Abstract
Several fatty acid alkyl esters were subjected to accelerated methods of oxidation, including EN 14112 (Rancimat method) and pressurized differential scanning calorimetry (PDSC). Structural trends elucidated from both methods that improved oxidative stability included decreasing the number of double bonds, introduction of trans as opposed to cis unsaturation, location of unsaturation closer to the ester head group, and elimination of hydroxyl groups. Also noticed with EN 14112 was an improvement in oxidative stability when a larger ester head group was utilized. Methyl esters that contained ten or less carbons in the fatty acid backbone were unacceptable for analysis at 110 °C (EN 14112) due to excessive sample evaporation. With respect to PDSC, a correlation was noticed in which the oxidation onset temperature (OT) of saturated fatty esters increased with decreasing molecular weight (R 2 0.7328). In the case of the monounsaturates, a very strong inverse correlation was detected between molecular weight and OT (R 2 0.9988), which was in agreement with EN 14112. Lastly, a strong direct correlation (R 2 0.8759) was elucidated between OT and oil stability index (OSI, EN 14112, 80 °C). The correlation was not as strong (R 2 0.5852) between OSI obtained at 110 °C and OT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel, an alternative fuel prepared by transesterification of vegetable oils or animal fats, is susceptible to autoxidation. The rate of autoxidation (Fig. 1) is dependant on the number and location of methylene-interrupted double bonds contained within fatty acid methyl or ethyl esters (FAME or FAEE) that comprise biodiesel. Polyunsaturated materials are particularly vulnerable to autoxidation, as evidenced by the relative rates of oxidation of the unsaturates: 1 for ethyl oleate, 41 for ethyl linoleate, and 98 for ethyl linolenate [1]. The American (ASTM D6751 [2]) and European biodiesel standards (EN 14214 [3]) contain an oxidative stability specification whereby biodiesel must resist oxidation for at least 3 (ASTM D6751) or 6 h (EN 14214) according to the Rancimat method (EN 14112, 110 °C) [4]. Not only will oxidized biodiesel fail oxidative stability requirements, but oxidative degradation negatively impacts acid value and kinematic viscosity [5–7], both of which are specified in ASTM D6751 and EN 14214 (Table 1).
Measurement of oxidative stability can be accomplished with accelerated methods whereby various experimental parameters are influenced to yield results in a reasonable period of time. Such parameters may include elevated temperature, pressure, and/or flow rate of air (oxygen) through the sample, among others. Accelerated methods for determination of oxidative stability include, but are not limited to, the Rancimat method (EN 14112, [3]), AOCS official method Cd 12b-92 [8], and differential scanning calorimetry (DSC), which are summarized in Table 2.
Previous literature [9–21] on the oxidative stability of oleochemicals utilizing DSC studied a variety of vegetable oils and biodiesel, along with selected fatty acids and their corresponding ethyl esters. However, individual FAME were not investigated. Other reports [21–28] on the oil stability index (OSI) of various fatty acid alkyl esters (including biodiesel) and vegetable oils were accomplished following AOCS official method Cd 12b-92 at a variety of block temperatures (50–110 °C), but these studies did not include a number of FAME commonly found in biodiesel, nor were EN 14112 or DSC methods utilized. A more recent report [29] on the OSI of selected FAME and FAEE employed both EN 14112 and AOCS official method Cd 12b-92 at 110 °C, but once again did not include a number of FAME commonly found in biodiesel, nor were the effects of chain length, double bond location or orientation investigated.
The aim of the current study was to measure and compare the oxidative stability of FAME typically found in biodiesel (Table 3) by accelerated methods such as EN 14112 and pressurized DSC (PDSC). Of particular interest were the effects of chain length, type of ester head group, and double bond content, orientation, and location on oxidative stability. The Rancimat method (EN 14112) was selected because it is specified in ASTM D6751 and EN 14214. Although PDSC is not specified in ASTM D6751 or EN 14214, it is rapid, requires very little sample (<5 mg) and provides good precision for measurement of oxidative degradation [9, 10]. Also of interest were potential correlations between methods and the influence of other factors on the oxidative stability of FAME and FAEE, such as molecular weight and boiling point.
Materials and Methods
Materials
The following alkyl esters were purchased from Nu-Chek Prep, Inc. (Elysian, MN) and used as received soon after arrival: methyl hexanoate (1, 99 + %, methyl caproate), methyl octanoate (2, 99 + %, methyl caprylate), methyl decanoate (3, 99 + %, methyl caprate), methyl dodecanoate (4, 99 + %, methyl laurate), methyl tetradecanoate (5, 99 + %, methyl myristate), methyl hexadecanoate (6, 99 + %, methyl palmitate), methyl 9Z-hexadecenoate (7, methyl palmitoleate), methyl octadecanoate (8, 99 + %, methyl stearate), ethyl octadecanoate (9, 99 + %, ethyl stearate), methyl 12-hydroxyoctadecanoate (10, 99 + %, methyl 12-hydroxystearate), methyl 6Z-octadecenoate (11, 99 + %, methyl petroselinate), methyl 9Z-octadecenoate (12, 99 + %, methyl oleate), ethyl 9Z-octadecenoate (13, 99 + %, ethyl oleate), methyl 9E-octadecenoate (14, 99 + %, methyl elaidate), methyl 9Z-12-hydroxyoctadecenoate (15, 99 + %, methyl ricinoleate), methyl 9Z,12Z-octadecadienoate (16, 99 + %, methyl linoleate), ethyl 9Z,12Z-octadecadienoate (17, 99 + %, ethyl linoleate), methyl 9E,12E-octadecadienoate (18, 99 + %, methyl linoelaidate), methyl 9Z,12Z,15Z-octadecatrienoate (19, 99 + %, methyl linolenate), ethyl 9Z,12Z,15Z-octadeca-trienoate (20, 99 + %, ethyl linolenate), methyl eicosanoate (21, 99 + %, methyl arachidate), methyl 11Z-eicosanate (22, 99 + %, methyl gondoate), methyl tricosanoate (23, 99 + %, methyl behenate), and methyl 13Z-tricosenoate (24, 99 + %, methyl erucate). The samples were stored at −15 °C until needed to mitigate unwanted autoxidation. Soybean oil methyl esters (SME) were obtained from Ag Environmental Products, LLC (Omaha, NE) and were found to contain by GC (wt.%) 10.5% 6, 4.1% 8, 22.5% 12, 1.6% methyl 11Z-octadecenoate, 53.6% 16, and 7.7% 19, along with a trace amount (in summation less than 1%) of other methyl esters.
EN 14112
OSI (h) was measured following EN 14112 [3] at 110 and 80 °C utilizing a Metrohm (Herisau, Switzerland) model 743 Rancimat instrument provided by Brinkmann Instruments, Inc. (Westbury, NY). The flow rate of air through 3 ± 0.01 g of sample was 10 L/h. The block temperature was set to 110 or 80 °C with correction factors (ΔT) of 1.5 and 0.9 °C, respectively. The conductivity measuring vessel contained 50 ± 0.1 mL of deionized water. Each sample was run in triplicate and mean values are reported (Tables 4, 5, 6). OSI was mathematically determined as the inflection point of a computer-generated plot of conductivity (µS/cm) of deionized water versus time (h).
Pressurized Differential Scanning Calorimetry
Oxidation onset temperature (OT, °C) was determined using a DSC 2910 thermal analyzer from TA Instruments (Newcastle, DE). Typically, a 2 μL sample, resulting in a film thickness of <1 mm, was placed in an aluminum pan hermetically sealed with a pinhole lid and oxidized with pressurized (1378.95 kPa; 200 psi) dry air (Gateway Airgas, St. Louis, MO) in the module with a heating rate of 10 °C/min from 50 to 350 °C. A computer-generated plot of heat flow (W/g) versus temperature was used to graphically determine OT. Each sample was run in triplicate and average values rounded to the nearest tenth of a degree are reported (Table 4).
Results and Discussion
Oil Stability Index
The American (ASTM D6751) and European Union (EN 14214) biodiesel standards contain oxidative stability specifications utilizing the Rancimat (EN 14112) method. Consequently, this method was chosen for investigation of the oxidative stability of FAME in the current study. The Rancimat (EN 14112) method specifies that samples are to be analyzed at 110 °C (block temperature) with an air flow rate of 10 L/h through the sample. However, these parameters do not approximate conditions which are likely to be encountered during typical storage of biodiesel. Under the conditions of the standard EN 14112 method, unsaturated compounds generally undergo oxidative degradation in a relatively short (<3 h) period of time [23, 26, 28, 29, and results reported herein]. Therefore, block temperatures of 80 and 110 °C were selected for investigation. Earlier reports [21, 22] demonstrated that variances in OSI tended to increase with increased OSI values. Accordingly, experiments conducted in the present study were terminated at 40 h.
Compounds that are otherwise similar but contain a greater number of methylene-interrupted double bonds undergo oxidative degradation at faster rates [1, 27, 29, 30], which is confirmed in the present study through comparison of the OSI values (Table 4) of methyl stearate (8), methyl oleate (12), methyl linoleate (16), and methyl linolenate (19). There is a strong inverse relationship between the number of double bonds contained in a series of compounds and the resultant OSI values. For instance, for the series 12, 16, and 19, R 2 values of 0.8955 and 0.9700 were obtained for block temperatures of 80 and 110 °C, respectively (graphs not shown). Compounds that do not contain double bonds, such as 8, have not undergone oxidative degradation by 40 h at either 80 or 110 °C. Applying these results to biodiesel, one may expect superior oxidative stability from biodiesel fuels that were prepared from feedstocks relatively high in saturated fatty acid content and/or relatively low in polyunsaturated fatty acid content. For example, palm oil methyl esters are known to be considerably more stable to oxidation than soybean oil methyl esters according to EN 14112 [31, 32].
The effect of double bond location on oxidative stability was investigated through comparison of the OSI values of methyl petroselinate (11, 6Z-monoene) and 12 (9Z-monoene). As seen from Table 4, 11 was more stable to oxidation than 12, as evidenced by higher OSI values for 11 at block temperatures of 80 and 110 °C. A possible explanation for this result may be the comparatively close proximity of the electron rich carboxylate moiety to unsaturation in 11, which may introduce mild stereoelectronic repulsive effects during initial radical formation not present in the case of 12. Applying these results to biodiesel, biodiesel fuels enriched in FAME with unsaturation located close to the ester head group would have superior oxidative stability (as measured by EN 14112) to those with 9-ene FAME, such as 12, 16, and 19.
The influence of double bond orientation was ascertained through comparison of materials that contain trans double bonds [methyl elaidate (14) and methyl linoelaidate (18)] to the corresponding cis isomers (12 and 16). As seen from Table 4, 14 exhibited superior stability to oxidation (OSI: 7.7 and >40 h at 110 and 80 °C) in comparison to 12 (OSI: 2.5 and 15.1 h at 110 and 80 °C). Likewise, a similar observation was made between 18 and 16 at 80 °C (OSI: 4.4 vs. 3.4 h), but at 110 °C they were essentially indistinguishable. These results are not surprising, as it is known that trans isomers are generally thermodynamically more stable than the corresponding cis isomers. Applying these results to biodiesel, biodiesel fuels that contain at least some trans constituents would exhibit somewhat enhanced stability to oxidation according to EN 14112 than biodiesel fuels that contain a similar number of entirely cis double bonds. Although naturally occurring trans fatty acid-containing vegetable oils are rare, trans isomers can be chemically introduced through catalytic partial hydrogenation. Partially hydrogenated soybean oil methyl esters (7.7% trans FAME, 16.4% saturated FAME, 44.7% polyunsaturated FAME) yielded an OSI value of 6.2 h (110 °C, AOCS Cd 12b-92) versus 2.3 h for SME [33].
Due to the nature of AOCS official method Cd 12b-92 and EN 14112, a bias is introduced whereby compounds of higher molecular weight will appear to be more stable to oxidation than compounds of similar double bond and other functional group (such as ester) content but lower molecular weight. The parameters of the methods are the cause of this bias: a specified mass (3.0 g in the case of EN 14112) of material is required as opposed to a specified molar amount. This shortcoming has been discussed previously [27, 29]. Illustrative of this point is methyl palmitoleate (7, MW 268.44), methyl oleate (12, MW 296.49), and methyl gondoate (22, MW 324.54), which have similar double bond content but increasing OSI values (110 °C) of 2.1, 2.5, and 2.9 h (R 2 1.0000, graph not shown), respectively (Table 4). This trend was also evident when compared at 80 °C, which provided OSI values of 14.3, 15.1, and >40 h for 7, 12, and 22. Concomitant with these results was variation in ester group among otherwise similar compounds. Ethyl oleate (13, MW 310.52) demonstrated greater oxidative stability than 12, as evidenced by OSI values for 13 of 3.5 (110 °C) and 15.7 h (80 °C). The effect of ester head group on OSI was less pronounced for polyunsaturated compounds such as methyl and ethyl linoleates (16 and 17) and methyl and ethyl linolenates (19 and 20). The increased double bond content of these materials resulted in an accelerated rate of oxidation which essentially masked the aforementioned molecular weight effect on OSI.
Relatively short chain saturated FAME, such as methyl hexanoate (1, methyl caproate), methyl octanoate (2, methyl caprylate) and methyl decanoate (3, methyl caprate) are found in coconut, cuphea and babassu oils (Table 2), among others. These materials (1–3) were stable to oxidation due to the absence of unsaturation, which was verified in Table 4 at 80 °C (OSI of 1–3: >40 h). However, sample loss as a result of evaporation was noticed when OSI was determined at 110 °C for 1–3, which yielded unexpectedly low and unreliable OSI values. Although 1–3 possess boiling points in excess of 110 °C [34, 35, Table 5], prolonged exposure to elevated temperature in combination with a constant flow of air through the sample during experimentation resulted in evaporation. To explore this phenomenon more carefully, 1–3 and methyl laurate (4) were re-evaluated at 110 °C with concomitant determination of sample loss at 3, 6, and 24 h. As can be seen from Table 5, only 62% (wt.%) of 1 remained after 3 h. The remainder of the sample evaporated into the conductivity measuring vessel. After 6 h, only 32% of 1, 81% of 2, and 83% of 3 remained in the tube containing the sample. After 24 h, nearly all of 1 (2% remaining) had evaporated, along with a majority of 2 (28%) and a significant percentage of 3 (55%). Only after 24 h did 4 exhibit substantial sample evaporation (65% remaining). These results indicated that oxidative stability determined at 110 °C (AOCS or EN methods) of biodiesel containing 1–3 should be viewed with skepticism. Accordingly, soybean oil methyl esters (SME) were enriched in 1–4 at 5, 10, and 20 wt.% to ascertain the influence of these relatively volatile constituents on OSI at 110 °C of biodiesel. Soybean oil methyl esters were chosen because they do not naturally contain these constituents, so the effect(s) of adding 1–4 on oxidative stability could be clearly determined. As indicated in Table 6, addition of 1–4 did not influence OSI of SME at 5 wt.%. However, at 10 and especially 20 wt.% a deleterious effect on OSI was observed, as evidenced by the decreasing OSI values of SME enriched with methyl caproate at 0, 5, 10, and 20 wt.% (6.6, 6.4, 6.0, 5.2 h, respectively, Table 6). As anticipated, the negative impact on OSI of SME was greatest for the lowest molecular weight species (1), as indicated by the OSI values at 20 wt.% of 1 (5.2 h), 2 (5.4 h), 3 (5.5 h), and 4 (5.8 h).
Hydroxylated fatty acids occur naturally in castor and lesquerella oils. Ricinoleic acid (9Z-12-hydroxyoctadecanoic acid) constitutes roughly 80–90% of castor oil (Ricinus communis) and lequerolic acid (11Z-14-hydroxyeicosenoic acid), the C20 homolog of ricinoleic acid, comprises about 50–70% of lesquerella oil (Lesquerella fendleri) [36]. Both methyl 12-hydroxystearate (10) and 8 (Table 4) yielded indistinguishable OSI results (>40 h) at both 80 and 110 °C, but methyl 9Z-12-hydroxyoctadecenoate (15, methyl ricinoleate) exhibited inferior stability to oxidation when compared to 12. These results indicate that hydroxyl moieties in unsaturated FAME may impart mild pro-oxidant behavior to biodiesel.
Pressurized Differential Scanning Calorimetry
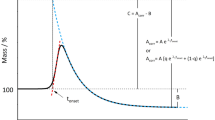
The OT (°C) is defined as the temperature at which a rapid increase in the rate of oxidation is observed [37], which is obtained from extrapolation of the tangent line drawn on the steepest slope of the reaction exotherm of a plot of heat flow versus temperature (Fig. 2). Higher OT values indicate greater stability to oxidation [37]. As with EN 14112, the parameters of the PDSC method do not approximate conditions encountered during typical storage of biodiesel. However, tests that do not employ accelerated oxidation conditions are unacceptably long to be of practical industrial value.
Results from PDSC analysis of 1–24 (Table 4) were generally in agreement with those obtained from EN 14112. Similar trends noticed from both methods included the effects of the number of double bonds, double bond orientation, and double bond position on stability to oxidation. In contrast to OSI, PDSC failed to provide a clear trend with regard to the effect of ester head group on oxidative stability. In fact, the effect of ester head group was minimal. For example, methyl esters 8 (OT 197.2 °C), 16 (OT 142.6 °C), and 19 (OT 129.8 °C) were more stable to oxidation than the corresponding ethyl esters (9, OT 195.9 °C; 17, OT 142.2 °C; 20, OT 129.2 °C). However, in the case of the oleates, the ethyl ester (13, OT 176.8 °C) was more stable than the methyl ester (12, OT 174.9 °C).
A statistically significant inverse relationship (R 2 0.7328, Fig. 3) was elucidated between OT and molecular weight in the case of the saturated esters (1–6, 8, 9, 21, 23). In the case of the mono-unsaturated methyl esters (7, 12, 22, 24) a statistically significant direct relationship (R 2 0.9988, Fig. 4) was discovered, which was in accordance with the OSI results obtained from EN 14112. The opposite trends noticed for oxidation of the saturated and unsaturated esters can be attributed to molecular weight effects. The PDSC method, like EN 14112, utilizes the same mass of material for every experiment. As with the OSI results, compounds with a similar number of double bonds but greater molecular weight yielded higher OT values. In the case of the saturated esters, the mechanistic pathway for oxidative degradation is considerably slower and may occur at any point along the fatty acid backbone with the exception of the terminal methyl group. A sample of molecules of lower molecular weight contains more unreactive terminal methyl groups than a sample of higher molecular weight. The general rule is that the ease of oxidation of C–H bonds follow the order: –CH3 (most stable) > –CH2– ≫ CH– (most reactive).
Another area in which disparate results were obtained with PDSC was the effect of hydroxyl group on stability to oxidation. In the case of methyl 12-hydroxystearate (10, OT 208.4 °C) a higher OT was obtained than for methyl stearate (8, OT 197.2 °C). This result is not comparable to the aforementioned OSI data, since both 8 and 10 yielded OSI values in excess of 40 h. With regard to the unsaturated alcohols, the reverse trend was noticed: methyl (12, OT 174.9 °C) and ethyl (13, OT 176.8 °C) oleates exhibited higher OT values than methyl ricinoleate (15, OT 170.2 °C). This result is in agreement with the OSI data, which indicated that the presence of the hydroxyl group may result in a mild pro-oxidant effect.
Relationship between Methods
A plot of OSI at 80 °C of esters that exhibited OSI values less than 40 h (7, 11–13,15–20) versus OT yielded a statistically significant direct relationship (R 2 0.8759, Fig. 5) in which samples with higher OSI values also yielded higher OT values. A plot of OSI at 110 °C of esters that yielded OSI values of less than 40 h (7, 11–20, 22, 24) versus OT yielded a similar trend, but the relationship was not as strong (R 2 0.5852, Fig. 6). This was likely due to the compression of OSI data at higher block temperatures. For instance, 16 and 18 only differed by 0.2 h at a block temperature of 110 °C, but the difference was 1.0 at 80 °C.
References
Holman RA, Elmer OC (1947) The rates of oxidation of unsaturated fatty acids and esters. J Am Oil Chem Soc 24:127–129
ASTM (2008) Standard specification for biodiesel fuel (B100) blend stock for distillate fuels. In: Annual Book of ASTM Standards, ASTM Press, West Conshohocken, Method D6751
European Committee for Standardization (2003) Automotive fuels fatty acid methyl esters (FAME) for diesel engines requirements and test methods. European Committee for Standardization Press, Brussels, Method EN 14214:2003
European Committee for Standardization (2003) Fat and oil derivatives—fatty acid methyl esters (FAME)—determination of oxidation stability (accelerated oxidation test). European Committee for Standardization Press, Brussels, Method EN 14112:2003
Du Plessis LM, de Villiers JBM, van der Walt WH (1985) Stability studies on methyl and ethyl fatty acid esters of sunflower oil. J Am Oil Chem Soc 62:748–752
Mittelbach M, Gangl S (2001) Long storage stability of biodiesel made from rapeseed and used frying oil. J Am Oil Chem Soc 78:573–577
Bondioli P, Gasparoli A, Della Bella L, Tagliabue S, Toso G (2003) Biodiesel stability under commercial storage conditions over one year. Eur J Lipid Sci Technol 105:735–741
AOCS (1999) Oil stability index. In: Firestone D (ed) Official methods and recommended practices of the American oil chemists’ society, 5th edn. AOCS Press, Champaign, Method Cd 12b-92
Sharma BK, Stipanovic AJ (2003) Development of a new oxidation stability test method for lubricating oils using high-pressure differential scanning calorimetry. Thermochim Acta 402:1–18
Litwinienko G, Kasprzycka-Guttman T (1998) A DSC study on thermoxidation kinetics of mustard oil. Thermochim Acta 319:185–191
Kowalski B (1989) Determination of oxidative stability of edible vegetable oils by pressure differential scanning calorimetry. Thermochim Acta 156:347–358
Kowalski B (1991) Thermal oxidative decomposition of edible oils and fats. DSC studies. Thermochim Acta 184:49–57
Kowalski B (1993) Evaluation of activities of antioxidants in rapeseed oil matrix by pressure differential scanning calorimetry. Thermochim Acta 213:135–146
Litwinienko G, Daniluk A, Kasprzycka-Guttman T (2000) Study on autoxidation kinetics of fats by differential scanning calorimetry. 1. Saturated C12–C18 fatty acids and their esters. Ind Eng Chem Res 39:7–12
Litwinienko G, Kasprzycka-Guttman T (2000) Study on autoxidation kinetics of fat components by differential scanning calorimetry. 2. Unsaturated fatty acids and their esters. Ind Eng Chem Res 39:13–17
Litwinienko G (2001) Autoxidation of unsaturated fatty acids and their esters. J Therm Anal Cal 65:639–646
Litwinienko G, Daniluk A, Kasprzycka-Guttman T (1999) A differential scanning calorimetry study on the oxidation of C12–C18 saturated fatty acids and their esters. J Am Oil Chem Soc 76:655–657
Dunn RO (2006) Oxidative stability of biodiesel by dynamic mode pressurized-differential scanning calorimetry (P-DSC). Trans ASABE 49:1633–1641
Dunn RO (2002) Effect of oxidation under accelerated conditions on fuel properties of methyl soyate (biodiesel). J Am Oil Chem Soc 79:915–920
Dunn RO (2005) Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process Technol 86:1071–1085
Dunn RO (2000) Analysis of oxidative stability of methyl soyate by pressurized-differential scanning calorimetry. Trans ASABE 43:1203–1208
Dunn RO (2008) Effect of temperature on the oil stability index of biodiesel. Energy Fuels 22:657–662
Akoh CA (1994) Oxidative stability of fat substitutes and vegetable oils by the oxidative stability index method. J Am Oil Chem Soc 71:211–216
Knothe G, Dunn RO (2003) Oxidative stability of biodiesel in blends with jet fuel by analysis of oil stability index. J Am Oil Chem Soc 80:1047–1048
Dunn RO (2005) Oxidative stability of soybean oil fatty acid methyl esters by oil stability index (OSI). J Am Oil Chem Soc 82:381–387
Canakci M, Monyem A, Van Gerpen JH (1999) Accelerated oxidation processes in biodiesel. Trans ASAE 42:1565–1572
Knothe G, Dunn RO (2003) Dependence of oil stability index of fatty compounds on their structure and concentration in the presence of metals. J Am Oil Chem Soc 80:1021–1025
Isbell TA, Abbott TP, Carlson KD (1999) Oxidative stability index of vegetable oils in binary mixtures with meadowfoam oil. Ind Crop Prod 9:115–123
Knothe K (2008) “Designer” Biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels 22:1358–1364
Frankel EN (2005) Lipid oxidation, 2nd edn. The Oily Press, Bridgewater
Park J-T, Kim D-K, Lee J-P, Park S-C, Kim Y-J, Lee J-S (2008) Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour Technol 99:1196–1203
Moser BR (2008) Influence of blending canola, palm, soybean, and sunflower oil methyl esters on fuel properties of biodiesel. Energy Fuels 22:4301–4306
Moser BR, Haas MJ, Winkler JK, Jackson MA, Erhan SZ, List GR (2007) Evaluation of partially hydrogenated methyl esters of soybean oil as biodiesel. Eur J Lipid Sci Technol 109:17–24
Dean JA (1987) Handbook of organic chemistry. McGraw-Hill, New York
Lide DR (2008) Handbook of chemistry and physics, 89th edn. CRC Press, Boca Raton
Gunstone FD, Harwood JL (2007) Occurrence and characterization of oils and fats. In: Gunstone FD, Harwood JL, Dijkstra AJ (eds) The lipid handbook, 3rd edn. CRC Press, Boca Raton, pp 37–142
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM (2000) Oxidation kinetics studies of unmodified and genetically modified vegetable oils using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta 364:87–97
Acknowledgment
The author acknowledges Benetria N. Banks for technical assistance and Drs. Kenneth M. Doll and Brajendra K. Sharma (USDA ARS NCAUR) for discussion of data and results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer: Product names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
About this article
Cite this article
Moser, B.R. Comparative Oxidative Stability of Fatty Acid Alkyl Esters by Accelerated Methods. J Am Oil Chem Soc 86, 699–706 (2009). https://doi.org/10.1007/s11746-009-1376-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1376-5