Abstract
A high-performance liquid chromatographic (HPLC) method is described for the determination of conjugated linoleic acids (CLA) and conjugated linolenic acids (CLN). Methyl esters prepared from purified lipid fractions of soybean oil were analyzed using an HPLC system equipped with photodiode-array detector to detect peaks having maximum absorption around 233 and 275 nm. These peaks were concentrated by AgNO3-silicic acid column chromatography and reversed-phase HPLC. The structural analysis, of dimethyloxazoline (DMOX) derivatized methyl esters, using gas chromatography–mass spectrometry (GC–MS) showed the occurrence of 9,11- and 10,12-CLA and 8,10,13-, 8,10,12-, and 9,11,13-CLN. The comparison of these conjugated fatty acids with authentic isomers by HPLC revealed the presence of isomeric mixtures of CLA [cis (c),trans(t) or t,c and t,t] and CLN (c,t,t or t,t,c and t,t,t). Traces of 9,11- and 10,12-CLA (c,t or t,c) were found in crude oil. CLN isomers (8,10,12-18:3 and 9,11,13-18:3) were found to be forming during the bleaching phase of soybean oil processing. 8,10,13-CLN and 9,11- and 10,12-CLA (t,t) were only found in soybean oil after the deodorization step. CLN contents in commercial soybean oil varied from 387 to 1,316 mg/kg oil. A decreased level of bleaching earth and temperature resulted in a reduced CLN content. It is possible that CLN would be derived from the linoleate hydroperoxides formed during the processing and storage of soybean oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conjugated fatty acids have evoked increased interest due to the beneficial effects they afford in terms of human health. Among these, conjugated linoleic acid (CLA) has been researched and reviewed extensively in relation to its occurrence [1], metabolism, and physiological effects [1–4]. However, various other conjugated fatty acids, including trienes, tetraenes, and pentaenes, have also been reported in different plant resources from both terrestrial and aquatic environments.
Conjugated linolenic acids (CLN) have been reported to occur in terrestrial plant lipids, especially seed oils. Important CLN from plant sources include α-eleostearic acid [9cis(c),11trans(t),13t-18:3], catalpic acid (9t,11t,13c-18:3), punicic acid (9c,11t,13c-18:3), calendic acid (8t,10t,12c-18:3), and jacaric acid (8c,10t,12c-18:3) [5]. Conversely, Yurawecz et al. [6] only could find traces (up to 0.2%) of CLN in their study of 27 vegetable oils for CLN content using UV measurement. The isomers were identified as α-eleostearic acid (9c,11t,13t-18:3), β-eleostearic acid (9t,11t,13t-18:3), and 8t,10t,12t-18:3. The possible mechanism for the formation of these CLN isomers involves linoleate oxidation, reduction of hydroperoxide to hydroxide, and dehydration [7, 8]. CLN exhibits less oxidative stability, as reported in our previous study [9], indicating that CLN may influence the oxidative deterioration of vegetable oils, despite its presence in only trace amounts.
Crude oil is refined by a series of processes to remove impurities that affect the taste, smell, appearance, and stability of the oil. The refining processes involve de-gumming, alkali refining, bleaching, and deodorization. Polyunsaturated fatty acids, such as linoleic acid and α-linolenic acid, found in vegetable oils are more susceptible to oxidation during the storage and refining stages of crude oil processing, resulting in oxidation products, mainly hydroperoxides. Hydroperoxides may also be formed by enzymatic oxidation of linoleate and linolenate by lipoxygenase during storage of the oil seeds. Any oxidation is injurious to the flavor and oxidative stability of refined oils as it results in the formation and accumulation of oxidation products. The quality of a processed vegetable oil always depends on the quality of the crude oil used and the processing parameters selected. Although indicators such as peroxide value in the final products can be reduced by lowering the storage temperature and minimizing exposure to pro-oxidants like air, light, and heat during processing, the complete removal of all oxidation products from oils is impossible. The linoleate hydroperoxides formed during the storage and refining stages of oils may get converted to 9,11,13-CLN or 8,10,12-CLN due to the heat and/or acid treatments involved in the processing [7, 8].
In the present study, we analyzed CLN in both crude soybean oil and processed soybean oil by high-performance liquid chromatography (HPLC) equipped with a photodiode-array detector. This detection system enabled us to determine c,t,t (or t,t,c) and t,t,t isomers of 9,11,13-CLN and 8,10,12-CLN in these soybean oils.
Materials and Methods
Materials
Crude soybean oil, purified soybean oil, and soybean oil at different processing stages were prepared by Nisshin Oillio, Tokyo, Japan. Commercial vegetable oils were obtained from oil companies in Japan or from local grocery stores. CLA (9c,11t- and 10t,12c-isomers) were purchased from Matreya (State College, Pa.). Each CLA was converted to its methyl ester with a H2SO4-methanol solution [10]. Standard CLN [α-eleostearic acid (9c,11t,13t-18:3), β-eleostearic acid (9t,11t,13t-18:3), catalpic acid (9t,11t,13c-18:3), and calendic acid (8t,10t,12c-18:4)] were separated by HPLC from methyl esters of CLN-containing seed oils as described previously [11, 12]. Seeds were powdered in an electric mill and extracted twice with n-hexane at room temperature. The seed oil was trans-esterified to its methyl esters with 0.5 M sodium methoxide in methanol. Each CLN isomer was separated from the mixed methyl esters by reversed-phase HPLC system using a column packed with C30 (Develosil C30 UG-5, 250 × 8.0 mm i.d., 5.0-μm particle size; Nomura Chem Co, Seto, Aichi, Japan).
Preparation of Methyl Esters from Vegetable Oils
Thin-layer chromatographic (TLC) analysis of the vegetable oils used in this study revealed that these vegetable oils mainly consisted of triacylglycerol with a trace of free fatty acid, monoacylglycerol, and diacylglycerol. TLC was carried out on 0.25-mm silica gel plates (Merck, Darmstadt, Germany) with diethyl ether/n-hexane/acetic acid (40:60:1, v/v/v) as the developing solvent. Triolein, oleic acid, monoolein, and diolein were used as standards. Anhydrous HCl/methanol or BF3/methanol is often used for the methylation of vegetable oils, but this acid-catalyzed condition causes extensive isomerization of the original CLA or CLN as well as artifact formation [13–15]. Methyl esters from vegetable oils were, therefore, prepared by trans-esterification using 0.5 M sodium methoxide in methanol [9]. The methyl esters thus prepared were subjected to gas chromatography (GC) to determine the fatty acid composition of vegetable oils under study. A Shimadzu GC-14B gas chromatograph (Shimadzu Seisakusho, Kyoto, Japan) equipped with a flame-ionization detector and a capillary column (Omegawax 320, 30 m × 0.32 mm i.d.; Supelco, Bellefonte, Pa.) was employed for the GC analysis. The column temperature was set at 200°C, while the injector and detector were held at 250° and 260°C, respectively, for the analysis. Helium with a flow of 50 kPa was used as the carrier gas.
Purification of Soybean Oil Methyl Esters
Methyl esters from purified soybean oil (approx. 10 g) were refined on a silicic acid column (40 × 2.7 cm i.d.) (Silicagel 60; Merck, Germany) by successive elution with n-hexane (300 ml) and a solution of diethyl ether/n-hexane (5:95, 800 ml; 10:90, 600 ml; v/v). Refined methyl esters (>9.5 g) eluted with diethyl ether/n-hexane (5:95, v/v) were used for the analytical HPLC separation of esters of conjugated fatty acids.
Analytical and Preparative HPLC
Analytical HPLC was carried out on an analytical reversed-phase column (Develosil C30 UG-5; 250 × 4.6 mm i.d., 5.0-μm particle size; Nomura Chem. Co) protected with a 10 × 4.0 mm i.d. guard column containing the same stationary phase. A mixture of methanol and water (85:15, v/v) at a flow rate of 1.0 ml/min was used as a mobile phase. All of the HPLC analyses were carried out on a Hitachi L-7000 system equipped with pump (L-7100) and an auto-sampler (L-7200). The instrument also housed a photodiode-array spectrophotometric detector (Hitachi L-7455) and an online analysis software (Hitachi HPLC system-5-manager; model D-7000). A preparative HPLC was carried out for the separation of the conjugated fatty acid-containing fraction obtained by AgNO3-silicic acid column chromatography. The HPLC conditions were the same as those mentioned in the analytical HPLC except that the column size was 250 × 8.0 mm i.d. and the solvent flow was 3.0 ml/min.
Fractionation and Identification of Conjugated Fatty Acids in Purified Soybean Oil
Conjugated fatty acid methyl esters were concentrated initially by argentation column chromatography. The soybean oil methyl esters (approx. 20 g) were refined on a 20% AgNO3-silicic acid column (50 × 4 cm i.d.) by eluting with n-hexane (500 ml) and a mixture of diethyl ether/n-hexane solution [2:98 (500 ml), 5:95 (500 ml), 10:90 (500 ml), 20:80 (500 ml), 30:70 (500 ml), and 40:60 (500 ml), v/v]. The fatty acid profile of each fraction was monitored by an analytical HPLC. Most of conjugated fatty acids were eluted with diethyl ether/n-hexane 5:95 (v/v), although this fraction (>1 g) mainly consisted of oleic acid and linoleic acid. Preparative HPLC was used for the fractionation of the conjugated fatty acid-containing fraction eluted with diethyl ether/n-hexane solution (5:95, v/v). The fraction separated by the reversed-phase HPLC still contained non-conjugated fatty acids, such as saturated fatty acids, oleic acid, and linoleic acid. The identification of conjugated fatty acids in the fraction was, therefore, carried out using GC–mass spectrometry (MS) after conversion of the methyl esters to dimethyloxazoline (DMOX) derivatives [16]. GC–MS was performed on a Hewlett-Packard HPG1800A instrument (Hewlett-Packard, Palo Alto, Calif.) under the following conditions: Omegawax-250 column, 30 m × 0.25 mm i.d. (Supelco, Bellefonte, Pa.); helium carrier gas, 40 ml/min; injector, 230°C; detector, 240°C; column, 198°C. Conjugated fatty acid isomers in the separated fraction were also characterized by comparison with the reference CLA and CLN by HPLC under similar conditions as those mentioned in the analysis of conjugated fatty acids.
Quantitative HPLC Analysis of CLN Content in Vegetable Oils
A known amount (100–1000 μg) of standard CLN methyl esters (9c,11t,13t; 9t,11t,13t; 9t,11t,13t; 8t,10t,12c) was diluted in 1 ml n-hexane solution containing 500 μg n-butyl benzoate as an internal standard. Ten microliters of the solution was injected into HPLC system equipped with an octadecylsilica (ODS) column (Cosmosil 5C18-AR, 4.6 × 150 mm i.d., 5-μm particle size; Nacalai Tesque, Kyoto, Japan) connected to a guard column (Develosis ODS-UG; 4.0×10 mm, 5-μm particle size; Nomura Chem. Co). The analysis was carried out at 20°C using methanol/water (85:15, v/v) as the mobile phase at a flow rate of 1.0 ml/min . Peaks were monitored with a Hitachi L-7400 UV detector set at 275 nm. HPLC was carried out in a Hitachi L-7000 system as mentioned. A calibration curve for each CLN isomer was made from the peak ratio of the CLN to the internal standard, and an amount of CLN was injected. For the quantification of CLN in vegetable oil, methyl esters of the oil (approx. 5.0 mg) were dissolved in 1 ml n-hexane solution containing 500 μg internal standard, and then 10 μl of the solution was injected into HPLC. The CLN in the oil was quantified using calibration curves for CLN isomers. The analysis was duplicated, and there was little difference in the CLN content for each determination.
Results and Discussion
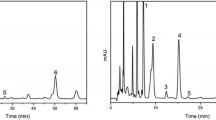
The major fatty acids of purified soybean oil from Nisshin Oillio were linoleic acid (18:2n-6; 52.1%), oleic acid (18:1n-9; 23.3%), palmitic acid (16:0; 11.2%), α-linolenic acid (18:3n-3; 5.2%), stearic acid (18:0; 3.8%), and 18:1n-7 (2.1%). Trans isomers of linoleic acid (<1.0%) and α-linolenic acid (<1.0%) were also detected by GC analysis. The HPLC chromatogram of the soybean oil methyl esters (Fig. 1) indicates the presence of unsaturated fatty acids (18:2n-6, 18:3n-3, 18:1n-9), as shown by UV detection at 210 nm. Two peaks near 18:2n-6 and 18:3n-3 could be due to the presence of their trans isomers. Saturated fatty acids were not observed, as indicated by UV detection, due to the absence of double bonds in the molecule. A number of other peaks were observed by means of UV detection at 233 or 274 nm. These peaks showed a maximum absorption at 229–240 nm and 266–270 nm, respectively, on the UV spectrum, as indicated by photodiode-array detection. The absorbance spectrum of these two peaks was almost identical to that of the CLA and CLN standards. The HPLC chromatogram of the soybean oil methyl esters fraction obtained by elution with diethyl ether/n-hexane (5:95, v/v) on the AgNO3-silicic acid column is presented in Fig. 2. Peaks showing a maximum absorption around 233 and 275 nm could be concentrated in this fraction, although GC analysis revealed 18:2n-6, 18:1n-9, 18:0, and 16:0 to be the main fatty acids of this fraction. Most of the linoleic acid and α-linolenic acid were eluted with diethyl ether/n-hexane solution (10:90 and 20:80, v/v) and with diethyl ether/n-hexane solution (30:70 and 40:60, v/v), respectively. As shown in Fig. 2, the conjugated fatty acid concentrated fraction was further fractionated by reversed-phase HPLC to F1–F5. These HPLC fractions still contained non-conjugated fatty acid esters. Therefore, the structural analysis of conjugated fatty acid of HPLC fraction (F1–F5) was carried out by GC–MS following the conversion of methyl esters to DMOX derivatives and by comparison with authentic CLA and CLN isomers by means of HPLC [16, 17].
Analysis of purified soybean oil methyl esters by analytical HPLC; chromatograph was equipped with a photodiode-array detector. Procedures for the analytical HPLC are described in the Materials and methods
Analytical HPLC of the conjugated fatty acid-concentrated fraction obtained by AgNO3-silicic acid column chromatography. The procedures for the analytical HPLC are described in the Materials and methods
GC–MS analysis of the DMOX derivatives of F1 showed peaks which gave a parent ion at m/z 331 and characteristic fragmentation ions at 260 and 248, 220 and 208, 194 and 182, showing that the double bonds were located at positions 8–9, 10–11, and 13–14. GC–MS analysis also indicated the presence of two another CLN isomers, 9,11,13–18:3 and 8,10,12–18:3, in both the F2 and F4 fractions. Double bond positions could be identified by the characteristic loss of 12 Da; 260 and 248, 234 and 222, 208 and 196 for the 9,11,13-isomer, and 246 and 234, 220 and 208, 194 and 182 for the 8,10,12-isomer. Conjugated fatty acids in F3 and F5 were identified by GC–MS to be 9,11- and 10,12-18:2. The DMOX derivatives of the CLA isomers gave a characteristic loss of 12 Da; 234 and 222, 208 and 196 for 9,11-18:2, 220 and 208, 194 and 182 for 10,12-18:2.
A comparison of the F2 and F4 fractions with that of authentic CLN isomers on reversed-phase HPLC showed that the CLN peaks in F2 eluted on the same retention time as 9c,11t,13t-18:3 and 9t,11t,13c-18:3, while those in F4 corresponded to that of 9t,11t,13t-18:3. Fatty acids with cis double bonds elute faster than those with trans double bonds. The CLN peak in F2 may possibly consist of either c,t,t- or t,t,c-isomers of 8,10,12- or 9,11,13–18:3. Similarly, those in F4 might be t,t,t-isomers of 8,10,12- or 9,11,13–18:3. The absorption maxima of the CLN peaks in F2 and F4 were 268.8 and 266.6 nm, respectively. The wavelength and the spectrum of CLN in F2 and F4 were identical to those of 9c,11t,13t-18:3 or 9t,11t,13c-18:3 and 9t,11t,13t-18:3, respectively. Consequently, 8,10,12- and 9,11,13-18:3 (c,t,t or t,t,c, and t,t,t) were found as CLN isomers with conjugated trienes in the purified soybean oil (Table 1). Geometrical isomers of 8,10,13-18:3 were found in the oil as CLN isomers with conjugated diene. CLA isomers detected in the purified soybean oil were c,t- or t,c-, and t,t-isomers of 9,11- and 10,12–18:2.
The HPLC chromatogram of crude soybean oil and processed soybean oil at different stages of the processing is shown in Fig. 3. Traces of 9,11- and 10,12-CLA isomers (c,t or t,c) were found in the crude oil. The sources of these c,t- or t,c-CLA isomers are not clear, although one possibility is that these may be formed during the oil and meal separation processes involving heating. Another CLA isomer (t,t) appeared after deodorizing. The peak areas of c,t- or t,c-CLA isomers increased after deodorization. The presence of CLA in vegetable oils has been reported [18]. The formation of conjugated fatty acids has been observed during heating and acid treatment of linoleate [19, 20]. The present study also showed an increase in CLA content during the heat treatment of the deodorization step. CLN isomers with conjugated trienes were observed during bleaching, but they were not found after de-gumming and alkali refining (Fig. 3). However, CLN with conjugated diene (8,10,12-18:3) was detected after deodorization. Yurawecz et al. [6] reported the presence of CLN (9,11,13-18:3 and 8,10,12-18:3) in edible oils. The HPLC analysis employing the photodiode-array detector in the present study provides an overall perspective of the formation of conjugated fatty acids during the refining of soybean oil. In this scheme, linoleate hydroperoxides would be the source of the 9,11,13- and 8,10,12-CLN isomers. CLN with conjugated diene (8,10,12–18:3) may result from CLN with conjugated trienes. Only one positional isomer (8,10,13-18:3) was found by the GC–MS analysis of the DMOX derivatives of F1 (Fig. 2), although many peaks appeared on the chromatogram of F1. Further studies are required to identify the structure of conjugated dienoic acids in F1.
a–e Analytical HPLC of methyl esters from crude soybean oil (a), soybean oil after degumming (b), alkali refining (c), bleaching (d), and deodorization (e). Procedures for analytical HPLC are described in the Materials and methods
We identified c,t,t- or t,t,c-CLN isomers (8,10,12 and 9,11,13) in the F2 fraction and t,t,t-CLN isomers (8,10,12 and 9,11,13) in the F4 fraction of purified soybean oil methyl esters (Figs. 1, 2). The quantification of these CLN was carried out by quantitative HPLC using a calibration curve for CLN (9c,11t,13t-18:3; 9t,11t,13c-18:3; 9t,11t,13t-18:3; 8t,10t,12c-18:3). The calibration curve for 9t,11t,13c-18:3, 9c,11t,13t-18:3, and 8t,10t,12c-18:3 was almost the same, but slightly different from that for 9t,11t,13t-18:3, indicating that the molecular extinction coefficient of CLN would be dependent on the number of the trans configuration. Thus, the contents of both types of geometrical isomers (c,t,t or t,t,c and t,t,t) found in soybean oil were determined by HPLC using calibration curves for the CLN standard with the c,t,t (or t,t,c) and t,t,t configurations.
Table 2 shows the CLN contents of crude soybean oil and processed soybean oil. While CLN could barely be detected in crude soybean oil or in the oil after de-gumming and alkali refining, a significant amount of CLN (8,10,12 or 9,11,13) was found in soybean oil after bleaching. A slight decrease in CLN after deodorization may be due to the isomerization of the CLN to CLN with conjugated dienes (8,10,13–18:3). CLN contents in purified soybean oil from different companies in Japan are shown in Table 3; these can be seen to vary from 387 to 1316 mg/kg oil, which corresponds to 0.039–0.13% (w/w). These values are agreement with the results of Yurawecz et al. [6]. CLN content was affected by the bleaching conditions (Table 4). Combinations of higher percentages of bleaching earth and lower bleaching temperatures resulted in a reduced CLN content. Similar effects of bleaching temperature and earth combinations have been reported by Van Den Bosch [19, 20]. Table 5 shows the content of CLN in commercial vegetable oils in Japan. High levels of CLN were found in soybean and corn oils, while moderate levels were observed in safflower, sesame, and rapeseed oils. On the other hand, no CLN was detected in olive oil. The CLN level was strongly associated with linoleic acid content in these oils (Table 6), suggesting that18:2n-6 is the possible source of CLN [7, 8]. There were no difference in the content of linoleic acid between soybean oil and corn oil, with the former containing 9.5% 18:3n-3, while corn oil had less than 1%. 18:3n-3 is more easily oxidized than 18:2n-6, and its oxidation products can promote the oxidation of 18:2n-6 to produce linoleate hydroperoxides. Hence, CLN may possibly originate from linoleate hydroperoxides. The higher level of CLN in soybean oil than in corn oil may be due to the difference in the content of 18:3n-3 among them.
References
Pariza MW (1999) The biological activities of conjugated linoleic acid. In: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ (eds) Advances in conjugated linoleic acid research, vol 1. AOCS Press, Champaign, pp 12–20
Pariza MW, Park Y, Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40:283–298
Pariza MW, Park Y, Xu X, Ntambi J, Kang K (2003) Speculation on the mechanisms of action of conjugated linoleic acid. In: Sébédio J-L, Christie WW, Adlof R (eds) Advances in conjugated linoleic acid research, vol 2. AOCS Press, Champaign, pp 251–258
Fritsche J, Steinhart H (1998) Analysis, occurrence, and physiological properties of trans fatty acids (TFA) with particular emphasis on conjugated linoleic acid isomers (CLA): a review. Fett Lipid 100:190–210
Smith Jr CR (1971) Occurrence of unusual fatty acids in plants. Prog Chem Fats Other Lipids 11:137–177
Yurawecz MP, Molina AA, Mossoba M, Ku Y (1993) Estimation of conjugated octadecatrienes in edible fats and oils. J Am Oil Chem Soc 70:1093–1099
Parr LJ, Swoboda PAT (1976) The assay of conjugable oxidation products applied to lipid deterioration in stored foods. J Food Technol 11:1–12
Fishwick MJ, Swoboda PAT (1976) Measurement of oxidation of polyunsaturated fatty acids by spectrophotometric assay of conjugated derivatives. J Sci Food Agric 28:387–393
Suzuki R, Abe M, Miyashita K (2004) Comparative study of the autoxidation of TAG containing conjugated and nonconjugated C18 PUFA. J Am Oil Chem Soc 81:563–569
Yamasaki M, Kishihara K, Ikeda I, Sugano Yamada MK (1999) A recommended esterification method for gas chromatographic measurement of conjugated linoleic acid. J Am Oil Chem Soc 76:933–938
Takagi T, Itabashi Y (1981) Occurrence of mixtures of geometrical isomers of conjugated octadecatrienoic acids in some seed oils: analysis by open-tublar gas liquid chromatography and high performance liquid chromatography. Lipids 16:546–551
Suzuki R, Noguchi R, Ota T, Abe M, Miyashita K, Kawada T (2001) Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells. Lipids 36:477–482
Park SJ, Park CW, Kim SJ, Kim JK, Kim YR, Park KA, Kim JO, Ha YL (2002) Methylation methods for the quantitative analysis of conjugated linoleic acid (CLA) isomers in various lipid samples. J Agric Food Chem 50:989–996
Yurawecz MP, Hood JK, Mossoba MM, Roach JAG, Ku Y (1995) Furan fatty acids determined as oxidation products of conjugated octadecadienoic acid. Lipids 30:595–598
Noguchi R, Yasui Y, Suzuki R, Hosokawa M, Fukunaga K, Miyashita K (2001) Dietary effects of bitter gourd oil on blood and liver lipids of rats. Arch Biochem Biophys 396:207–212
Sehat N, Yurawecz MP, Roach JAG, Mossoba MM, Kramer JKG, Ku Y (1998) Silver-ion high-performance liquid chromatographic separation and identification of conjugated linoleic acid isomers. Lipids 33:217–221
Destaillats F, Sebedio J-L, Berdeaux O, Junaneda P, Angers P (2005) Gas chromatography – mass spectrometry determination of metabolites of conjugated cis-9, trans-11, cis-15 18:3 fatty acid. J Chromatogr B 820:15–22
Parodi PW (2003) Conjugated linoleic acid in food. In: Sébédio J-L, Christie WW, Adlof R (eds) Advances in conjugated linoleic acid research, vol 2. AOCS Press, Champaign, pp 101–122
Van Den Bosch G (1973) Bleaching of vegetable oils: I. Conversions in soybean oil, triolein and trilinolein. J Am Oil Chem Soc 50:421–423
Van Den Bosch G (1973) Bleaching of vegetable oils: II. Conversions of methyl oleate and linoleate. J Am Oil Chem Soc 50:487–493
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kinami, T., Horii, N., Narayan, B. et al. Occurrence of Conjugated Linolenic Acids in Purified Soybean Oil. J Amer Oil Chem Soc 84, 23–29 (2007). https://doi.org/10.1007/s11746-006-1005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-006-1005-5