Abstract
Despite several studies aimed at evaluating the positional and fatty acid specificity of fish triacylglycerol (TAG) digestive lipases, there is still much uncertainty regarding these issues. The aim of the present study was therefore to address these questions in Atlantic salmon (Salmo salar L.). Crude luminal midgut extracts were collected from fed salmon and the hydrolysis studied for various substrates including triolein (Tri-18:1), trilinolein (Tri-18:2), trilinolenin (Tri-18:3), trieicosapentaenoin (Tri-20:5), tridocosahexaenoin (Tri-22:6) and natural fish oil TAG. Using Tri-18:1, in a time-curve model showed an initial high degree of sn-1 or sn-3 specificity where sn-1,2(2,3)-diacylglycerol (1,2(2,3)-DAG) and free fatty acid (FFA) were the main hydrolytic products up to 15 min. Lack of initial sn-2 specificity was confirmed by negligible sn-1,3-diacylglycerol (1,3-DAG) being produced. During the further hydrolysis of DAG, all positions appeared susceptible to attack causing a concomitantly small increase in sn-1(3)-monoacylglycerol (1(3)-MAG) and 2-MAG, but not at the level expected for an exclusively sn-1,3-specific lipase. Oleic acid (18:1n-9) and eicosapentaenoic acid (20:5n-3) were preferred substrates for hydrolysis using both fish oil and acyl-homogeneous TAGs with FFA as the main product of lipolysis. Hydrolysis of the natural fish oil TAG appeared slower yet produced proportionally more MAG and DAG after 5 min, and similar specificities, as for synthetic TAG substrates, were exhibited with 18:1n-9 and 20:5n-3 accumulating in the FFA fraction after 30 min. Notably, 16:0 was particularly conserved in MAG. As TAG resynthesis of absorbed lipid in salmon enterocytes proceeds preferably with 2-MAG as templates, the absorption of 2-MAG, produced during initial stages of TAG hydrolysis, would need to occur rapidly to be effectively utilised via the MAG pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the pancreatic juice of mammals there are two major lipolytic enzymes responsible for neutral lipid digestion: pancreatic lipase (PL; E.C. 3.1.1.3.) and bile salt-dependent lipase (BSDL; E.C. 3.1.1.1.). In the intestinal lumen, triacylglycerol (TAG) is mainly hydrolysed by PL. This enzyme has several distinctive characteristics. It possesses good activity towards lipid emulsions, yet is inhibited by the presence of bile salts and other amphipathic lipids. Inhibition is only overcome by the addition of colipase, which acts as an anchor for PL at the lipid droplet interface [1]. Furthermore, PL has a characteristic sn-1,3-specificity towards TAG producing free fatty acids (FFA) and sn-2-monoacylglycerols (2-MAG) for subsequent absorption.
In comparison, BSDL is dependent on bile salts for its hydrolytic activity towards TAG, cholesteryl esters, wax esters, vitamin esters, and phospholipids [2, 3]. BSDL is also less discriminatory, than PL, towards sn-fatty acyl position yielding mainly glycerol and FFA as hydrolytic products of TAG. Lower specificity is also apparent in that it has good activity towards all fatty acids including long chain polyunsaturated fatty acids (PUFA) that are not effectively hydrolysed by PL. Hence, intestinal BSDL is non-specific towards fatty acyl moiety and sn-position of TAG, displays a preference for micellar substrates, yet possesses 10–60 times less activity than PL in mammals. Thus, PL and BSDL appear to act sequentially where partial acylglycerols are released from lipid droplets by the PL-colipase complex for incorporation into mixed micelles and possible complete hydrolysis by BSDL [4, 5].
The advantage of producing 2-MAG during digestion, as is the case for PL, is that when absorbed into the enterocytes, resynthesis of TAG can proceed rapidly via the MAG pathway using 2-MAG as a template. This process is catalyzed by monoacylglycerol acyltransferase (MGAT) with relatively low expenditure of energy [6]. If TAG is completely hydrolysed to FFA and glycerol, TAG resynthesis in enterocytes must proceed through the slower and energy-consuming de novo glycerol-3-phosphate (G3P) pathway [7].
Dietary lipid for fish species in the marine environment is characteristically high in wax esters and TAG containing n-3 highly unsaturated fatty acids (HUFA) such as eicosapentaenoic (20:5n-3) and docosahexaenoic (22:6n-3) acids [8]. As PL does not possess wax ester hydrolytic activity and has low activity towards ester bonds containing pentaenoic and hexaenoic fatty acids, the predominant lipid-digesting enzyme in fish may be BSDL [2, 9]. This is furthermore evident in the failure to prove the existence of a PL-colipase system in fish [9, 10] and the cessation of all lipolytic activity upon removal of bile, that is subsequently restored by addition of bile salts in rainbow trout (Oncorhynchus mykiss), red sea bream (Pagrus major) and Atlantic salmon, Salmo salar [10–12].
Recent reports have however, shown the existence of MGAT in both Atlantic salmon [13, 14] and seabream, Sparus aurata [15], which is most likely required for a fast and efficient transfer of absorbed intestinal lipid to the blood as lipoproteins. This again indicates that the hydrolysis of luminal TAG may proceed through sn-1,3-specificity. This possibility is strengthened by the observation of some sn-1,3-specificity of digestive lipases in cod, Gadus morhua [16, 17] and rainbow trout [10, 18]. Furthermore, Gjellesvik [16] showed evidence of BSDL with sn-1,3-specificity using partially-purified caecal tissue from cod. This opens for the possibility that BSDL in fish may actually possess sn-1,3-specific lipase capability.
As previous studies in fish have shown both sn-1,3-specificity and complete hydrolysis of different TAG substrates, the present study was conducted to elucidate possible discrepancies in luminal lipase specificity with regard to sn-acyl position and fatty acid species. Complete hydrolysis to FFA and glycerol has especially been noted in fish if the fatty acid in sn-2 of TAG is PUFA [18–21]. Thus, we hypothesised that the luminal formation of 2-MAG is dependent on the species of fatty acid present at sn-2 position of TAG. The present study utilised a crude intestinal lipase preparation from the midgut lumen of Atlantic salmon as a model for studying TAG hydrolase activity [10, 12]. As BSDL activity is dependent on bile salt concentration [10], intestinal extracts were desalted and known concentrations added in the assay to avoid concentration dependent errors. Utilising synthetic acyl-homogeneous TAG substrates in the assay allowed for the relative determination of lipase sn-positional specificity, with respect to fatty acid species, by quantifying the isomeric products released. Furthermore, fish oil was also used to represent a natural TAG substrate, with a more typical distribution of fatty acids that salmon intestinal lipases would encounter.
Materials and Methods
Chemicals
Triolein (Tri-18:1), trilinolein (Tri-18:2), trilinolenin (Tri-18:3), trieicosapentaenoin (Tri-20:5), tridocosahexaenoin (Tri-22:6) and TLC standard (>99%) were all purchased from Nu-Chek Prep Inc. (Minnesota, USA). Chloroform, methanol, hexane, sulphuric acid, ethanol, acetone, sodium taurocholate, boric acid, 2,7-dichlorofluorescein were purchased from Sigma–Aldrich (St Louis, USA).
Substrate
The substrates of Tri-18:1, Tri-18:2, Tri-18:3, Tri-20:5 or Tri-22:6 were made to a concentration of 100 mg/mL in chloroform. Triacylglycerols from fish oil (Möllers Tran; MöllerCollett AS, Lysaker, Norway) were purified on TLC plates (20 × 20 cm × 0.5 mm, Merck 1.13894, Darmstadt, Germany) using hexane: diethyl ether: acetic acid (80:20:2) as developing solvent. Lipid classes were visualized using 0.1% (w/v) 2,7-dichlorofluorescein in 95% methanol. The TAG band was extracted from the silica with hexane: diethyl ether (1:1), evaporated under a stream of nitrogen and stored at −80 °C in chloroform. To prepare lipase substrates, 10 mg of the respective substrate was evaporated under a stream of nitrogen and the residue dissolved in 200 μL taurocholate (400 mM, 4 °C) and 1,800 μL of 100 mM potassium phosphate buffer (pH 8) containing 1 mM EDTA (ethylene diamine tetraacetic acid), 1 mM DTT (1,4-dithiothreitol) and 1 mM benzamidine (4 °C). The substrate mixture was stirred for 5 min, sonicated in ice-cold water for 2 min and vortexed for 1 min before use.
Enzyme
Atlantic salmon of 1,000 g, fed commercial diets (Biomar, Trondheim) 1 h twice a day, were anaesthetised in 4 g/kg benzocaine (Braine-Lálleud, Belgium) 1 hour after the last meal and killed by a sharp blow to the head. Four fish were weighed, and the intestinal tract dissected. Luminal contents were collected from the midgut, defined as the section from the last pyloric caeca to the start of the distal intestine, as recognised by its larger diameter [22]. The enzyme preparation was made once from pooling the intestinal contents from the four salmon. The pooled content were further diluted twofold (w/v) in 100 mM potassium phosphate buffer (pH 8) containing 1 mM EDTA, 1 mM DTT and 1mM benzamidine. The suspension was centrifuged at 3,220×g for 10 min at 4 °C. The lipid layer was aspirated from the surface and the infranatant collected and designated “crude extract”. To remove endogenous bile salts, the extract was transferred to Zeba™ Desalt Spin Columns (Pierce, Rockford, USA) and centrifuged at 200×g for 2 min at 4 °C. The protein contents of the desalted extract was analysed by the method of Lowry et al. [23] using an Ultrospec 4000 spectrophotometer (Pharmacia Biotech, Cambridge, England) and adjusted to 1.0 mg/mL. The enzyme preparation was then prepared in cryo tubes each containing materials for 1 day work and stored at −80 °C prior to use, as preliminary tests had shown no loss of activity when stored under these conditions. By this procedure, all experiments were carried out on the same enzyme preparation.
Incubation
The incubation assay was performed according to a modification of the method of Bogevik et al. [12]. In brief, 0.2 mL of the enzyme extract was added to 0.2 mL substrate suspension and incubated for 5–30 min at the ambient temperature of the fish (10 °C). The reaction was terminated by the addition of 8 ml chloroform. The tubes were vortexed for 15 s and centrifuged for 2 min at 300×g after which the upper aqueous layer was aspirated off and the lower chloroform layer evaporated under a stream of nitrogen. The residue was resuspended in 30–50 μL chloroform and applied to TLC plates (20 × 20 cm × 0.25 mm, Merck 1.05721, Darmstadt, Germany) pre-impregnated with 2.3% boric acid in ethanol:water (1:1) and developed in 50 ml chloroform:acetone (96:4), according to Thomas [24]. Lipid spots were detected by spraying the plate with 0.1% 2,7-dichlorofluorescein in 95% methanol and visualized under UV light.
Lipid Extraction
Two-hundred microlitres either of the pooled desalted midgut extract or the substrate suspension were added to 4 mL chloroform and extracted as above. The desalted midgut extract was separated into neutral lipid classes as described above.
Quantification of Lipid Classes
The lipid class bands representing 1(3)-MAG, 2-MAG, FFA, 1,2(2,3)-DAG, 1,3-DAG, and TAG were scraped into tubes, added internal standard (19:0) and subjected to acid-catalyzed transesterification using 1% (v/v) H2SO4 in methanol [25]. The resulting fatty acid methyl esters (FAME) were extracted and quantified by gas liquid chromatography using a HP 5890 gas chromatograph equipped with a J&N Scientific Inc. DB-23 fused silica column (30 m × 0.25 mm id). Hydrogen was used as carrier gas and temperature programming was from 50 to 150 °C at a rate of 40 °C/min, from 150 to 195 °C at 1.5 °C/min, and then to a final temperature of 205 °C at 0.5 °C/min. Individual components were identified by comparison with known standards.
Calculation and Statistics
The amount of fatty acids in neutral lipid classes were converted to the amount of lipid by a correction factor which takes into account the amount of glycerol [25]. The amount of lipid were converted into moles for the respective lipid classes. From this, individual lipid classes were calculated as a percentage of total lipid classes recovered from each substrate. Data is given as ±SD for replicate incubations. All statistical analysis was performed using STATISTICA 8.0 software for Windows (StatSoft. Inc., Tulsa, USA). The statistical significance of differences was tested using one-way ANOVA followed by the Tukey post hoc test for comparison of the different acyl-homogenous substrate in each lipid class after hydrolysis. Significance was accepted at P < 0.05. The fish oil TAG substrate contained different levels of each fatty acid, and the fatty acid profile in each lipid class was therefore compared to the original TAG substrate after hydrolysis.
Results
In order to test for possible interference from the lipid contained in the digesta, neutral lipid content of the desalted midgut extract was estimated. The distribution of lipid classes was: FFA (10 μg) > MAG (2 μg) > DAG (2 μg) > TAG (0.5 μg). The main fatty acids of the lipid classes were palmitic acid (16:0) in MAG (0.73 μg/200 μL), 1,2/2,3-DAG (0.40 μg/200 μL) and TAG (0.24 μg/200 μL), while 1,3-DAG had oleic acid, 18:1n-9 (0.79 μg/200 μL) and FFA had 20:5n-3 (2.84 μg/200 μL) as the major fatty acid (Table 1). However, the total amount of each of these fatty acids only accounted for a small fraction compared to the amount of substrate (1 mg) added in the incubation, and was subtracted from the fatty acids calculated in the lipid classes after hydrolysis.
The time course digestion of Tri-18:1 is shown in Fig. 1. The hydrolysis was very high over the first 5 min and then stabilized at a relative constant rate throughout. FFA was the major product reaching a 1:1 ratio of FFA and TAG after 20 min hydrolysis, and increased to account for almost 60% of the lipid classes at the termination of the study, showing a 10-times higher concentration than MAG and DAG. Sn-1,2(2,3)-DAG increased to a maximum of 14% of the lipid classes after 15 min followed by a reduction towards the termination of the study. Both 1(3)-MAG and 2-MAG increased slowly towards the end, but neither contributed to more than 9% of the lipids.
The hydrolysis of various acyl-homogeneous TAGs was assessed after 5 (Fig. 2) and 30 min of incubation (Fig. 3). After 5 min, hydrolysis of Tri-18:1 and Tri-20:5 proceeded at a higher rate than the other synthetic TAG substrates. The main products were in both cases increased levels of FFA compared to the other TAG substrates. The 1,2(2,3)-DAG were the second largest hydrolysis products, while 1,3-DAG and various MAG products were in minute amounts. There was also a notable but non-significant tendency of more 2-MAG to be produced using Tri-18:1 and Tri-20:5 as substrates. The tendency of Tri-18:1 as the best substrate for lipolysis was verified after 30 min incubation where more than 80% was hydrolysed compared to less than 70% for the other substrates. The lowest rate of hydrolysis was found with Tri-22:6, where less than 50% was hydrolysed, which was significantly lower than for the other synthetic substrates (Fig. 3). In each case, FFA was the main product of hydrolysis, but there was also an increase in 1,2(2,3)-DAG and MAG isomers, particularly 2-MAG that reached around 10% of the lipid classes. Very low amounts of 1,3-DAG was produced regardless of substrate.
The mol% of total lipid class upon 5 min hydrolysis of either 1 mg triolein (Tri-18:1), trilinolein (Tri-18:2), trilinolenin (Tri-18:3), trieicosapentaenoin (Tri-20:5), tridocosahexaenoin (Tri-22:6) with desalted midgut extract (118 μg) from Atlantic salmon in 100 mM sodium phosphate-buffer (pH 8.0) and 20 mM sodium taurocholate. The column represents the mean of 6 replicate incubations ±SD, values not having the same superscript letter are significantly different (P < 0.05) as determined by one-way-ANOVA
The mol% of total lipid class hydrolysis of either 1 mg triolein (Tri-18:1), trilinolein (Tri-18:2), trilinolenin (Tri-18:3), trieicosapentaenoin (Tri-20:5), tridocosahexaenoin (Tri-22:6) with desalted midgut extract (118 μg) from Atlantic salmon in 100 mM sodium phosphate-buffer (pH 8.0) and 20 mM sodium taurocholate at 30 min. The column represents the mean of 6 replicate incubations ±SD, values not having the same superscript letter are significantly different (P < 0.05) as determined by one-way-ANOVA
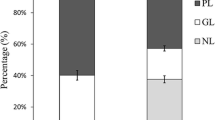
The hydrolysis of fish oil TAG appeared to progress at a different rate than for the structured substrates. After 5 min of hydrolysis, only 40% of the TAG remained with the main hydrolysis products being 1,2(2,3)-DAG (25%), 1(3)-MAG (15%) and 2-MAG (15%). Only 3% was found in FFA (Fig. 2). After 30 min of incubation, the FFA content had increased to 49% while the content of the different MAGs was notably decreased while the level of 1,2(2,3)-DAG was at 13% (Fig. 3).
The fish oil TAG contained oleic acid (18:1n-9, 29.0%) as the major fatty acid followed by palmitic acid (16:0, 21.5%), the fatty acids of the 20-carbon series (gadoleic acid (20:1n-9), 14.6%), eicosapentaenoic acid (20:5n-3, 14.6%) and docosahexaenoic acid (22:6n-3, 11.9%). Linoleic acid (18:2n-6, 4.8%) and linolenic acid (18:3n-3, 3.5%) were only found in small amounts (Fig. 4).
Total fatty acid composition of the substrate and fatty acid specificity within each lipid class after hydrolysis of 1 mg of fish oil TAG with desalted midgut extract (118 μg) from Atlantic salmon in 100 mM sodium phosphate-buffer (pH 8.0) and 20 mM sodium taurocholate at 5 min (mol% fatty acid within each lipid class/1 mg TAG/118 μg desalted midgut extract/5 min). Means, n = 6 ± SD, values not having the same superscript letter are significantly different (P < 0.05) as determined by one-way-ANOVA
After 5 min of incubation, the pattern of fatty acids in the remaining TAG was very similar to that of the substrate with the exception that the level of 20:5n-3 appeared to be somewhat reduced (Fig. 4). The fatty acid pattern in 1,2(2,3)-DAG had many similarities to that of TAG but with some notable differences. Firstly, the content of 22:6n-3 was increased to 5% of the fatty acids. Secondly, the content of 20:5n-3 was reduced further in relation to TAG. All MAGs were significantly altered compared to the substrate where 1(3)-MAG and 2-MAG were enriched in 16:0 by circa 6%. Furthermore, there was also a notable retention of 18:3n-3 in all MAGs, while the levels of 18:2n-6 and 20:5n-3 were very low. Comparing the different MAGs showed that 22:6n-3 accumulated somewhat in 2-MAG while 18:1n-9 and 18:3n-3 were higher in 1(3)-MAG. Of the minor lipid classes, FFA appeared to be enriched in 18:3n-3 and 20:1n-9 while 1,3-DAG was enriched in 16:0, 18:3n-3 and 20:1n-9.
After 30 min, unreacted TAG still had many similarities to the original substrate except for a lower content of 20:5n-3 and a tendency to increased levels of 22:6n-3 and 20:1n-9 (Fig. 5). In the FFA fraction, 18:1n-9 and 20:5n-3 was significantly increased in relation to the substrate while 22:6n-3 was reduced. As shown after 5 min, all MAGs were enriched in 16:0. In 2-MAG, there was still a clear accumulation of 22:6n-3. Docosahexaenoic acid (22:6n-3) was significantly increased in 1,2(2,3)-DAG compared to the substrate while the content of 18:1n-9 was reduced (Fig. 5).
Total fatty acid composition of the substrate and fatty acid specificity within each lipid class after hydrolysis of 1 mg of fish oil TAG with desalted midgut extract (118 μg) from Atlantic salmon in 100 mM sodium phosphate-buffer (pH 8.0) and 20 mM sodium taurocholate at 30 min (mol% fatty acid within each lipid class/1 mg TAG/118 μg desalted midgut extract/30 min). Means, n = 6 ± SD, values not having the same superscript letter are significantly different (P < 0.05) as determined by one-way-ANOVA
Discussion
Dietary lipid is well utilized in most fish species [9]. In order to accomplish this, fish require an efficient system for the intestinal digestion of TAG for absorption across the brush border membrane (BBM) and subsequent resynthesis of hydrolytic products in enterocytes for transportation. The MAG pathway has recently been established as the predominant pathway for TAG resynthesis in the intestine of Atlantic salmon [13, 14] and sea bream [15], which challenges the presence of a non-specific bile salt-dependent lipase (BSDL) as the major lipolytic enzyme of the luminal content in fish. There is a general assumption that BSDL does not discriminate towards sn-acyl position on the glycerol ‘backbone’ of TAG thus yielding glycerol and FFAs that must be resynthesized by the slower and more energetically-consuming glycerol-3-phosphate (G3P) pathway in enterocytes [6].
Therefore, there is some uncertainty as to which lipase system predominates in fish. Although several studies have attempted to verify the existence of a pancreatic sn-1,3-specific lipase, no such enzyme has ever been proven [9]. In most fish species studied so far, desalting appears to halt all lipolytic activity while it is regained by the addition of bile salts [10–12]. This clearly points to the prevalence of a bile salt dependent lipase (BSDL) in fish [9]. However, some studies have reported this lipase to be sn-1,3-specific [10, 16] which, concomitant with the presence of the MAG pathway in enterocytes, opens up the possibility that BSDL in fish may actually possess sn-1,3-specific hydrolytic activity. Furthermore, this specificity has especially been reported for Tri-18:1n-9 [16] and in the presence of shorter chain fatty acyl esters or saturated fatty acids [26].
The present study showed that the lipase has a high degree of sn-1 or sn-3 specificity initially where sn-1,2(2,3)-DAG and FFA were the main hydrolytic products of Tri-18:1n-9 up to 15 min, while sn-1,3-DAG was produced in minute amounts showing low activity towards the sn-2 position in the intermediate hydrolysis. During further hydrolysis of DAG, all sn-positions appeared to be susceptible to hydrolytic attack causing a concomitant small increase in sn-1(3)-MAG and sn-2-MAG in roughly equimolar concentrations. However, the occurrence of 1(3)-MAG could be an isomerisation product from 2-MAG due to the instability of 2-MAG in biological systems having a reported half-life of 2–10 min dependent on protein content and pH [27]. This does suggest the possibility for the existence of a 1,3-specific lipase. However, the total level of MAG (including 2-MAG) never reached the concentrations that would be expected with a 1,3-specific lipase (see Mattson and Volpenhein [4] and Gjellesvik et al. [16]) indicating that a step-wise hydrolysis from TAG through DAG and MAG will eventually proceed to glycerol and FFA as final products. In this respect, our data appear to comply with in vivo feeding studies of Atlantic salmon that has suggested that FFA and glycerol are the main hydrolysis products [28].
There was also an interesting difference in the rate of hydrolysis of synthetic substrates and fish oil. While the digestion of the synthetic substrates was faster producing mainly FFA, the natural substrate had a slower rate producing more intermediate products of MAG and DAG after 5 min. The reason for this could be the fatty acid complexity of the natural oil, where the enzyme will most likely prefer certain fatty acids in a certain position over another, as seen previously where complete hydrolysis to FFA and glycerol has especially been noted if the fatty acid in sn-2 position is a monounsaturated fatty acid [2, 18] or PUFA [18–21].
If the high activity of the MAG pathway is to be effective in the resynthesis of TAG, it is essential that the 2-MAG being produced at an early stage of hydrolysis is absorbed rapidly. In this respect it is interesting to note that the hydrolysis of fish oil over the first 5 min actually did produce substantial amounts of MAG. So even if salmon lipase does not have the traditional sn-1,3-specificity, the MAG pathway would still have sufficient material providing that the absorption is rapid. This would not be the case for the synthetic substrates where the hydrolysis seems to proceed to FFA and glycerol. Fish fed on these diets would consequently rely on the G3P pathway for TAG synthesis, and would probably have lower growth rates. Glycerol-3-phosphate (G3P) is normally produced from amino acids/pyruvate through pathways of glyceroneogenesis, or alternatively generated from glycerol via glycerol kinase, which has extremely low activity in mammalian intestine compared to adipose and has yet to be investigated in fish.
The salmon lipase in the present study also possessed some fatty acid specificity, at least during parts of the hydrolysis process. The most notable effect using synthetic substrates was the very rapid hydrolysis of Tri-18:1 and Tri-20:5n-3 over the first 5 min of incubation compared to the other substrates. This led to a significantly increase in FFA but not other acylglycerols. After 30 min of incubation all differences had evened out, and there was no significant difference between these substrates. This may indicate that FFA has a feedback inhibition on the lipase activity. The only notable effect after 30 min was the high level of Tri-22:6n-3 compared to the other substrates indicating low substrate specificity of the lipase. Fish oil was also very rapidly degraded over the first 5 min of incubation. But in this case, the main products were MAG and 1,2(2,3)-DAG with little occurring in FFA and 1,3-DAG. The build up of 22:6n-3 in 1,2(2,3)-DAG and 16:0 in the MAG fractions do however, show that these were difficult to digest by the lipase. At 30 min this tendency was maintained to some degree, although they were more hydrolysed to FFA, while the build up of 18:1n-9 and 20:5n-3 in the FFA fraction clearly demonstrates that these were preferred over the other fatty acids for hydrolysis.
These data do in some respects agree with previous results from fish. As in our study, Halldorsson et al. [29] showed that 16:0 accumulated in the DAG and MAG fractions while 20:5n-3 increased in the FFA fraction when caecal lipase was incubated with various fish oils. They did however, not observe a similar preference for 18:1n-9 as seen in the present study. Furthermore, they found a significant accumulation of 22:6n-3 in the FFA fraction that was not the case in the present study. Cod digestive lipase also seems to have many similarities with the salmon lipase in that there is a preference for unsaturated fatty acids, 20:5n-3 and a lower activity towards 22:6n-3 [17, 19, 25]. However, cod lipase also seems to have the same preference for shorter chain PUFA like 18:2n-6, which does not seem to be the case in salmon. It thus appears that although fish digestive lipases are similar with regard to preference for 20:5n-3, there are also notable differences. This may indicate some adaptations to natural habitats and the presence of different fatty acids in their natural diets.
However, it is also possible that some of the differences observed are due to variations in experimental conditions. In the present study for example, we used ambient temperature of the fish (10 °C) for incubations in order to mimic natural conditions. Other studies like that of Gjellesvik [26] and presumably Halldorsson et al. [29] used room temperature for their studies. As lipase specificity is known to change at higher temperatures, in cod lipase towards saturated fatty acids [26] this may explain some of the differences observed. Furthermore, assay conditions may also affect the specificities. This was elegantly shown using cod lipase where there was a dramatic change in specificity when the substrates were added in alcoholic or taurocholate solutions [26]. In the present study, we solubilised the TAG substrate in a taurocholate solution before use.
In conclusion, Atlantic salmon lipase does possess some specificity towards sn-1 or sn-3 fatty acyl positions on TAG, at least with Tri-18:1n-9 due to initial increases in 1,2(2,3)-DAG but not 1,3-DAG. However, this specificity is not as pronounced as for mammalian pancreatic lipase or as suggested for BSDL in other fish species. The lack of MAG as a major product of hydrolysis poses a problem for the MAG pathway for reconstituting TAG in the enterocytes when utilising acyl-homogeneous TAG substrates. However, by using natural TAG substrates in the present study, fairly large amounts of MAG were produced during the early stages of digestion that is not seen using synthetic substrates. Provided that MAG is absorbed rapidly, fish enterocytes may still be supplied with sufficient 2-MAG for the MAG pathway to function effectively. Atlantic salmon digestive lipases also have a clear specificity towards certain fatty acids like 20:5n-3 and 18:1n-9, regardless of their positional distribution. This lipase has therefore similarities to that found in other fish species like cod and rainbow trout [19, 29] but there are also some differences that may indicate species adaptations to specialized feed habitats, or simply variations in the in vitro experimental setup.
Abbreviations
- DAG:
-
Diacylglycerol
- FFA:
-
Free fatty acid
- FO:
-
Fish oil
- MAG:
-
Monoacylglycerol
- MGAT:
-
Monoacylglycerol acyltransferase
- Tri-18:1:
-
Triolein
- Tri-18:2:
-
Trilinolein
- Tri-18:3:
-
Trilinolenin
- Tri-20:5:
-
Trieicosapentaenoin
- Tri-22:6:
-
Tridocosahexaenoin
- PUFA:
-
Polyunsaturated fatty acid
- TAG:
-
Triacylglycerol
- TLC:
-
Thin-layer chromatography
References
Borgstrom B (1977) Action of bile-salts and other detergents on pancreatic lipase and interaction with colipase. Biochim Biophys Acta 488:381–391
Patton JS, Nevenzel JC, Benson AA (1975) Specificity of digestive lipases in hydrolysis of wax esters and triglycerides studied in anchovy and other selected fish. Lipids 10:575–583
Lombardo D (2001) Bile salt-dependent lipase: its pathophysiological implications. Biochim Biophys Acta 1533:1–28
Mattson FH, Volpenhein RA (1964) The digestion and absorption of triglycerides. J Biol Chem 239:2772–2777
Wang CS, Kuksis A, Manganaro F, Myher JJ, Downs D, Bass HB (1983) Studies on the substrate-specificity of purified human-milk bile salt-activated lipase. J Biol Chem 258:9197–9202
Lehner R, Kuksis A (1996) Biosynthesis of triacylglycerols. Prog Lipid Res 35:169–201
Johnston JM (1977) Gastrointestinal tissue. In: Snyder F (ed), Lipid metabolism in mammals, vol 1. Plenum Press, New York, pp 151–187
Cowey CB, Sargent JR (1977) Lipid nutrition in fish. Comp Biochem Physiol B Biochem Mol Biol 57:269–273
Olsen RE, Ringo E (1997) Lipid digestibility in fish: a review. Recent Res Dev Lipids Res 1:199–265
Tocher DR, Sargent JR (1984) Studies on triacylglycerol, wax ester and sterol ester hydrolases in intestinal ceca of rainbow-trout (Salmo-Gairdneri) fed diets rich in triacylglycerols and wax esters. Comp Biochem Physiol B Biochem Mol Biol 77:561–571
Iijima N, Tanaka S, Ota Y (1998) Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physlog Biochem 18:59–69
Bogevik AS, Tocher DR, Waagbo R, Olsen RE (2008) Triacylglycerol-, wax ester- and sterol ester-hydrolases in midgut of Atlantic salmon (Salmo salar). Aquacult Nutr 14:93–98
Oxley A, Torstensen BE, Rustan AC, Olsen RE (2005) Enzyme activities of intestinal triacylglycerol and phosphatidylcholine biosynthesis in Atlantic salmon (Salmo salar L.). Comp Biochem Physiol B Biochem Mol Biol 141:77–87
Oxley A, Jutfelt F, Sundell K, Olsen RE (2007) Sn-2-monoacylglycerol, not glycerol, is preferentially utilised for triacylglycerol and phosphatidylcholine biosynthesis in Atlantic salmon (Salmo salar L.) intestine. Comp Biochem Physiol B Biochem Mol Biol 146:115–123
Caballero MJ, Gallardo G, Robaina L, Montero D, Fernandez A, Izquierdo M (2006) Vegetable lipid sources affect in vitro biosynthesis of triacylglycerols and phospholipids in the intestine of sea bream (Sparus aurata). Br J Nutr 95:448–454
Gjellesvik DR, Raae AJ, Walther BT (1989) Partial-purification and characterization of a triglyceride lipase from cod (Gadus-Morhua). Aquaculture 79:177–184
Gjellesvik DR, Lombardo D, Walther BT (1992) Pancreatic bile-salt dependent lipase from Cod (Gadus-Morhua)—purification and properties. Biochim Biophys Acta 1124:123–134
Leger C (1985) Digestion, absorption and transport of lipids. In: Cowey CB, Mackie AM, Bell JG (eds) Nutrition and feeding in fish. Academic Press, London, pp 299–331
Lie O, Lambertsen G (1985) Digestive lipolytic enzymes in cod (Gadus-Morhua)—fatty-acid specificity. Comp Biochem Physiol B Biochem Mol Biol 80:447–450
Lie O, Lied E, Lambertsen G (1987) Lipid digestion in cod (Gadus-Morhua). Comp Biochem Physiol B Biochem Mol Biol 88:697–700
Koven WM, Henderson RJ, Sargent JR (1994) Lipid Digestion in Turbot (Scophthalmus-Maximus). 1. Lipid class and fatty acid composition of digesta from different segments of the digestive-tract. Fish Physiol Biochem 13:69–79
Olsen RE, Myklebust R, Kaino T, Ringo E (1999) Lipid digestibility and ultrastructural changes in the enterocytes of Arctic char (Salvelinus alpinus L.) fed linseed oil and soybean lecithin. Fish Physiol Biochem 21:35–44
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Thomas AE, Scharoun JE, Ralston H (1965) Quantitative estimation of isomeric monoglycerides by thin-layer chromatography. J Am Oil Chem Soc 42:789–792
Christie WW (2003) LIPID ANALYSIS: isolation, separation, identification and structural analysis of lipids. 3rd edn. The Oily Press, Bridgwater
Gjellesvik DR (1991) Fatty acid specificity of bile salt-dependent lipase—enzyme recognition and super-substrate effects. Biochim Biophys Acta 1086:167–172
Rouzer CA, Ghebreselasie K, Marnett LJ (2002) Chemical stability of 2-arachidonylglycerol under biological conditions. Chem Phys Lipids 119:69–82
Sigurgisladottir S, Lall SP, Parrish CC, Ackman RG (1992) Cholestane as a digestibility marker in the absorption of polyunsaturated fatty acid ethyl-esters in Atlantic salmon. Lipids 27:418–424
Halldorsson A, Kristinsson B, Haraldsson GG (2004) Lipase selectivity toward fatty acids commonly found in fish oil. Eur J Lipid Sci Technol 106:79–87
Acknowledgments
This work was supported by the Norwegian Research Council (Grant no. 165051/S40). The authors are also grateful for the skillful assistance of Ivar Helge Matre, Institute of Marine Research, in looking after the fish.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bogevik, A.S., Oxley, A. & Olsen, R.E. Hydrolysis of Acyl-Homogeneous and Fish Oil Triacylglycerols Using Desalted Midgut Extract from Atlantic Salmon, Salmo salar . Lipids 43, 655–662 (2008). https://doi.org/10.1007/s11745-008-3185-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3185-2