Abstract
We report here a two-step process for the high-yield enzymatic synthesis of 2-monoacylglycerides (2-MAG) of saturated as well as unsaturated fatty acids with different chain lengths. The process consists of two steps: first the unselective esterification of fatty acids and glycerol leading to a triacylglyceride followed by an sn1,3-selective alcoholysis reaction yielding 2-monoacylglycerides. Remarkably, both steps can be catalyzed by lipase B from Candida antarctica (CalB). The whole process including esterification and alcoholysis was scaled up in a miniplant to a total volume of 10 l. With this volume, a two-step process catalyzed by CalB for the synthesis of 1,3-oleoyl-2-palmitoylglycerol (OPO) using tripalmitate as starting material was established. On a laboratory scale, we obtained gram quantities of the synthesized 2-monoacylglycerides of polyunsaturated fatty acids such as arachidonic-, docosahexaenoic- and eicosapentaenoic acids and up to 96.4% of the theoretically possible yield with 95% purity. On a technical scale (>100 g of product, >5 l of reaction volume), 97% yield was reached in the esterification and 73% in the alcoholysis and a new promising process for the enzymatic synthesis of OPO was established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arachidonic acid (ARA or C20:4n-6), docosahexaenoic acid (DHA or C22:6n-3) and eicosapentaenoic acid (EPA or C20:5n-3) are polyunsaturated fatty acids (PUFA) involved in a wide range of biologically relevant functions and have strong effects on human health [1, 2]. ARA is an essential fatty acid in human nutrition and a major component of human milk and necessary for the cognitive development of infants. ARA is also a precursor of biologically active prostaglandins and leucotrienes, involved in inflammatory processes [3–5]. DHA is important for the development of the central nervous system [3]. EPA shows significant effects in preventing heart diseases and lowering blood cholesterol levels and thus reduces the risk of arteriosclerosis [6, 7]. The fatty acid distribution on the glycerol backbone influences the adsorption and tissue uptake of glycerides [8, 9]. Generally speaking, 2-monoacylglycerides (2-MAG) are most readily absorbed through the intestinal mucosa and are also the most rapidly absorbed among PUFA derivatives. 2-MAG have very good emulsifying properties and are physiologically essential molecules involved in lipid absorption. In vertebrates, 2-MAG and not glycerol is preferentially utilised for triacylglycerol and phosphatidylcholine biosynthesis [10]. Up to now, these molecules only have limited industrial applications because of difficulties in their synthesis. The chemical synthesis takes several steps, a costly purification and often results in very low yields [11]. Enzymatic synthesis of 2-MAG usually depends on two types of lipase: an unspecific enzyme for the esterification and an sn1,3-specific lipase for the alcoholysis (usually ethanolysis) reaction to the 2-MAG.
The type of fatty acid in the 1,3-position of triglycerides [12] also influences the intestinal adsorption as 2-MAG after the TAG are regiospecifically hydrolyzed in mouth, stomach and small intestine. Symmetrically structured triglycerides incorporate the same fatty acids in sn1 and sn3 position (ABA type). ABA with medium chain fatty acid groups (C6–C10) in the outer positions and a PUFA in the middle position have excellent dietary and absorption characteristics. Additionally the PUFA residue is protected against oxidation by the two saturated fatty acid residues protecting the more oxidizable PUFA in the middle position from oxygen (sterical reasons). Several experiments by Endo and colleagues proved that 1,2-dipalmitoyl-3-PUFA-glycerol is oxidised much more readily than 1,3-dipalmitoyl-2-PUFA-glycerol [13, 14]. The medium-chain fatty acids are easily hydrolyzed in the gastrointestinal tract by the pancreatic lipase. The resulting 2-MAG are readily absorbed and used either as a high-energy resource or for several health-protecting actions [8, 15, 16].
Fish oil, especially tuna and salmon oils, are well-known and inexpensive natural sources of PUFAs. A large variety of health products are made of or contain fish oil-derived compounds, e.g., the encapsulation of these oils in gelatine is of interest as a health food supplement for the prevention and treatment of cardiovascular diseases, neurodegenerative disorders and cancer [9, 17].
1,3-Oleoyl-2-palmitoylglycerol (OPO) is an important ABA-type TAG in infant nutrition. Human milk fats contain palmitic acid predominantly in the sn2-position of TAG. Often infant formulas contain palmitic acid in sn1,3-positions, which leads to the formation of calcium soaps after their release. These soaps are only poorly absorbed by the intestine, which results in indigestion and loss of calcium [18].
We have developed a two-step process leading to 2-monoacylglycerides which is enzymatically catalyzed by lipases. Lipases [triacylglycerol-hydrolases (EC 3.1.1.3)] catalyze the hydrolysis of triacylglycerols at the interface between water and the hydrophobic substrate. Besides the hydrolysis of triacylglycerols, lipases also catalyze the enantio- and regioselective hydrolysis or synthesis of a wide range of natural and unnatural esters [19, 20]. Especially lipases from microorganisms have received a lot of interest because they are useful catalysts for many industrial applications [21, 22] such as ester synthesis, optical resolution [23–25], transesterification or washing processes [26]. In the process described here the same lipase was used for both steps - the esterification as well as the following alcoholysis: Lipase B from Candida antarctica (CalB) [27]. CalB reveals high enantioselectivity against secondary alcohols and, due to its extraordinary stability in organic solvents and at high temperature, has become one of the most frequently used enzymes in industrial applications [28]. For hydrolysis or transesterification of TAG, CalB is classified as an sn1,3-specific lipase. On the other side it is known that in esterification, CalB forms homogeneous TAG (AAA type). Starting from this knowledge, our strategy was first to synthesize a homogeneous TAG of PUFAs followed by the conversion to the desired 2-MAG. In the present work we synthesized the 2-MAG of three different PUFA: ARA, DHA and EPA all in gram quantities and high purities. We also showed that 2-MAG of short and medium chain length can be synthesized by the same method.
After this process was successfully established on a laboratory scale it was transferred and scaled up to a miniplant (total reaction volume up to 10 l). A miniplant is a minimized production line involving all processing steps regarding different parameters such as temperature, pH value and mass transfer.
For the synthesis of OPO, a CalB-driven two-step miniplant process was established. Starting with tripalmitate, the substrate was first converted to the corresponding 2-monopalmitate in an alcoholysis reaction followed by an esterification with oleic acid. Again only CalB was used as the biocatalyst.
Materials and Methods
Lipases and Chemicals
Lipase CalB (Novozym 435) was from Novozymes (Bagsvaerd, Denmark). The lipase gene was derived from Candida antarctica and transferred to the host organism Aspergillus oryzae in which the lipase was expressed. The purified lipase was immobilized on a macroporous acrylic resin. All lipases were purchased from Fluka. Immobilized CalB was removed after the reaction by filtration, washed three times, dried in a desiccator and afterwards could be used again. All chemicals except the PUFAs and solvents used were of analytical reagent grade and purchased from normal suppliers (Sigma, Fluka and Riedel de Haën). PUFAs were supplied by Nu-Chek Prep (Elysian, USA). References of MAG, DAG and TAG for GC/MS analysis were also supplied by Nu-Chek Prep.
Esterification Reaction
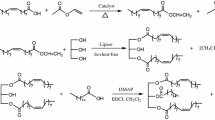
Purified 2-MAG (100 mg, obtained from alcoholysis reactions) or glycerol and fatty acids (molar ratio 1:3) [29] were dissolved in 20 ml n-hexane. The water, generated during the reaction was removed by addition of 200 mg activated molecular sieve (pore diameter 3 Å, bead diameter ~2 mm, UOP Type 3A, Fluka, Buchs, Switzerland). The mixture was stirred magnetically (level 10 on a RCTbasic, IKAlabortechnik, Staufen, Germany) and incubated at 50 °C. The reaction was started by adding 100 mg immobilized lipase (CalB). After 24 h the reaction was stopped by removing the lipase (Fig. 1) as described above.
Two-step synthesis of 2-monoglyceride of DHA catalyzed by CalB. The process consists of two steps. First esterification of glycerol and free fatty acids leads to homogeneous TAG and water. TAGs are further converted in an alcoholysis reaction to the corresponding 2-MAGs and ethyl esters. The ethyl esters are removed in the following purification step in a two-phase (acetonitrile/water and n-hexane) system via a separatory funnel. Both steps are catalyzed by CalB which acts unspecific in the esterification and sn1,3-specific in the following alcoholysis. Synthesis of the 2-MAGs of AA and EPA was achieved according to the same scheme (D, DHA: Docosahexaenoic acid; DHAEt: DHA ethyl ester)
Alcoholysis Reaction of Triglycerides
The homogeneous TAG of PUFA or other TAG (100 mg) were mixed with pure ethanol (molar ratio TAG:ethanol is 1:4) and emulsified for 30 min at 25 °C. The reaction was started by adding CalB (100 mg) and stopped after 4–5 h by filtration of the catalyst (Fig. 1). The reaction process was monitored using TLC.
Reactions on a Technical Scale (Reaction Volume: 8 l)
The miniplant was built by the Institute of Biochemical Engineering (IBVT, University of Stuttgart, Germany) within a Bosch-Rexroth frame (UTZ Ratio Technik, Korb, Germany). Process control equipment and software were supplied by National Instruments Germany GmbH, München and National Instruments (Labview®, Austin, TX, USA), respectively. The enzyme reactions were performed in a 10 l glass-vessel (Type BDAV, HWS Labortechnik, Mainz, Germany). The reaction mixture was stirred by air-driven stirrers (Gebr. Buddeberg GmbH, Mannheim, Germany). The temperature was controlled by a thermostat (F33, Julabo, Seelbach, Germany) [30].
The reactions in the miniplant were performed as batch processes under a nitrogen atmosphere. The amounts of chemicals and lipase were calculated to the working volume of the miniplant which was 8 litres.
Because of the high price of highly purified PUFA, erucic acid (EA or C22:1n-9), a cheaper, long-chain unsaturated fatty acid was used as a model substrate for the esterification with glycerol. 8 l n-hexane, 304 g erucic acid, 37 g glycerol, 60 g immobilized CalB and 500 g molecular sieves (pore diameter 3 Å, beads diameter ~2 mm, UOP Type 3A, Fluka, Buchs, Switzerland) were applied.
In the alcoholysis reaction tripalmitate was converted to 2-monopalmitate. TAG and ethanol were emulsified in a molar ratio of 1:10 and then the vessel was filled up with acetone to the working volume (8 l acetone, 283 g tripalmitate, 161 g ethanol and 60 g immobilized CalB).
This product was afterwards esterified with oleic acid leading to OPO (8 l n-hexane, 84 g 2-monopalmitate, 260 g oleic acid, 60 g immobilized CalB and 500 g molecular sieves; Fig. 2).
Two-step synthesis of OPO catalyzed by CalB. Starting substrate for OPO synthesis is tripalmitate, which is converted to 2-monopalmitate in an alcoholysis reaction and afterwards purified by crystallization. The following reaction consisted of esterification of 2-monopalmitate with oleic acid to OPO, which was purified via a silica-gel column. Both steps were catalyzed by CalB (PAEt: Palmitic acid ethyl ester)
Purification of 2-Monoacylglycerides and Triglycerides
For purification of 2-MAG of PUFA, the solvent was evaporated and the concentrate was dissolved in acetonitrile/water (95:5 v/v, 10-fold volume compared to the concentrate) and washed three times with the same volume of hexane for ethyl ester removal [31]. The pure 2-MAG are found in the acetonitrile/water phase.
2-Monopalmitate was purified via crystallization. After filtering off the catalyst, the excess solvent was evaporated and the residue was dissolved in n-hexane:methyl-tert-butylether (MTBE) (70:30 v/v, 10-fold volume compared to the concentrate) and stored for 1 h at −25 °C. After this period, the white crystals formed were collected by filtration at −25 °C. The supernatant containing ethyl esters, fatty acids, and a small amount of diglycerides was discarded. The 2-MP was recrystallized until the TLC plate showed only one band of 2-MP. The purity of 2-MP was confirmed by GC/MS analysis.
For purification of TAG, the solvent was evaporated and the TAG purified by silica gel column chromatography eluted with n-hexane/methyl-tert-butylether (MTBE) (70:30 v/v). The TAG are eluted first followed by ethyl ester, fatty acids, di- and finally monoacylglycerides.
TLC Analysis
For rapid analysis during the process, thin-layer-chromatography (TLC) was performed using aluminium sheets with silica gel (Merck KGaA, Darmstadt, Germany) as the stationary phase; n-hexane/MTBE (70:30 v/v) was the mobile phase. TLCs were developed in a staining solution (10 g cer(IV)-sulphate, 2 g molybdatophosphoric acid, 10 ml concentrate sulphuric acid, 100 ml water) for 30 s and afterwards dried with a heat gun. The spots were identified using the corresponding references.
GC/MS Analysis
For GC/MS analysis, samples were derivatized with 1% of trimethylchlorosilane in N,O-bis(trimethylsilyl)trifluoroacetamide. The derivatization reagent converts all hydroxyl- and carboxyl groups to the corresponding trimethylsilyl ethers and -esters. After addition of the derivatization solution, samples were incubated for 30 min at 60 °C.
Products were identified on a Shimadzu GC/MS-QP2010 (Shimadzu Corporation, Kyoto, Japan) equipped with a 30 m FS-Supreme-5 column (5% diphenyl polysiloxane/95% dimethyl polysiloxane, internal diameter 0.25 mm, film thickness 0.25 μM) using helium as the carrier gas at a linear velocity of 30 cm s−1. Analysis of mono-, di- and triglycerides was performed using the following program: 200 °C followed by heating (8 °C/min) to 360 °C. Using the GC/MS software GC/MS-solution® (Shimadzu Corporation, Kyoto, Japan) the amounts of substrate and products as well as the yield were calculated. For product identification the respective references were measured on the GC/MS and the reaction products compared in terms of retention time and mass spectra.
Results
Synthesis of PUFA 2-Monoacylglycerides
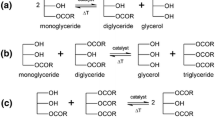
So far, direct esterification of PUFA and glycerol or an adequate interesterification leading to 2-MAG of PUFA has been impossible because there is no sn-2 specific lipase known. Therefore we developed a two-step process including esterification of glycerol and PUFA leading to TAG followed by ethanolysis resulting in the desired 2-MAG (Fig. 1). The potential of CalB for synthesis of triglycerides in an unspecific manner has already been described [29]. A glycerol:PUFA ratio of 1:3 was found to be the optimum for the reaction. Excess of glycerol or fatty acids is inappropriate because of the production of large amounts of MAG and diacylglycerols (DAG) decreasing the yield of TAG.
For the sn1,3-specific alcoholysis, several lipases classified as sn1,3-specific were tested: Candida antarctica B lipase (CalB), Penicillium camembertii lipase, Pseudomonas fluorescens lipase, Rhizomucor mihei lipase, Rhizopus javanicus lipase and Rhizopus oryzae lipase [32]. Only CalB was able to convert all three TAG of PUFA to the desired 2-MAG. The lipase of Rhizopus oryzae was able to convert the TAG of ARA to the 2-MAG, but failed in the case of TAG of DHA (DDD) and EPA (EEE). For most lipases, the substrates seem to be too sterically demanding for their substrate-binding-site and therefore cannot be converted. Interestingly CalB, which acts in the first reaction as an unspecific catalyst is able to convert the substrates regioselectively to 2-MAG.
The alcoholysis reaction was very fast (Fig. 3). After 240 min the reaction was completed and almost no TAG was detected by TLC anymore. The reaction rate was constant from the beginning till the end of the reaction after 240 min. No DAG or free fatty acids were detectable. Fatty acid ethyl ester was able to be completely removed by liquid-liquid extraction. No isomerization of MAG was observed. TLC-analysis revealed a single spot of 2-MAG.
No acyl migration was observed when the alcoholysis reaction was performed in a temperature range from 25 to 30 °C. Temperatures higher than 40 °C led to increasing acyl migration and partial deacylation to glycerol.
The 2-MAG of the three model PUFA (ARA, DHA, EPA) were synthesized in gram quantity with purities higher than 85% and yields up to 96% (Table 1).
The same process was proven to be usable for the synthesis of 2-MAG of short and middle-chain fatty acids such as caprylic- (C8), palmitic- (C16) and oleic- (C18:1) acid. The corresponding 2-MAG were not purified as these studies were only performed to explore the range of 2-MAG accessible by the technology described herein. So the substrate spectra of the 1,3-specific alcoholysis reaction with CalB covers all chain lengths from short to long and saturated or polyunsaturated.
Synthesis of Triglycerides in the Miniplant
For upscaling, erucic acid was chosen as a cheap substrate since it is available in large quantities with high purity. The CalB-catalyzed esterification yielded 352 g of triglyceride of erucic acid corresponding to 97% of the theoretically possible yield (Table 2). The glycerol:fatty acid ratio of 1:3 led to a highly efficient esterification and less than 5% of by-products like mono- and diglycerides.
Synthesis of OPO in the Miniplant
OPO was synthesized in the miniplant starting with tripalmitate as substrate. In the first step, tripalmitate was converted to 2-monopalmitate (2-MP) in an alcoholysis reaction, followed by esterification of 2-MP with oleic acid to OPO (Fig. 2). The yield of 2-MP was 73% corresponding to 84 g (77% purity). The esterification led to 198 g of OPO (yield 90%) with a purity of 95% (Table 2).
During the alcoholysis reaction no acyl migration was detectable.
In general, immobilized CalB could be reused after filtration, washing and drying at least three times without any loss of activity.
Discussion
In our study we showed that it is possible within a two-step process to synthesize several 2-MAG without limitations regarding chain-length and saturation degree with just one biocatalyst. The reactions were successfully scaled up to 8 l scale and also a two-step process for the synthesis of OPO was established.
The easiest and most effective route towards 2-MAG would be the application of an sn2-specific lipase which would allow to synthesize these desired molecules in a one-step reaction. With such an outstanding catalyst it would be possible to perform a direct esterification of glycerol and fatty acids (glycerolysis) leading to 2-MAG or to perform an interesterification reaction between two homogeneous triglycerides leading to an ABA type TAG. Such an enzyme would be an outstanding innovation in the field of enzyme technology. Up to now only very few lipases like Lipase A from Candida antarctica (CalA) [33] have been described to show significant preference for position sn2 of glycerides. For other enzymes this characteristic is discussed, as, for example, the lipases from Vernonia anthelminitica [34] or Candida parapsilosis [35, 36]. Recently the expression of CalA in E. coli on a microtiter plate scale has been described allowing directed evolution of this lipase [37]. So the prerequisites for a possible tailoring of CalA towards an sn2-specific lipase via directed evolution are fulfilled.
The results of the esterification and alcoholysis reactions showed that CalB displays either an unspecific or a very strict sn1,3-specific behaviour depending on the reaction conditions. We also observed that, after complete conversion of TAG to 2-MAG, the catalyst starts to hydrolyze the 2-MAG leading to glycerol and ethyl esters. So the reaction has to be stopped by removing the lipase directly after the TAG are converted quantitatively. These results indicate that CalB is not a “classical” or “totally” sn1,3-specific lipase because it is also able to convert 2-MAG. 2-MAG are not hydrolyzed at all by highly sn1,3-specific lipases, e.g., the Rhizomucor mihei lipase. It is probable that the addition of ethanol converts CalB from an unspecific to a more 1,3-regiospecific enzyme. A possible reason for this behaviour might be decreased flexibility of the tertiary structure of CalB caused by ethanol, hindering the substrate from accessing the catalytic binding pocket with the acyl group in the middle position [31]. This increased rigidity should also be favoured by decreasing the reaction temperature to 25 °C. Thus CalB should be classified as an unspecific lipase which can be tailored by reaction engineering to a more sn1,3-specific enzyme. The most important advantage over all other sn1,3-specific lipases tested in our work is the ability of CalB to convert the sterically demanding TAG of PUFAs which do not fit into the substrate binding pockets of other lipases (e.g., the Rhizopus lipases).
Reduced acyl migration has been reported when lowering the water content of the reaction mixture or adjusting the water activity [38]. In the reactions described here the water content was adjusted to a low level by addition of molecular sieve. Even though in other processes the water activity is exactly adjusted [39] we did not detect any effect of acyl migration in our miniplant reactions. Accordingly the temperature was the most determinant parameter for acyl migration.
In a first attempt the alcoholysis reaction in the miniplant was performed in pure ethanol as solvent and co-substrate, which ended in very weak product formation and low yield. Ethanol inhibits the lipase activity: after a certain amount of time the reaction stopped although most of tripalmitate was not converted. After filtration of the lipase and removing the ethanol, the enzyme was active again. This indicates a kind of inhibitory complex that is formed between the enzyme and ethanol. Still the alcoholysis reaction in pure ethanol worked fine on a small scale. This may be due to significantly higher lipase concentration on a small scale compared to technical scale. Consequently, the amount of ethanol was decreased in the miniplant reactions. In this case no inhibition of the lipase was detectable.
Inhibition of the alcoholysis reaction by ethanol in the miniplant was surprising. It is even more surprising that the reaction performed well in an acetone/ethanol mixture since this kind of mixture has been said to inactivate lipases [40, 41]. The results presented here show that high-yield production of 2-monopalmitate in acetone is possible. As acetone is regarded as food-safe, the process is suitable for the production of nutrition-related materials.
The purification, taking advantage of the high solubility of 2-MAG in acetonitrile/water (95:5 v/v), while more hydrophobic by-products are more soluble in n-hexane, allowed an easy and fast purification of the 2-MAG of PUFA. Monoacylglycerides and ethyl esters could be clearly separated. Usually the purification of 2-MAG is performed via crystallization as in the case of 2-MP. This is unsuitable in the case of PUFA because of their very deep freezing point. A possible alternative that also has been tested is the purification via a silica gel column treated with boric acid. In that case, large amounts of organic solvents were necessary and significant acyl migration was detected (up to 40%).
Yield and purity of OPO synthesized in the miniplant with CalB was slightly lower than in a similar process catalyzed by Rhizopus (Rhizopus delemar, Rhizopus oryzae and Rhizomucor mihei) lipases [39]. In the alcoholysis reaction a yield of 85% with a purity >95% was described. Still this process was performed on a laboratory scale whereas in our case we reached a technical scale (>100 g of product) indicating further upscaling for industrial production is possible. Processes for OPO synthesis have been described using two different lipases with distinct regioselectivity for the ethanolyis and esterification reaction [42]. The use of only one enzyme as suggested in our work will facilitate industrial applications.
The potential of our approach is further underlined by comparison of the results to the latest literature on 2-MAG synthesis. A lab-scale process for the enzymatic synthesis (by ethanolysis) of 2-MAG described by Shimada and colleagues yielded only 28–29 mol% of 2-MAG content [43]. Yang and colleagues described a CalB-driven glycerolysis process yielding 2-MAG of PUFA [44]. Still the yield in the stirred tank just reached 70% and unfortunately they had to work with a multiphase-system.
With respect to process development and further upscaling, the use of only one lipase for the unspecific esterification and the sn1,3-selective alcoholysis is a big advantage of the process described here. Reusability of immobilized CalB in the batch processes indicates that establishment of continuous production processes is possible. Thus new promising applications of this outstanding biocatalyst might arise.
References
Biscione F, Pignalberi C, Totteri A, Messina F, Altamura G (2007) Cardiovascular effects of omega-3 free fatty acids. Curr Vasc Pharmacol 5:163–172
Gill I, Valivety R (1997) Polyunsaturated fatty acids .1. Occurrence, biological activities and applications. Trends Biotechnol 15:401–409
Innis SM (1991) Essential fatty-acids in growth and development. Prog Lipid Res 30:39–103
Koletzko B, Schmidt E, Bremer HJ, Haug M, Harzer G (1989) Effects of dietary long-chain poly-unsaturated fatty-acids on the essential fatty-acid status of premature-infants. Eur J Pediatr 148:669–675
Koletzko B, Decsi T, Demmelmair H (1996) Arachidonic acid supply and metabolism in human infants born at full term. Lipids 31:79–83
Weylandt KH, Kang JX, Leaf A (1996) Polyunsaturated fatty acids exert antiarrhythmic actions as free acids rather than in phospholipids. Lipids 31:977–982
Simopoulos AP (1991) Omega-3-fatty-acids in health and disease and in growth and development. Am J Clin Nutr 54:438–463
Christensen MS, Hoy CE, Becker CC, Redgrave TG (1995) Intestinal-absorption and lymphatic transport of eicosapentaenoic (Epa), docosahexaenoic (Dha), and decanoic acids—dependence on intramolecular triacylglycerol structure. Am J Clin Nutr 61:56–61
Sadou H, Leger CL, Descomps B, Barjon JN, Monnier L, Depaulet AC (1995) Differential incorporation of fish-oil eicosapentaenoate and docosahexaenoate into lipids of lipoprotein fractions as related to their glyceryl esterification—a short-term (Postprandial) and long-term study in healthy humans. Am J Clin Nutr 62:1193–1200
Oxley A, Jutfelt F, Sundell K, Olsen RE (2007) Sn-2-monoacylglycerol, not glycerol, is preferentially utilised for triacylglycerol and phosphatidylcholine biosynthesis in Atlantic salmon (Salmo salar L.) intestine. Comp Biochem Physiol B Biochem Mol Biol 146:115–123
Yamane T (1999) Monoacylglycerols. Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation 4:1810–1818
Mutsuda M, Michel KP, Zhang X, Montgomery BL, Golden SS (2003) Biochemical properties of CikA, an unusual phytochrome-like histidine protein kinase that resets the circadian clock in Synechococcus elongatus PCC 7942. J Biol Chem 278:19102–10
Endo Y, Hoshizaki S, Fujimoto K (1997) Oxidation of synthetic triacylglycerols containing eicosapentaenoic and docosahexaenoic acids: effect of oxidation system and triacylglycerol structure. J Am Oil Chem Soc 74:1041–1045
Endo Y, Hoshizaki S, Fujimoto K (1997) Autoxidation of synthetic isomers of triacylglycerol containing eicosapentaenoic acid. J Am Oil Chem Soc 74:543–548
Babayan VK (1987) Medium chain triglycerides and structured lipids. Lipids 22:417–420
Mascioli EA, Bistrian BR, Babayan VK, Blackburn GL (1987) Medium chain triglycerides and structured lipids as unique nonglucose energy-sources in hyperalimentation. Lipids 22:421–423
Davis TA, Gao L, Yin HY, Morrow JD, Porter NA (2006) In vivo and in vitro lipid peroxidation of arachidonate esters: the effect of fish oil omega-3 lipids on product distribution. J Am Oil Chem Soc 128:14897–14904
Lien EL, Boyle FG, Yuhas R, Tomarelli RM, Quinlan P (1997) The effect of triglyceride positional distribution on fatty acid absorption in rats. J Pediatr Gastroenterol Nutr 25:167–174
Okumura S, Iwai M, Tsujisaka Y (1979) Synthesis of various kinds of esters by four microbial lipases. Biochim Biophys Acta 575:156–165
Nakano H, Kitahata S, Tominaga Y, Takenishi S (1991) Esterification of glycosides with glycerol and trimethyloipropane moieties by Candida cylindracea lipase. Agric Biol Chem 55:2083–2089
Björkling F, Godtfredsen SE, Kirk O (1991) The future impact of industrial lipases. Trends Biotechnol 9:360–363
Schmid RD, Verger R (1998) Lipases, interfacial enzymes with attractive applications. Agnew Chem Int Ed Engl 37:1608–1633
Ghanem A (2006) Trends in lipase-catalyzed asymmetric access to enantiomerically pure/enriched compounds. Tetrahedron 63:1721–1754
Kirchner G, Scollar MP, Klibanov AM (1985) Resolution of racemic mixtures via lipase catalysis in organic solvents. J Am Chem Soc 107:7072–7076
Langrand G, Secchi M, Buono G, Baratti J, Triantaphylides C (1985) Lipase-catalyzed ester formation in organic solvents. An easy preparative resolution of alpha-substituted cyclohexanols. Tetrahedron Lett 26:1857–1860
Kojima Y, Yokoe M, Mase T (1994) Purification and characterization of an alkaline lipase from Pseudomonas fluorescens AK102. Biosci Biotechnol Biochem 58:1564–1568
Hoegh I, Patkar S, Halkier T, Hansen M.T (1995) Two lipases from Candida antarctica: cloning and expression in Aspergillus oryzae. Can J Bot 73:869–875
Anderson EM, Larsson KM, Kirk O (1998) One biocatalyst–many applications: the use of Candida antarctica lipase B–lipase in organic synthesis. Biocat Biotransform 16:181–204
Medina AR, Cerdán LE, Giménez AG, Páez BC, González MJI, Grima EM (1999) Lipase-catalyzed esterification of glycerol and polyunsaturated fatty acids from fish and microalgae oils. J Biotechnol 70:379–391
Berendsen WR, Gendrot G, Freund A, Reuss M (2006) A kinetic study of lipase-catalyzed reversible kinetic resolution involving verification at miniplant-scale. Biotechnol Bioeng 95:883–892
Irimescu R, Iwasaki Y, Hou CT (2002) Study of TAG ethanolysis to 2-MAG by immobilized Candida antarctica lipase and synthesis of symmetrically structured TAG. J Am Oil Chem Soc 79:879–883
Bornscheuer UT, Kazlauskas RJ (1999) Hydrolases in organic synthesis
Rogalska E, Cudrey C, Ferrato F, Verger R (1993) Stereoselective hydrolysis of triglycerides by animal and microbial lipases. Chirality 5:24–30
Olney CE, Jensen RG, Sampugna J, Quinn JG (1968) Purification and Specificity of a Lipase from Vernonia anthelmintica Seed. Lipids 3:498–502
Brunel L, Neugnot V, Landucci L, Boze WN, Moulin G, Bigey F, Dubreucq E (2004) High-level expression of Candida parapsilosis lipase/acyltransferase in Pichia pastoris. J Biotechnol 111:41–50
Neugnot V, Moulin G, Dubreucq E, Bigey F (2002) The lipase/acyltransferase from Candida parapsilosis: molecular cloning and characterization of purified recombinant enzymes. Eur J Biochem 269:1734–1745
Pfeffer J, Rusnak M, Hansen CE, Rhlid RB, Schmid RD, Maurer SC (2007) Functional expression of Lipase A from Candida antarctica in Escherichia coli—a prerequisite for high-throughout screening and directed evolution. J Mol Cat: B Enzymatic 45:62–67
Goderis HL, Fouwe BL, van Cauwenbergh SM, Tobback PP (1986) Measurements and control of water content of organic solvents. Anal Chem 58:1561–1563
Schmid U, Bornscheuer UT, Soumanou MM, McNeill GP, Schmid RD (1999) Highly selective synthesis of 1,3-oleoyl-2-palmitoylglycerol by lipase catalysis. Biotechnol Bioeng 64:678–684
Heinsman NWJT, Valente AM, Smienk HGF, van der Padt A, Franssen MCR, de Groot A, van’t Riet K (2001) The effect of ethanol on the kinetics of lipase-mediated enantioselective esterification of 4-methyloctanoic acid and the hydrolysis of its ethyl ester. Biotechnol Bioeng 76:193–199
Yamane T (1987) A comparison of assay on hydrolytic activity of lipase with and without surfactants. J Jpn Oil Chem Soc 36:402–408
Chen ML, Vali SR, Lin JY, Ju YH (2004) Synthesis of the structured lipid 1,3-dioleoyl-2-palmitoylglycerol from palm oil. J Am Oil Chem Soc 81:525–532
Shimada Y, Ogawa J, Watanabe Y, Nagao T, Kawashima A, Kobayashi T, Shimizu S (2003) Regiospecific analysis by ethanolysis of oil with immobilized Candida antarctica lipase. Lipids 38:1281–1286
Yang T, Rebsdorf M, Engelrud U, Xu X (2005) Enzymatic production of monoacylglycerols containing polyunsaturated fatty acids through an efficient glycerolysis system. J Agric Food Chem 53:1475–1481
Acknowledgments
We thank the Nestlé Company for the financial support of our work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pfeffer, J., Freund, A., Bel-Rhlid, R. et al. Highly Efficient Enzymatic Synthesis of 2-Monoacylglycerides and Structured Lipids and their Production on a Technical Scale. Lipids 42, 947–953 (2007). https://doi.org/10.1007/s11745-007-3084-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-007-3084-y