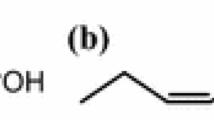Abstract
The present study demonstrates the separation of a critical pair of conjugated linolenic acid (CLN) isomers—jacaric acid (JA; c8, t10, c12-18:3) and punicic acid (PA; c9, t11, c13-18:3)—on a 60-m conventional Supelcowax 10 column. The alkyl esters of different alcohols (C1–C8) of JA and PA were prepared and analyzed isothermally at 220, 230 and 240 °C. The adequacy of their separation was determined from the separation factors (α) and peak resolutions (R s). Acceptable resolution (R s = 1.01) of JA and PA was obtained with their 2-ethyl-1-hexyl ester derivatives at a column temperature of 230 °C. In addition, the Gibbs energy of transfer from solution to gas of the three double bonds \((\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\)) could be used to describe the interactions of the double bond with the stationary phase. Characterization of 2-ethyl-1-hexyl esters of Jacaranda mimosifolia seed oil at 230 °C demonstrates that the oil contains JA and α- and β-calendic acid as a CLN without the presence of PA. The results suggested that JA could be resolved from PA on a 60-m Supelcowax 10 column as the ethyl hexyl ester.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jacaric acid (JA; c8, t10, c12-18:3) and its positional isomer, punicic acid (PA; c9, t11, c13-18:3) are conjugated linolenic acids (CLNs) found in jacaranda and pomegranate seed oil, respectively. Among CLN isomers, JA and PA are thought to be the most potent cytotoxicity agents. They induce apoptosis in various types of human prostate cancer cells. Their effectiveness is related to the similarities in their three-dimensional molecular structure [1]. Shinohara et al. [2] reported that among CLN isomers, JA had the strongest cytotoxic effect on human adenocarcinoma cells. In addition, JA might have anti-obesity and anti-diabetes properties by inhibiting the activity of stearoyl coenzyme A desaturase (SCD) and decreasing the expression of SCD-1 mRNA [3].
Silver-ion high-performance liquid chromatography (Ag+-HPLC) and gas chromatography (GC) are the most common methods for CLN analysis. However, nuclear magnetic resonance (NMR) is necessary for structural studies [4–7]. The methyl ester of JA could not be resolved from PA in the GC analysis. Thus, 13C NMR was used to confirm the presence or absence of JA in pomegranate seed oil [8].
The most common derivatives of fatty acids in GC analysis are methyl esters. However, some critical pairs of mono- and di-enoic fatty acid methyl esters could not be resolved on a GC column, but the longer chain alkyl esters could be separated. The pair of petroselinic (c6-18:1) and oleic (c9-18:1) acids is an example. The two isomers could not be separated as the methyl ester derivatives but they were well resolved as the phenylethyl [9], isopropyl [10] and longer alkyl esters [11, 12]. Similarly, separations of the four conjugated linoleic acid (CLA) isomers on a 50-m cyanopropyl polysiloxane column were improved when they were esterified with aliphatic alcohols of different chain length and adduct formation with 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) [13].
In order to find new natural sources of CLN isomers, which may be beneficial to human health, a reliable, cost-effective and single analytical method for separation and identification of CLN isomers is preferred. This work reports the method for improving the separation of JA from PA with GC using alkyl ester derivatives. The separations of the CLN alkyl esters can potentially be related to their interactions with the stationary phase, which are analyzed as the change in Gibbs energies. The proposed method would be very useful for rapidly screening the presence or absence of JA and PA in the seed oil.
Experimental Procedures
Materials
JA and PA standards were purchased from Larodan (Malmo, Sweden). Standard fatty acid methyl esters (FAMEs) and aliphatic alcohol (C1–C8) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals are of analytical grade. Jacaranda mimosifolia seed was purchased from a local garden seed store (Bangkok, Thailand).
Preparation of Fatty Acid Alkyl Esters
The standard JA and PA alkyl esters were prepared according to Park et al. [14] with a slight modification. The mixture of JA and PA standards in toluene (2–4 mg/mL each) was esterified with 1.5 mL of 0.5 M H2SO4 in different alcohols (methanol, ethanol, 1-butanol, 1-hexanol, 1-octanol and 2-ethyl-1-hexanol). The reactions were carried out at 55 °C for 1 h, stopped by adding 4% Na2CO3 and washed with distilled water (1.5 mL × 3). The toluene phase was separated and dried over anhydrous Na2SO4.
Transesterification of J. mimosifolia seed oil was carried out according to Chen et al. [4] with a slight modification. In brief, 30 mg of the dried seed were ground, extracted with 1 mL of toluene and transesterified with 2 mL of 0.5 M KOH in 2-ethyl-1-hexanol at 40 °C for 10 min. Residual free fatty acid was esterified with 2 mL of 1.5 M H2SO4 in 2-ethyl-1-hexanol for 30 min. The completeness of the reaction was checked by high-performance size exclusion chromatography (HPSEC) [15]. The obtained ester was dried under a stream of N2 and redissolved in 1 mL of n-hexane before analysis.
Gas Chromatography (GC)
GC analysis was carried out on a Shimadzu gas chromatograph model 2010 equipped with a flame-ionization detector (Shimadzu Inc., Tokyo, Japan). Alkyl esters of JA and PA standards were analyzed on a Supelcowax 10 column (polyethylene glycol, 60 m × 0.2-mm i.d., 0.2-μm film thickness) from Sigma-Aldrich Co. LLC (St. Loius, MO, USA). The standard mixture of JA and PA alkyl esters was analyzed isothermally at 220, 230 and 240 °C. Samples of 1 μL were injected with a split ratio of 70:1. The injector and detector temperatures were set at 250 °C. Helium was used as carrier gas. The GC was operated in constant pressure mode, with the pressure set at 472.1 kPa. The nitrogen makeup gas flow rate was 30 mL/min. The separations of the two chromatographic peaks were expressed in terms of separation factor (α) and peak resolution (R S) as follows:
where t R and t M are retention times of the solute and un-retained solvent, respectively. The αCLN/18: 0 and αPA/JA are the separation factor of CLN (JA or PA) and stearic acid and of PA and JA, respectively. W is a peak width at a baseline level.
Gibbs Energy of Transfer from Solution to Gas of a Triene Double Bond (\(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\))
The \(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\) of JA and PA alkyl esters were calculated according to Sansa-ard et al. [12] as shown in Eqs. (4) and (5), respectively.
where \(\Delta_{\text{sln}}^{\text{g}} G_{{ 1 8: 3~({\text{JA)}}}}^{0}\), \(\Delta_{\text{sln}}^{\text{g}} G_{{ 1 8:3~({\text{PA)}}}}^{0}\) and \(\Delta_{\text{sln}}^{\text{g}} G_{18:0}^{0}\) are the standard Gibbs energies of transfer from solution to gas of JA, PA and stearic acid alkyl esters, respectively. R is the gas constant (1.98588 cal K−1 mol−1). T is the absolute temperature (Kelvin). K is the equilibrium constant, which is related to the retention factor (k):
where
Beta (β) is the column phase ratio (volume of gas phase/volume of stationary phase).
Identification of Jacaranda mimosifiola Seed Oil Fatty Acid 2-Ethyl-1-Hexyl Ester
The equivalent chain length (ECL) was used to identify fatty acids in J. mimosifiola seed oil. The method for obtaining the ECL values was adopted from Krisnangkura et al. [16]. In brief, the mixed standard fatty acid 2-ethyl-1-hexyl esters were prepared by transesterification of mixed standard saturated fatty acid methyl esters (C16, C17, C18, C19, C20 and C22) with 0.5 M KOH in 2-ethyl-1-hexanol at 40 °C for 10 min. The 2-ethyl-1-hexyl esters were then analyzed isothermally at 5-°C intervals between 200 and 240 °C on the Supelcowax 10 column. Hexane was used as the t M marker. The t R data were collected and the numeric column constants (a, b, c, and d) of fatty acid 2-ethyl-1-hexyl esters on the Supelcowax 10 column were determined with Microsoft Excel 2010.
Statistical Analysis
All experiments were carried out in triplicate. Statistical analysis was performed using Microsoft Excel 2010 software.
Results and Discussion
Separation of Jacaric and Punicic Acid Alkyl Esters
The t R of alkyl esters (C1–C8) of JA and PA separated on a Supelcowax 10 column at 220, 230 and 240 °C are summarized in Table 1. The separation factor or selectivity (α) is used to express the relative interaction between the two solutes and the stationary phase. The α values for each ester pairs are calculated and summarized in the same table. The α value of CLN and stearic acid alkyl esters (αCLN/18:0) were in the range of 2.367–2.840, implying that the CLN and stearic acid were easily separated on this column. The αCLN/18:0 decreased as the column temperature was increased, suggesting that their separations depended on the intermolecular forces between the solutes (CLN and stearic acid) and the stationary phase [17]. On the other hand, the α value of JA and PA alkyl esters (αPA/JA) were in the range of 1.007–1.013 and they were relatively insensitive to the change in temperature (220–230 °C). Thus, separation would be difficult due to the similarity in boiling points and intermolecular interactions of the two isomers with the stationary phase. The polyethylene glycol stationary phase contains a molecule having a permanent dipole that can induce a counter dipole in compounds containing π-electrons [18]. Thus, electron donor–acceptor forces predominate in the intermolecular force between CLN and the stationary phase of the column [19]. The α value of greater than 1.02 is preferred for successful separation of any two compounds in the capillary GC column [17]. At the same column temperature, the αCLN/18:0 tended to decrease, while the αPA/JA was slightly increased with the size of the alkyl esters. Thus, it was speculated that better separation of JA and PA would be obtained by increasing the chain length of esters. The resolution (R s) of different alkyl esters in Table 1 clearly confirmed the speculation.
Figure 1 is the aligned chromatograms of alkyl esters (with different alcohols) of a mixture of JA and PA separated on a Supelcowax 10 capillary column at 230 °C. The methyl and ethyl esters of JA and PA were partially separated with R s of 0.61 and 0.66, respectively. Better separations were observed for the JA and PA butyl and hexyl esters with the R s of 0.81 and 0.83, respectively. For the C8 alcohol derivatives, the branched chain 2-ethyl-1-hexyl esters have a shorter t R and narrower peak width than those of the linear octyl esters. Acceptable resolution (R s = 1.01) of the JA and PA 2-ethyl-1-hexyl esters was obtained. The results agreed with the reports of Isbell et al. [11] and Sansa-ard et al. [12] on the separations of petroselinate and oleate esters. Generally, baseline separation (R s ≥1.5 or 6σ) is ideal for accurate quantitative analysis. However, for qualitative or identification purpose, a resolution of 1.0 or 4σ is acceptable [20].
Gibbs Energy Contribution to Gas Chromatographic Separation of JA and PA Alkyl Esters
To clarify the effects of alkyl esters on the separation of CLN isomers, Gibbs energy of transfer from solution to gas of the double bond \((\Delta_{\text{sln}}^{\text{g}} G_{\text{u}} )\) proposed by Sansa-ard et al. [12] was evaluated. The Microsoft Excel worksheet for the calculation of the standard Gibbs energies of transfer from solution to gas of saturated fatty acid (\(\Delta_{\text{sln}}^{\text{g}} G_{18:0}^{0}\)) and the unsaturated fatty acid (\(\Delta_{\text{sln}}^{\text{g}} G_{{ 18:3~( {\text{JA)}}}}^{0}\) and \(\Delta_{\text{sln}}^{\text{g}} G_{{ 1 8: 3 ~( {\text{PA)}}}}^{0} )\) and the differences in \(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\) at 230 °C is demonstrated and available in the supplemental data file. The results are summarized in Table 2. As mentioned above, separations of JA and PA alkyl esters gradually increased as the carbon numbers of the alcohols increased. The Gibbs energies of transfer from solution to gas of the whole molecule (\(\Delta_{\text{sln}}^{\text{g}} G^{0}\)) of stearic acid, JA and PA alkyl esters increased (ignoring the sign) as the carbon numbers of the alcohols increased. Figure 2 shows the molecular structure of esters of stearic (Fig. 2a), JA (Fig. 2b) and PA (Fig. 2c) which are C18:0, 18:3 (c8, t10, c12) and 18:3 (c9, t11, c113), respectively. The difference in C–C bonding in the alkyl chain determines their three-dimensional behavior and lets them interact differently with the stationary liquid phase. The \(\Delta_{\text{sln}}^{\text{g}} G^{0}\) of stearic acid alkyl esters was lower than those of the JA and PA alkyl esters. The difference in \(\Delta_{\text{sln}}^{\text{g}} G^{0}\) values between saturated and unsaturated fatty acid alkyl esters resulted from the double bonds and is assigned as \(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\). The \(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\) of JA and PA decreased as the alkyl sizes increased, suggesting that the interactions between the triene double bonds and stationary phase were reduced by the interference of the bulky alkyl groups of the alcohol. The \(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\) of JA alkyl esters were lower than those of PA alkyl esters due to the double bonds of JA (c8, t10, c12) being closer to the bulky alkyl group than those of PA (c9, t11, c13). The highest difference in \(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\) of the two fatty acids was found on 2-ethyl-1 hexyl ester (−11.8 cal/mol). Accordingly, \(\Delta_{\text{sln}}^{\text{g}} G_{\text{u}}\) might be a valuable tool for looking into the molecular or fragment of molecular interactions to its environment.
Tentative Identification of Fatty Acids in Jacaranda mimosifiola Seed Oil
The GC chromatograms of standard JA and PA and J. mimosifiola seed oil 2-ethyl-1-hexyl ester separated on a polyethylene glycol (Supelcowax 10) capillary column at 230 °C are shown in Fig. 3a, b, respectively. The peak of PA was not observed in Fig. 3b, suggesting that no PA is present in J. mimosifiola seed oil. To confirm the absence of PA and the separation of JA and PA in the extracted oil, standard PA was spiked into J. mimosifiola seed oil prior to derivatization. The GC chromatogram in Fig. 4 shows the separation of JA and PA with acceptable resolution (R s = 1.0). The fatty acid compositions in J. mimosifiola seed oil were tentatively identified by their ECL value according to Krisnangkura et al. [16]. The numeric column constants (a, b, c, and d) of fatty acid 2-ethyl-1-hexyl esters on the Supelcowax 10 column were −10.337, −0.360, 3653.626 and 317.480, respectively. The equation for calculation of ECL is shown in Eq. (8).
GC chromatogram of a JA and PA 2-ethyl-1-hexyl ester and b J. mimosifiola seed oil 2-ethyl-1-hexyl ester on a 60-m Supelcowax 10 column at 230 °C (For identification, see Table 3)
Table 3 summarizes the t R, retention factor, the calculated ECL and tentative identification of fatty acids in J. mimosifiola seed oil. The results show that fatty acids in this seed oil are palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), linolenic acid (C18:3), eicosanoic acid (C20:0) and eicosenoic acid (C20:1). The major CLN isomer is JA, with a trace amount of α-calendic acid (α-CA) and β-calendic acid (β-CA). Results from the present study suggested that the 2-ethyl-1-hexyl ester could be used to verify the presence or absence of positional CLN isomers, JA and PA in the oil sample. The ECL values of JA, PA, α-CA and β-CA on the Supelcowax 10 column at 230 °C were 21.49, 21.53, 21.89 and 22.30, respectively. These values are in agreement with the ECL values of JA, PA and α-CA on a Carbowax 20M column at 180 °C which were 21.50, 21.56 and 21.88, respectively [6]. However, the JA and PA in that study were not injected in the same run.
Conclusion
The separation and identification of JA and PA as 2-ethyl-1-hexyl ester derivatives can be obtained on a 60-m Supelcowax 10 column. The improved resolution is relative to the interference of a 2-ethyl-1-hexyl moiety to the conjugated triene double bonds. The method is effective, universal and it can be used to identify the presence or absence of JA and PA in J. mimosifiola seed oil. This method may be useful for the separation of other critical un-resolved peaks.
References
Gasmi J, Thomas Sanderson J (2013) Jacaric acid and its octadecatrienoic acid geoisomers induce apoptosis selectively in cancerous human prostate cells: a mechanistic and 3-D structure–activity study. Phytomedicine 20:734–742
Shinohara N, Tsuduki T, Ito J, Honma T, Kijima R, Sugawara S, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, Nishiyama K, Nakagawa K, Miyazawa T, Ikeda I (2012) Jacaric acid, a linolenic acid isomer with a conjugated triene system, has a strong antitumor effect in vitro and in vivo. Biochim Biophys Acta 1821:980–988
Shinohara N, Ito J, Tsuduki T, Honma T, Kijima R, Sugawara S, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, Nishiyama K, Nakagawa K, Miyazawa T, Ikeda I (2012) Jacaric Acid, a linolenic acid isomer with a conjugated triene system, reduces stearoyl-CoA desaturase expression in liver of mice. J Oleo Sci 61:433–441
Chen J, Cao Y, Gao H, Yang L, Chen Z-Y (2007) Isomerization of conjugated linolenic acids during methylation. Chem Phys Lipids 150:136–142
Cao Y, Gao H-L, Chen J-N, Chen Z-Y, Yang L (2006) Identification and characterization of conjugated linolenic acid isomers by Ag+-HPLC and NMR. J Agric Food Chem 54:9004–9009
Gaydou EM, Miralles J, Rasoazanakolona V (1987) Analysis of conjugated octadecatrienoic acids inmomordica balsamina seed oil by GLC and13C NMR spectroscopy. J Am Oil Chem Soc 64:997–1000
Cao Y, Yang L, Gao H-L, Chen J-N, Chen Z-Y, Ren Q-S (2007) Re-characterization of three conjugated linolenic acid isomers by GC–MS and NMR. Chem Phys Lipids 145:128–133
Sassano G, Sanderson P, Franx J, Groot P, van Straalen J, Bassaganya-Riera J (2009) Analysis of pomegranate seed oil for the presence of jacaric acid. J Sci Food Agric 89:1046–1052
Liu L, Hammond E (1995) Phenylethyl esters of fatty acids for the analytical resolution of petroselinate and oleate. J Am Oil Chem Soc 72:749–751
Wolff R, Vandamme F (1992) Separation of petroselinic (cis-6 18:1) and oleic (cis-9 18:1) acids by gas-liquid chromatography of their isopropyl esters. J Am Oil Chem Soc 69:1228–1231
Isbell TA, Green LA, DeKeyser SS, Manthey LK, Kenar JA, Cermak SC (2006) Improvement in the gas chromatographic resolution of petroselinate from oleate. J Am Oil Chem Soc 83:429–434
Sansa-ard C, Aryusuk K, Lilitchan S, Krisnangkura K (2011) Free energy contribution to gas chromatographic separation of petroselinate and oleate esters. Chromatogr Res Int 2011:9
Blasi F, Giua L, Lombardi G, Codini M, Simonetti MS, Damiani P, Cossignani L (2011) Improved HRGC separation of cis, trans CLA isomers as diels-alder adducts of alkyl esters. J Chromatogr Sci 49:379–383
Park SJ, Park CW, Kim SJ, Kim JK, Kim YR, Park KA, Kim JO, Ha YL (2002) Methylation methods for the quantitative analysis of conjugated linoleic acid (CLA) isomers in various lipid samples. J Agric Food Chem 50:989–996
Kittirattanapiboon K, Krisnangkura K (2008) Separation of acylglycerols, FAME and FFA in biodiesel by size exclusion chromatography. Eur J Lipid Sci Technol 110:422–427
Krisnangkura K, Tancharoon A, Konkao C, Jeyashoke N (1997) An alternative method for the calculation of equivalent chain length or carbon number of fatty acid methyl esters in gas chromatography. J Chromatogr Sci 35:329–332
McNair HM, Miller JM (1998) Basic gas chromatography. Wiley, New York
Cazes J, Scott RPW (2002) Chromatography theory. Marcel Dekker Inc., New York
James AT (1963) Methods of separation of long-chain unsaturated fatty acids. A review. Analyst 88:572–582
Scott RPW (1995) Techniques and practice of chromatography. Marcel Dekker Inc, New York
Acknowledgements
This work was supported by a grant from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0048/2556); and the Higher Education Research Promotion and National Research University (NRU) Project of Thailand, Office of the Higher Education Commission.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Pojjanapornpun, S., Aryusuk, K., Jeyashoke, N. et al. Gas Chromatographic Separation and Identification of Jacaric and Punicic 2-Ethyl-1-Hexyl Esters. J Am Oil Chem Soc 94, 511–517 (2017). https://doi.org/10.1007/s11746-017-2965-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-2965-3