture homogeneity has been shown to have favorable effects on process efficiency, additional unfavorable phenomena such as glycerol adsorption should be the focus of further research.
References
Mittelbach M (1990) Lipase catalyzed alcoholysis of sunflower oil. J Am Oil Chem Soc 67:168–170
Noureddini H, Zhu D (1997) Kinetics of transesterification of soybean oil. J Am Oil Chem Soc 74:1457–1463
Yuan W, Hansen AC, Zhang Q, Tan Z (2005) Temperature-dependent kinematic viscosity of selected biodiesel fuels and blends with diesel fuel. J Am Oil Chem Soc 82:195–199
Knothe G, Gerpen JV, Krahl J (2005) The biodiesel handbook. AOCS Press, Champaign
Shimada Y, Watanabe Y, Sugihara A, Tominaga Y (2002) Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J Mol Catal B Enzym 17:133–142
Iso M, Chen BX, Eguchi M, Kudo T, Shrestha S (2001) Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J Mol Catal B Enzym 16:53–58
Nelson LA, Foglia TA, Marmer WN (1996) Lipase-catalyzed production of biodiesel. J Am Oil Chem Soc 73:1191–1195
Dossat V, Combes D, Marty A (1999) Continuous enzymatic transesterification of high oleic sunflower oil in a packed bed reactor: influence of the glycerol production. Enzyme Microb Technol 25:194–200
Nielsen PM, Brask J, Fjerbae L (2008) Enzymatic biodiesel production: Technical and economical considerations. Eur J Lipid Sci Technol 110:692–700
Shimada Y, Watanabe Y, Samukawa T, Sugihara A, Noda H, Fukuda H, Tominaga Y (1999) Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. J Am Oil Chem Soc 76:789–793
Wei D, Yuanyuan X, Dehua L, Jing Z (2003) Lipase-catalysed transesterification of soya bean oil for biodiesel production during continuous batch operation. Biotechnol Appl Biochem 38:103–106
Selmi B, Thomas D (1998) Immobilized lipase-catalyzed ethanolysis of sunflower oil in a solvent-free medium. J Am Oil Chem Soc 75:691–695
Watanabe Y, Shimada Y, Sugihara A, Noda H, Fukuda H, Tominaga Y (2000) Continuous production of biodiesel fuel from vegetable oil using immobilized Candida antarctica lipase. J Am Oil Chem Soc 77:355–360
Watanabe Y, Shimada Y, Sugihara A, Tominaga Y (2002) Conversion of degummed soybean oil to biodiesel fuel with immobilized Candida antarctica lipase. J Mol Catal B: Enzym 17:151–155
Du W, Xu Y, Liu D, Zeng J (2004) Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J Mol Catal B Enzym 30:125–129
Komers K, Tichý J, Skopal F (1995) Ternäres Phasendiagramm Biodiesel-Methanol-Glyzerin. J Pract Chem 337:328–331
Zhou H, Lu H, Liang B (2006) Solubility of multicomponent systems in the biodiesel production by transesterification of Jatropha curcas L: oil with methanol. J Chem Eng Data 51:1130–1135
Makareviciene V, Sendzikiene E, Janulis P (2005) Solubility of multi-component biodiesel fuel systems. Bioresour Technol 96:611–616
Standard UNE-EN 14103 (2003) Determinación de los contenidos de éster y éster metílcio del ácido linolénico, issued by Asociación Española de Normalización y Certificación, Madrid, Spain
Standard UNE-EN 14105 (2003) Determinación de los contenidos de glicerol libre y total y de mono-, di- y triglicéridos, Asociación Española de Normalización y Certificación, Madrid, Spain
Acknowledgments
Special acknowledgments to CSIC (Comisión Sectorial de Investigación Científica) of Uruguay’s Universidad de la República for financial support and scholarships; and to Novozymes Latinoamerica Ltda, for the supply of lipase samples. Text edited by Chem. Eng. Eduardo Speranza.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jachmanián, I., Dobroyán, M., Moltini, M. et al. Continuous Lipase-Catalyzed Alcoholysis of Sunflower Oil: Effect of Phase-Equilibrium on Process Efficiency. J Am Oil Chem Soc 87, 45–53 (2010). https://doi.org/10.1007/s11746-009-1478-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1478-0