Abstract
The dynamic interfacial properties and dilational rheology of gemini sulfonate surfactant (SGS) and its mixtures with quaternary ammonium bromides (DTAB, CTAB) at the air–water interface were investigated using drop shape analysis. Results suggest that the adsorption process of these surfactants is diffusion-controlled at dilute concentrations, whereas the adsorption mechanism gradually shifts to a mixed kinetic-diffusion control with increasing surfactant concentration. The mixed surfactant system possesses the best surface activity when the molar ratios of SGS/DTAB and SGS/CTAB mixtures are 9:10. The formation of catanionic complexes shields the electrostatic repulsion between surfactant molecules and lowers the electrostatic adsorption barrier. Therefore, SGS/DTAB and SGS/CTAB mixtures exhibit higher adsorption rates than either component alone. The effects of oscillating frequency and surfactant concentration on the surface dilational properties of SGS, DTAB, CTAB, SGS/DTAB, and SGS/CTAB mixtures were also determined. As the oscillating frequency increases, the dilational elasticity of these surfactants gradually increases. The dilational elasticity peaks at a certain concentration, which is less than the critical micelle concentration (CMC). Results show that the dilational elasticity of SGS/DTAB and SGS/CTAB mixtures is higher than that of either component, resulting from the formation of a denser monomolecular adsorption layer at the air–water interface. Our study provides a basis for understanding the interaction mechanism of catanionic surfactant mixtures containing Gemini surfactant at the air–water interface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When interfacial processes proceed at a rapid rate [1], the dynamic interface properties of surfactant and biomolecule solution are more important than the equilibrium properties in many industries, such as printing and dyeing, tertiary oil recovery, and pharmaceuticals [2, 3]. Therefore, many studies have been done on the dynamic interface properties of surfactants and biomolecules during the past few decades. Dilational interfacial rheology is described as the resistance of the interface to deformation, which has been shown to be important for the stability of foam and emulsion [4]. When the interface is perturbed, different relaxation processes occur, including the diffusion process from the bulk solution to the interface region, re-orientation, and aggregation and re-arrangement of surfactant molecules inside the adsorbed layer [5]. Dilational interfacial rheology measurements can provide more information about the microcosmic processes of the adsorbed layer [6]. Moreover, this information is beneficial to elaborate the interaction mechanism of adsorbed molecules at the interface [7].
The dilational interfacial rheology of surfactants and macromolecule solutions has been extensively investigated in recent years [8, 9]. Langevin indicated the stability of foam and emulsion is strongly dependent on the value of dilational elasticity and viscosity [10]. Maldonado-Valderrama et al. investigated the relation between the stability of foams and emulsions stabilized by β-casein and the dilational properties of the interface [11]. They proved that the interfacial viscosity of the protein adsorbed layer is crucial in the formation of the foam; however, the interfacial elasticity has a marked impact on the stability of emulsions and foams.
Gemini surfactants have two hydrophilic head groups and two hydrophobic alkyl chains. Due to this special molecule structure, gemini surfactants possess better interface properties than conventional single-chain surfactants. Therefore, gemini surfactants have attracted great interest and been extensively investigated for the past 20 years. Zhang et al. examined the dilational properties of anionic gemini surfactants (C8E8C8) at the air–water interface [12]. The dilational modulus of C8E8C8 passes through two peaks with surface aging, which is different from that of conventional surfactants. Xu et al. studied the dilational rheological properties of bisquaternary ammonium dibromides at the air–water interface [13]. The results show that the dilational modulus of C12C2C12·2Br− is higher than that of the single-chain cationic surfactant. In addition, the dilational rheological properties of gemini surfactant/protein [14, 15], and gemini surfactant/polymer mixtures [16–18] were studied.
In general, catanionic surfactant mixtures are regarded as a taboo, because these mixtures are prone to precipitation [19]. However, some studies have proved catanionic surfactant mixtures may exhibit outstanding properties when these surfactants are mixed in a proper proportion [20]. Molecular interactions between catanionic surfactant mixtures are mainly governed by the electrostatic forces between their hydrophilic head groups; therefore, gemini surfactants are expected to have stronger synergistic interactions with surfactants of different charge types than single-chain surfactants. Studies have illustrated the stronger synergistic effects of catanionic gemini surfactants [20–22]. Parekh et al. examined the surface properties of the mixed surfactant systems of anionic conventional surfactant (sodium dodecyl trioxyethylene sulfate) and cationic gemini surfactant (N, N′-bis-(dimethyldodecyl)-α,ω-dialkane diammonium dibromide) [23]. Results confirm that the mixed surfactant systems exhibit a very low critical micelle concentration (CMC) value, which results from the weakening of the electrostatic head group repulsion which favors mixed micelle formation. Cationic gemini surfactants bind tightly with anionic surfactants by electrostatic, hydrophobic and ion–dipole interactions. The synergistic effects between catanionic surfactant mixtures have been extensively examined; however, only a few reports are available on the interfacial dynamic properties and dilational rheology of catanionic surfactant mixtures of different charge types.
In the present study paper, we investigated the interfacial dynamic properties and dilational rheology of sulfonate gemini surfactant (SGS) and its mixtures with quaternary ammonium bromides [hexadecyltrimethylammonium bromide (CTAB) and dodecyltrimethylammonium bromide (DTAB)] at the air–water interface using the drop shape analysis. Our study provides a basis for understanding the interaction mechanism of catanionic surfactant mixtures at the air–water interface. The results also give helpful information about the design and application of catanionic surfactant mixtures containing gemini surfactants.
Experimental
Materials
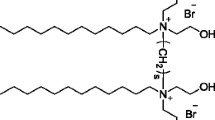
The sulfonate gemini surfactant was synthesized in our laboratory. The chemical structure is presented in Fig. 1. The synthetic method of the surfactant has been reported elsewhere [24], and its purity was identified by 1H NMR and elemental analysis. CTAB, DTAB were purchased from Aladdin Reagent (Shanghai, China). Ultrapure water with 18.2 MΩ cm (Millipore) was used as solvent in all experiments.
Determination of Equilibrium Surface Tension
The equilibrium surface tensions of different surfactant solutions were measured with a JK99B tensiometer (Shanghai Zhongchen Digital Technic Apparatus Co., Ltd., China) using the du Nouy ring. The surface tension was determined at 25 °C until a constant value was attained.
Determination of Dynamic Surface Tension
For dynamic surface tension (DST) measurements, the surface tension was monitored as a function of time. DST of surfactant solution was measured using a commercial bubble profile tensiometer (Tracker, Teclis-IT Concept, France). In this instrument, the air–water interface was created by injecting 5 μL of air into an inverse stainless steel needle attached to a gas-tight syringe. The tip of the bent needle was immersed in a quartz cuvette containing the surfactant solution. The image of the bubble was captured by a charge coupled device (CCD) camera and analyzed by software employing the Laplace equation.
Rosen et al. demonstrated that the DST of surfactant solutions can be described well by the empirical Eq. 1 [1],
where \(\gamma_{0}\) is the surface tension of the solvent, \(\gamma_{\text{t}}\) is the surface tension of surfactant solutions at surface age t, \(\gamma_{\text{m}}\) is the mesoequilibrium surface tension, and n and \(t^{*}\) are constants. Based on the experimental data, n and \(t^{*}\) can be obtained from the slope and intercept of the plot of \(\lg [(\gamma_{0} - \gamma_{\text{t}} )/(\gamma_{\text{t}} - \gamma_{\text{m}} )]\) versus \(\lg t\).
In addition, values for an effective diffusion coefficient (D) for surfactants at short surface ages can be obtained using Eq. 2 based on the Ward and Tordai equation [25],
where \(c_{0}\) is the bulk surfactant concentration, \(\pi\) = 3.142, n = 1 for non-ionic surfactants, and n = 2 for ionics [25]. A linear plot of \(\gamma_{t}\) versus \(t^{1/2}\) indicates that adsorption of surfactant from the bulk to surface is diffusion-controlled and permits evaluation of D from the slope of the plot.
For a prolonged adsorption process, the Ward and Tordai equation can be stated as Eq. 3 [26],
where \(\varGamma_{\text{eq}}\) is the surface excess concentration and can be obtained from the equilibrium surface tension-concentration plot using the Gibbs adsorption equation.
Determination of Interfacial Dilational Viscoelasticity
The interfacial dilational viscoelasticity of surfactant solution was also measured using an automated bubble profile tensiometer (Tracker, Teclis-IT Concept, France). The initial volume of the bubble was 3 μL. Each sample was assumed to be equilibrated when the surface tension did not change with time. Interfacial rheological measurements were performed by oscillating the bubble volume to a periodic, small sinusoidal deformation of 10% of the original drop volume with different frequencies. The influences of surfactant concentration and dilatational frequency (0.005–0.5 Hz) on the interfacial dilational properties were studied.
When the interfacial area is imposed to a periodic compression and expansion at a given frequency (ω), the interfacial tension changes in response [27]. As shown in Eq. 4, the Gibbs interfacial dilational modulus (ε) is generally defined as the ratio of a small change in interfacial tension (γ) to a specific change in interfacial area (A) [14],
According to Eq. 5, the ε can also be expressed as a complex number, with real (ε d) and imaginary components (ε η = ωη d) [28]. ε d is the dilational elasticity or storage modulus, representing the elastic energy storage in the surface, and ε η is the dilational viscosity or loss modulus, accounting for the energy loss in the relaxation process [29].
The phase angle (θ) is calculated according to Eq. 6.
When there is no relaxation processes affecting the expansion modulus, θ is equal to 0, and the surface layer behaves as a purely elastic membrane. When the phase of interfacial tension oscillation is ahead of that of interface area oscillation, the value of the phase angle is positive; otherwise, the value of the phase angle is negative [7].
Results and Discussion
Dynamic Surface Tension of Quaternary Ammonium Bromide Solutions (DTAB and CTAB)
When a fresh interface is created, surfactant molecules will diffuse from the bulk solution to the interface region. The diffusion of surfactant molecules will result in surface tension to decrease from the initial value (γ 0) to the equilibrium value (γ eq) [12]. At low concentration, the surface tension gradually decreases because of the few surfactant molecules adsorbed onto the surface in a short period of time. When the surfactant concentration increases, the surface tension sharply decreases, and the equilibrium time is reduced [30].
The DST of quaternary ammonium bromides with different alkyl chain lengths (DTAB and CTAB) at the air–water interface was monitored. The DST curves for different concentrations of DTAB in aqueous solution are shown in Figure S1(a). The DTAB exhibits a pronounced induction region when the concentration is lower than 1 mM. Moreover, a decrease in the induction region is observed with increasing surfactant concentration. The critical micellar concentration (CMC) of DTAB is 10 mM. When the DTAB concentration is higher than the CMC value, the surface tension of DTAB solution attains the mesoequilibrium value within 200 s.
Two main models are available for the transport and adsorption of surfactant molecules, namely, the diffusion-controlled and mixed kinetic-diffusion models [31]. In the diffusion-controlled model, the diffusion of surfactant molecules from the bulk solution to the subsurface region is the rate-controlling step. When the surfactant molecules diffuse to the subsurface, the adsorption barrier prevents the molecules from being adsorbed into the surface. This barrier will cause diffusion of the surfactant molecules back into the bulk solution. The mixed kinetic-diffusion model assumes that the transfer of surfactant monomers to the interface is the rate-controlling process.
The Ward and Tordai equation accounts for the diffusion of surfactant molecules from the bulk solution to the subsurface, and also the diffusion back from subsurface to the bulk solution [12]. However, at the start of adsorption, the back diffusion can be neglected. Therefore, Eq. 2 can be applied. Figure S2 shows the variation of DST with t 1/2 for different concentrations of DTAB. The short-time experimental kinetics have been fitted according to Eq. 2. When the concentration of DTAB solution is lower than 1 mM, the DST curves for DTAB solutions are in good agreement with Eq. 2. The initial stages of transport and adsorption for surfactant molecules are diffusion-controlled.
A strong deviation from Eq. 2 is observed with increasing surfactant concentration. The adsorption process becomes kinetically controlled and governed by a surface barrier. Figure S3 shows the variation of DST with t −1/2 for different concentrations of DTAB. Following Eq. 3, the effective diffusion coefficients for different concentrations of DTAB are calculated and listed in Table S1. The effective diffusion coefficient of the surfactant molecules decreases with increasing concentration. The adsorption mechanism of surfactants is diffusion-controlled at dilute concentrations, whereas the adsorption process gradually shifts to a mixed kinetic-diffusion control as the surfactant concentration increases. The monomer self-diffusion coefficient (D) of di-(C6-Glu) was obtained by PFGSE-NMR measurements as described by Eastoe et al. [32]. According to the dependence of D on molecular weight, we estimate the value of D to be 4.07 × 10−10 m2 s−1 at 25 °C. The ratio of D eff/D decreases with increasing surfactant concentration, thereby indicating a significant adsorption barrier at high concentration [33].
Moreover, in Figure S4, DST curves can be fitted by the Rosen empirical Eq. (1). As shown in Table S2, values of n and t * for DTAB decrease with increasing surfactant concentration. The values of n represent the rates of diffusion from the bulk to the subsurface at the initial stage of adsorption (t → 0). As the concentrations of the bulk increase, the values of n decrease. More surfactant molecules diffuse from the bulk to the subsurface with increasing concentration, and then the diffusion coefficient increases as well. Therefore, the mesoequilibrium region is easier to achieve. The values of t * represent the rates of diffusion from the subsurface to the surface towards the end of the adsorption (t → ∞). Table S2 shows that the values of t * decrease as the concentration of bulk solution increases. This behavior is consistent with the presence of a weak adsorption barrier during prolonged adsorption.
Compared with DTAB, the surface tension of CTAB reaches equilibrium within less time (Figure S1 (b)). The adsorption rate of cationic surfactants increases as alkyl chain length increases. When CTAB concentration is lower than 0.2 mM, a pronounced induction time is observed. Figure S5 shows the variations of surface tension with t 1/2 for different concentrations of CTAB. When the concentration of CTAB solution is lower than 0.2 mM, the DST curves for CTAB solutions are in good agreement with Eq. 2. In addition, the variations of DST with t −1/2 for different concentrations of CTAB are shown in Figure S6. According to the dependence of D on molecular weight, we estimate the value of D for CTAB to be 3.46 × 10−10 m2 s−1. The effective diffusion coefficients for different concentrations of CTAB are presented in Table S3. The ratio of D eff/D decreases with increasing surfactant concentration, thereby indicating a significant adsorption barrier above the CMC. In Figure S7, the DST curves can be fitted by the Rosen empirical Eq. 1. The values of γ m, n, and t * for CTAB are obtained and listed in Table S4. At the same concentration, the surface tension of CTAB solution is lower than that of DTAB solution. Therefore, quaternary ammonium bromides are more effective in reducing surface tension as alkyl chain length increases.
Dynamic Surface Tension of Sulfonate Gemini Surfactant Solutions
The DST curves were measured at different concentrations of SGS in aqueous solutions (Figure S1 (c)). SGS exhibits a pronounced induction region when the surfactant concentration is lower than 10 μM, whereas the induction region is not observed with a concentration above 10 μM. Moreover, a decrease in the induction region is observed with increasing surfactant concentration. When the SGS concentration is higher than 10 μM, the surface tension of SGS solution attains the mesoequilibrium value within 200 s. Compared with the monomeric surfactants, SGS exhibits a faster adsorption rate and superior surface activity.
Figure S8 shows the variation of DST with t 1/2 for different concentrations of SGS. The short-time experimental kinetics have been fitted according to Eq. 2. When the concentration of SGS solution is lower than its CMC (2.0 × 10−5 mol L−1), the DST curves for SGS solutions are in good agreement with Eq. 2, thereby suggesting that the initial stages of transport and adsorption for surfactant molecules are diffusion-controlled. A strong deviation from Eq. 2 is observed with increasing surfactant concentration. Once the concentration of SGS solution becomes higher than its CMC, the equilibrium surface coverage increases, and the process then becomes kinetically controlled and governed by a surface barrier. Figure S9 shows the variation of DST with t −1/2 for different concentrations of SGS. Following Eq. 3, the effective diffusion coefficients for different concentrations of SGS are calculated and listed in Table S5. The effective diffusion coefficient of the surfactant molecules decreases with increasing surfactant concentration. The adsorption mechanism of surfactants is diffusion-controlled at dilute concentrations, whereas the adsorption process gradually shifts to a mixed kinetic-diffusion control as the surfactant concentration increases. According to the dependence of D on molecular weight, we estimate the value of D to be 2.53 × 10−10 m2 s−1 at 25 °C. The ratio D eff/D decreases with increasing surfactant concentration, thereby indicating a significant adsorption barrier above the CMC [33].
Moreover, DST curves could be modeled by the Rosen empirical Eq. 1 (Figure S10). The two parameters n and t * can be obtained by fitting the DST data to an appropriate linear plot (Eq. 1). As shown in Table S6, values of n and t * for SGS decrease with increasing surfactant concentration.
Dynamic Surface Tension of Catanionic Surfactant Mixtures
SGS/DTAB Mixtures
The DST of catanionic surfactant mixtures were monitored by drop shape analysis. For the SGS/DTAB mixtures, the DTAB concentration is fixed at 2 mM. A series of SGS/DTAB mixtures with different molar ratios are obtained by changing the SGS concentrations. The molar ratios of SGS to DTAB are 1:10, 1:5, 3:10, 2:5, 1:2, 7:10, 9:10, 11:10, 13:10 and 3:2, respectively. The DST curves of SGS/DTAB mixtures with different molar ratios are shown in Figure S11(a). For the SGS/DTAB mixtures, at all mixing ratio, a reduced surface tension and an enhanced surface adsorption are observed. When the molar ratio is 9:10, the mixture shows the best surface activity. The surface tension of the mixture is lower than that of either component. Moreover, the induction region is not observed for the mixture with a molar ratio of 9:10, whereas parent individual ionic surfactants exhibit a pronounced induction region. However, the difference in the dynamic surface tensions of SGS/DTAB mixtures with different molar ratios (from 2:5 to 3:2) is not significant. According to Eq. 1, the values of γ m, n, and t * for the SGS/DTAB mixtures are listed in Table S7. The values of γ m and n for the SGS/DTAB mixture at a molar ratio of 9:10 are less than those of other mixtures. The values of n represent the rates of diffusion from the bulk to the subsurface at the initial stage of adsorption (t → 0). The smaller the values of n are, the faster the diffusion process becomes. This condition indicates the formation of catanionic surfactant complexes, which are generated through the electrostatic interaction between the cationic and anionic surfactants. The formation of catanionic complexes shields the electrostatic repulsion between the surfactant headgroups and lowers the electrostatic adsorption barrier. Therefore, the catanionic surfactant complexes exhibit faster diffusion than either component. Moreover, the values of t * represent the rates of diffusion from the subsurface to the surface towards the end of the adsorption (t → ∞). The larger the values of t * are, the faster the adsorption process becomes. The reduced electrostatic repulsion between these catanionic complexes helps the formation of a denser monomolecular adsorption layer at the air–water interface. When the adsorption layer becomes denser, the adsorption barrier increases. Therefore, the values of t * for the SGS/DTAB mixture at a molar ratio of 9:10 are less than those of other mixtures.
SGS/CTAB Mixtures
We examined the surface tension curves of the SGS/CTAB mixtures with different molar ratios. For the SGS/CTAB mixtures, the CTAB concentration is fixed at 1 mM. The molar ratios of SGS to CTAB are 1:10, 1:5, 3:10, 2:5, 1:2, 7:10, 4:5, 9:10, 1:1, 6:5, 7:5, 8:5 and 2:1. Figure S11(b) shows the DST of SGS/CTAB mixtures with different molar ratios. Unlike the SGS/DTAB mixtures, the surface tension of SGS/CTAB mixtures increases with increasing molar ratio from 1:10 to 1:2. Moreover, the surface tension of SGS/CTAB mixtures increases with increasing SGS concentration. This condition results in the formation of precipitate. Thus, the surface activity of catanionic surfactant mixtures is reduced. However, a reduced surface tension is observed when the molar ratio ranges from 7:10 to 2:1. The surface tension of the mixtures at a molar ratio of 9:10 reaches the minimum value (23.28 mN/m), which is lower than those of the parent individual surfactants. This result indicates the optimum ratio of SGS/CTAB mixture, which is equal to that of SGS/DTAB mixtures. By fitting the DST data to Eq. 1, the values of γ m, n, and t * for the SGS/CTAB mixtures are listed in Table S8. The values of γ m, n, and t * for the SGS/CTAB mixtures at optimum ratio are less than those of other mixtures, which is similar to the SGS/DTAB mixtures. However, the value of γ m for the SGS/CTAB mixtures at a molar ratio of 9:10 is lower than that of SGS/DTAB mixtures at the same molar ratio. With increasing alkyl chain length, the hydrophobic interaction between the cationic and anionic surfactants will increase. Therefore, the catanionic surfactant mixtures are more effective in reducing surface tension with increasing alkyl chain length.
Equilibrium Surface Tension of Catanionic Surfactant Mixtures
The equilibrium surface tensions of pure surfactant solutions and catanionic surfactant mixtures were detected using the du Nouy ring method. As shown in Figure S12, the CMC values of DTAB, CTAB, and SGS solutions are 1.6 × 10−2, 1 × 10−3, and 2 × 10−5 mol L−1, respectively. However, the SGS/DTAB and SGS/CTAB mixtures possess higher surface activity. The CMC values of SGS/DTAB and SGS/CTAB mixtures are 1 × 10−5 and 9 × 10−6 mol L−1, respectively. Meanwhile, the surface tension of SGS/DTAB and SGS/CTAB mixtures can be reduce to ~26 and ~23 mN m−1 with increasing surfactant concentration. Klitzing et al. examined the surface tension isotherms of the SDS/C12TAB (5:5) mixture [34]. They indicated the enhanced surface activity resulting from the formation of catanionic surfactant complexes. In general, the formation of catanionic surfactant complexes will reduce the electrostatic repulsion between the surfactant molecules, thereby leading to a denser monomolecular adsorption layer at the air–water interface (Fig. 2). The SGS/DTAB and SGS/CTAB mixtures have lower CMC values than the SDS/C12TAB mixture (3 × 10−5 mol L−1).
Frequency Dependence of the Surface Dilational Properties
Measuring dilational interfacial rheology of surfactant solutions is an efficient means of evaluating the properties of interfacial films. When the interface is perturbed, different relaxation processes occur, including the diffusion from the bulk to the interface, re-orientation, aggregation, and re-arrangement of surfactant molecules inside the adsorbed layer. Figures 3, 4, and 5 show the effects of dilational frequency ranging from 0.005 to 0.5 Hz on the dilational viscoelasticity of DTAB, CTAB, and SGS, respectively. As the frequency increases, the dilational elasticity at the air–water interface gradually increases, whereas the phase angle decreases. At a low dilational frequency, the low vibration of the interface shape gives the surfactant molecules enough time to diminish the gradient of interfacial tension induced by the compressed interface. As the interfacial tension gradient decreases, the dilational modulus of surfactant molecules decreases. At high dilational frequency, the surfactant molecules will have insufficient time to diminish the interfacial tension gradient, resulting from interface deformation. Therefore, the interfacial film embodies the character of the insoluble film, and the dilational elasticity is higher. At moderate frequency, the dilational modulus at the air–water interfacial increases as the dilational frequency increases.
In Fig. 3a, the curves of lg|ε|–lgω are quasi-linear, and the slope of lg|ε|–lgω is lower than 0.5 at surfactant concentrations below 1 × 10−2 mol L−1 [35]. The slope of the lg|ε|–lgω curve is different when there are different relaxation processes [35]. The slope for the diffusion-controlled relaxation process was less than 0.5. When the surface has a special structure, the limiting slope is 1 [29]. For small surfactant molecules, the characteristic frequency of the diffusion process and rearrangement of interface molecules exceeds the highest frequency used in this experiment (0.5 Hz). Therefore, DTAB has a low frequency dependence of the dilational modulus (Fig. 3a). At 1 × 10−2 mol L−1 (near the CMC), the dilational modulus of DTAB exhibits a high frequency dependence. When the surfactant concentration is near the CMC, there are other slow relaxation processes. In Fig. 3b, the phase angle is positive over the studied concentration range and gradually decreases with increasing dilational frequency. According to Ref. [7], the lower the slope, the more elastic the film appears. The proportion of elasticity is reduced, and the exchange of surfactant molecules between the bulk and the interface increases with increasing surfactant concentration. The phase angle describes the phase difference between the interfacial tension and the interfacial area variations. The positive phase angle occurs when the phase of interfacial tension oscillation is ahead of interfacial area oscillation while the phase angle is negative when the phase of interfacial area oscillation is ahead of interfacial tension oscillation. A high phase angle reflects increased contribution of the viscosity component to the dilational modulus. Moreover, the exchange between the interface and the bulk is responsible for the viscosity of the film [28]. An increase in the dilational frequency adversely affects the diffusion of surfactant molecules [13]. Therefore, the phase angle gradually decreases as the dilational frequency increases.
Figure 4 shows the effects of dilational frequency ranging from 0.005 Hz to 0.5 Hz on the dilational viscoelasticity of CTAB. The effects of dilational frequency and surfactant concentration on the dilational viscoelasticity of CTAB are similar to those of DTAB. When the concentration is lower than 1 × 10−3 mol L−1, the dilational modulus of CTAB has low frequency dependence (Fig. 4). At 1 × 10−3 mol L−1 (above the CMC), the dilational modulus of CTAB exhibits a high frequency dependence.
Figure 5 shows the effects of dilational frequency, which ranged from 0.005 to 0.5 Hz on the dilational viscoelasticity of SGS. As the dilational frequency increases, the dilational modulus of SGS at the air–water interface gradually increases; however, the phase angle decreases [8]. Moreover, the dilational elasticity of SGS is higher than that of DTAB and CTAB. Compared with the conventional single-chain surfactants, gemini surfactants pack more tightly at the air–water interface, resulting from their special molecular structures. Therefore, gemini surfactants have far higher surface activities than single-chain surfactants. The value of the slope of lg|ε|–lgω in Fig. 5a is larger than that of DTAB and CTAB, thereby indicating that other relaxation processes exist. In Fig. 5b, the phase angle is positive in the studied concentration range and gradually decreases with increasing dilational frequency.
Concentration Dependence of the Interfacial Dilational Properties
The effects of bulk concentration on the dilational viscoelasticity of DTAB at 0.1 Hz are shown in Fig. 6. The dilational elasticity peaks at 2 × 10−3 mol L−1, which is less than the CMC values for DTAB (1.6 × 10−2 mol L−1). In general, an increase in bulk concentration has two effects on the dilational elasticity [7]. First, increasing surface concentration will induce a higher interfacial tension gradient of interface deformation, thereby increasing the dilational elasticity. Second, the surfactant molecular exchange between the bulk and the surface increases as the surfactant concentration increases [36]. The diffusion of surfactant molecules from the bulk to the interface may decrease the interfacial tension gradient, thereby resulting in a decrease in the dilational elasticity [8]. Therefore, at a low concentration, the dilational elasticity of DTAB increases, as the concentration increases. When the concentration of DTAB increases to 2 × 10−3 mol L−1, the dilational modulus peaks. A further increase in the bulk concentration mainly results in the increase of the diffusion of DTAB molecules from the bulk to the interface. Therefore, the dilational elasticity gradually decreases with decreasing interfacial tension gradient.
In addition, the dependence of the dilational viscous component of the bulk concentration has similar tendency with dilational elasticity. At concentrations below the CMC, the dilational surface viscosity is approximately zero; therefore, the interfacial films possess only elasticity. In Fig. 6, the dilational viscous component passes through a maximum near the CMC (8 × 10−3 mol L−1). Concurrently, the phase angles significantly increase. Theoretically, the dilational viscous component describes the summation of the various microscopic relaxation processes [16]. Therefore, the influence of the bulk concentration on the dilational viscous component is complex. Generally, the effect of bulk concentration on the dilational viscous component has two aspects. First, an increase of the surface concentration may increase the number of relaxation processes. Second, an increase in the bulk concentration induces a decrease in the surface tension gradient, which can decrease the total dilational modulus [13]. Moreover, the dilational viscous component is reduced. In Fig. 6, the phase angle increases steadily with increasing concentration. The phase angle increases significantly when the concentration rises above the CMC, resulting from the contribution of the fast exchange process between the interfacial surfactant molecules and the micelles near the interface. Simultaneously, this relaxation reduces the dilational modulus sharply.
In addition, the effects of bulk concentration on the dilational viscoelasticity of CTAB at 0.1 Hz are shown in Fig. 6. The dilational elasticity peaks at 4 × 10−5 mol L−1, which is far less than the CMC values for CTAB (1.0 × 10−3 mol L−1). The effects of surfactant concentration on the dilational viscoelasticity of CTAB were similar to those of DTAB. The dilational modulus of CTAB at the air–water interface is higher than that of DTAB, thereby suggesting that the strength against perturbation of an adsorption layer of CTAB is higher than that of DTAB [13]. Figure 6 shows the effect of bulk concentration on the dilational viscoelasticity of SGS. The dilational elasticity peaks at 2 × 10−6 mol L−1, which is less than the CMC value for SGS (2.0 × 10−5 mol L−1). In general, increasing bulk concentration has two effects on the dilational elasticity [7].
The surface elasticity of the SGS/DTAB mixtures at the molar ratio of 9:10 is higher than that of either component, thereby indicating the formation of a denser adsorption layer at the air–water interface. This result is consistent with the findings obtained from the surface tension determination. The formation of catanionic surfactant complexes reduces the electrostatic repulsion between the surfactant molecules, thereby leading to a denser monomolecular adsorption layer at the air–water interface. The strong attraction between the cationic and anionic surfactants causes the increase of dilational elasticity. Moreover, the formation of catanionic surfactant complexes reduces the exchange between the bulk solution and the surface phase [37]. Consequently, the surface elasticity of the SGS/DTAB mixtures increases with increasing surfactant concentration. However, when the concentration of SGS/DTAB is close to or higher than CMC, the dilation elasticity of SGS/DTAB mixtures decreases with increasing surfactant concentration, which is similar to dilation elasticity of pure surfactants. Similar to SGS/DTAB, the dilation elasticity of SGS/CTAB mixtures first increases, and then decreases with increasing surfactant concentration.
Effect of Ratio on the Adsorption Behavior of Catanionic Surfactant Mixture
Interfacial dilational elasticity of SGS/DTAB and SGS/CTAB mixtures at different molar ratios is shown in Fig. 7. The oscillation frequency is 0.1 Hz, and the total surfactant concentration is 5 × 10−5 mol L−1. In Fig. 7, the dilational elasticity of SGS/DTAB mixtures at the molar ratio from 2:5 to 3:2 is higher than that of SGS. As shown in Figure S11(a), the surface tension of SGS/DTAB mixtures at the molar ratio from 2:5 to 3:2 decreases faster than pure SGS. As mentioned above, the formation of catanionic surfactant complexes shields the electrostatic repulsion between the surfactant molecules. Therefore, the catanionic surfactant complexes exhibit a higher adsorption rate than either component. At the air–water interface, the formation of a denser monomolecular adsorption layer results from the reduced electrostatic repulsion between these catanionic complexes [37]. Consequently, the dilational elasticity of catanionic surfactant complexes is higher than that of SGS.
When the molar ratio is 9:10, the maximum dilational elasticity of SGS/DTAB mixtures is approximately 85 mN m−1, whereas the dilational elasticity of SGS is approximately 70 mN m−1. However, the difference in the dilational elasticity of SGS/DTAB mixtures with different molar ratios (from 2:5 to 3:2) is very small. As shown in Figure S11(a), the SGS/DTAB mixtures with the molar ratio of 9:10 possess the highest surface activity compared with other molar ratios. Meanwhile, the difference in the dynamic surface tensions of SGS/DTAB mixtures with different molar ratios (from 2:5 to 3:2) is not significant. The results of dynamic surface tension are consistent with the findings of interfacial dilational properties. Fauser et al. observed a symmetric distribution of the dilational elasticity for the SDS/DTAB mixtures. When the molar ratio is close to 1:1, the surface elasticity of SDS/DTAB mixtures reaches the maximum [34]. In addition, Zhang et al. also proved that the surface adsorption was controlled by the stoichiometric formation of catanionic surfactant complexes and independent of the mixing ratio in the bulk solution [37]. Therefore, when the molar ratio of SGS to DTAB is 9:10, the concentration of catanionic surfactant complexes formed reaches the maximum. For the mixtures of bisquaternary ammonium dibromide (BQAS) and SDS, we also found the dynamic surface activity and dilation elasticity of the mixtures peak at a molar ratio of approximately 1:1 (data not shown). Unlike conventional single-chain surfactant (e.g., DTAB and SDS), gemini surfactants (e.g., SGS and BQAS) have two hydrophilic headgroups. Given the charge neutralization of catanionic surfactant complexes, one gemini surfactant molecule will bind with two single-chain surfactant molecules. The difference between the experimental results and the structure analysis may be attributed to the stronger surface adsorption of SGS compared with DTAB.
For SGS/CTAB mixtures, the effects of molar ratios on the dilational elasticity are similar to that of SGS/DTAB mixtures. Considering the strong hydrophobic interaction, resulting from the longer alkyl chain of CTAB, the dilational elasticity of SGS/CTAB mixtures is slightly higher than that of SGS/DTAB mixtures. However, the dilational elasticity of SGS/CTAB mixtures peaks at the same molar ratio with SGS/DTAB mixtures (9:10), thereby suggesting that the stoichiometric formation of catanionic surfactant complexes may be independent of the length of alkyl chain. Consequently, as shown in Fig. 2, the catanionic surfactant complexes are generated through the electrostatic interaction between the cationic and anionic surfactants.
Conclusions
The dynamic surface tension of sulfonate gemini surfactant (SGS) and its mixtures with quaternary ammonium bromides (DTAB, CTAB) at the air–water interface were examined. Results suggest that the adsorption mechanism gradually shifts to a mixed kinetic-diffusion control with increasing surfactant concentration. The mixtures possess the best surface activity when the molar ratios of SGS/DTAB and SGS/CTAB mixtures are 9:10. The values of γ m, n, and t * for these mixtures at optimum ratios are less than those of other mixtures. The formation of catanionic complexes shields the electrostatic repulsion and lowers the electrostatic adsorption barrier. Consequently, these catanionic surfactant mixtures exhibit a higher adsorption rate than either component. The interfacial dilational rheology of SGS and its mixtures with quaternary ammonium bromides (DTAB, CTAB) at the air–water interface were investigated using drop shape analysis. As the oscillating frequency increases, the dilational elasticity of these surfactants increases gradually. The dilational elasticity peaks at a certain concentration, which is less than the critical micelle concentration. The addition of SGS induces an obvious increase in the dilational elasticity of DTAB or CTAB, which results from the formation of a denser monomolecular adsorption layer at the air–water interface.
References
Rosen MJ, Song LD. Dynamic surface tension of aqueous surfactant solutions 8. Effect of spacer on dynamic properties of gemini surfactant solutions. J Colloid Interface Sci. 1996;179:261–8.
Touhami Y, Hornof V, Neale GH. Dynamic interfacial tension behavior of acidified oil/surfactant-enhanced alkaline systems 1. Experimental studies. Colloids Surf A. 1998;132:61–74.
Chang H, Wang Y, Cui Y, Li G, Zhang B, Zhao X, Wei W. Equilibrium and dynamic surface tension properties of Gemini quaternary ammonium salt surfactants with hydroxyl. Colloids Surf A. 2016;500:230–8.
Erk KA, Martin JD, Schwalbe JT, Phelan FR, Hudson SD. Shear and dilational interfacial rheology of surfactant-stabilized droplets. J Colloid Interface Sci. 2012;377:442–9.
Karbaschi M, Lotfi M, Krägel J, Javadi A, Bastani D, Miller R. Rheology of interfacial layers. Curr Opin Colloid Interface Sci. 2014;19:514–9.
Pang J, Du S, Chang R, Pei Q, Cui D. Interfacial rheology of mixed surfactants at the oil/water interface. J Surfactants Deterg. 2015;18:747–53.
Tong L, Bao M, Li Y, Gong H. Interfacial dynamic and dilational rheology of polyelectrolyte/surfactant two-component nanoparticle systems at air–water interface. Appl Surf Sci. 2014;316:147–54.
Burke J, Cox A, Petkov J, Murray BS. Interfacial rheology and stability of air bubbles stabilized by mixtures of hydrophobin and β-casein. Food Hydrocoll. 2014;34:119–27.
Reichert MD, Alvarez NJ, Brooks CF, Grillet AM, Mondy LA, Anna SL, Walker LM. The importance of experimental design on measurement of dynamic interfacial tension and interfacial rheology in diffusion-limited surfactant systems. Colloids Surf A. 2015;467:135–42.
Langevin D. Influence of interfacial rheology on foam and emulsion properties. Adv Coll Interface Sci. 2000;88:209–22.
Maldonado-Valderrama J, Martín-Rodriguez A, Gálvez-Ruiz MJ, Miller R, Langevin D, Cabrerizo-Vílchez MA. Foams and emulsions of β-casein examined by interfacial rheology. Colloids Surf A. 2008;323:116–22.
Cao XL, Feng J, Guo LL, Zhu YW, Zhang L, Zhang L, Luo L, Zhao S. Dynamic surface dilational properties of anionic Gemini surfactants with polyoxyethylene spacers. Colloids Surf A. 2016;490:41–8.
Wu D, Feng Y, Xu G, Chen Y, Cao X, Li Y. Dilational rheological properties of gemini surfactant 1, 2-ethane bis (dimethyl dodecyl ammonium bromide) at air/water interface. Colloids Surf A. 2007;299:117–23.
Bos MA, Vliet TV. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Coll Interface Sci. 2001;91:437–71.
Grigoriev DO, Derkatch S, Krägel J, Miller R. Relationship between structure and rheological properties of mixed BSA/Tween 80 adsorption layers at the air/water interface. Food Hydrocoll. 2007;21:823–30.
Zhang L, Wang XC, Yan F, Luo L, Zhang L, Zhao S, Yu J-Y. Interfacial dilational properties of partly hydrolyzed polyacrylamide and gemini surfactant at the decane–water interface. Colloid Polym Sci. 2008;286:1291–7.
Rao A, Kim Y, Kausch CM, Thomas RR. Effect of binding of an oligomeric cationic fluorosurfactant on the dilational rheological properties of gelatin adsorbed at the air-water interface. Langmuir ACS J Surf Colloids. 2006;22:7964–8.
Noskov BA. Dilational surface rheology of polymer and polymer/surfactant solutions. Curr Opin Colloid Interface Sci. 2010;15:229–36.
Kume G, Gallotti M, Nunes G. Review on anionic/cationic surfactant mixtures. J Surfactants Deterg. 2008;11:1–11.
Fan H, Li B, Yan Y, Huang J, Kang W. Phase behavior and microstructures in a mixture of anionic Gemini and cationic surfactants. Soft Matter. 2014;10:4506–12.
Xu H, Liu B, Kang P, Bao X. Properties of a binary system containing anionic and cationic gemini surfactants. J Surfactants Deterg. 2015;18:297–302.
Liu Z, Chai J, Chai Z, Liu N, Chai H, Zhang H. Interfacial composition, solubility, and solubilization capacity of microemulsions containing cationic gemini and anionic surfactants. J Chem Eng Data. 2014;59:2230–4.
Parekh P, Varade D, Parikh J, Bahadur P. Anionic–cationic mixed surfactant systems: micellar interaction of sodium dodecyl trioxyethylene sulfate with cationic gemini surfactants. Colloids Surf A. 2011;385:111–20.
Lai L, Mei P, Wu XM, Hou C, Zheng YC, Liu Y. Micellization of anionic gemini surfactants and their interaction with polyacrylamide. Colloid Polym Sci. 2014;292:2821–30.
Göbel J, Joppien G. Dynamic interfacial tensions of aqueous triton X-100 solutions in contact with air, cyclohexane, n-Heptane, and n-Hexadecane. J Colloid Interface Sci. 1997;191:30–7.
Liu J, Messow U. Diffusion-controlled adsorption kinetics at the air/solution interface. Colloid Polym Sci. 2000;278:124–9.
Li Y, Xu G, Xin X, Cao X, Wu D. Dilational surface viscoelasticity of hydroxypropyl methyl cellulose and CnTAB at air–water surface. Carbohyd Polym. 2008;72:211–21.
Zhang L, Wang XC, Gong QT, Zhang L, Luo L, Zhao S, Yu JY. Interfacial dilational properties of tri-substituted alkyl benzene sulfonates at air/water and decane/water interfaces. J Colloid Interface Sci. 2008;327:451–8.
Wang H, Gong Y, Lu W, Chen B. Influence of nano-SiO2 on dilational viscoelasticity of liquid/air interface of cetyltrimethyl ammonium bromide. Appl Surf Sci. 2008;254:3380–4.
Zhmud B, Tiberg F, Kizling J. Dynamic surface tension in concentrated solutions of CnEm surfactants: a comparison between the theory and experiment. Langmuir. 2000;16:2557–65.
Ferri JK, Stebe KJ. Which surfactants reduce surface tension faster? A scaling argument for diffusion-controlled adsorption. Adv Coll Interface Sci. 2000;85:61–97.
Eastoe J, Dalton JS, Rogueda PG, Griffiths PC. Evidence for activation-diffusion controlled dynamic surface tension with a nonionic surfactant. Langmuir. 1998;14:979–81.
He Y, Yazhgur P, Salonen A, Langevin D. Adsorption–desorption kinetics of surfactants at liquid surfaces. Adv Coll Interface Sci. 2015;222:377–84.
Fauser H, Uhlig M, Miller R, et al. Surface adsorption of oppositely charged SDS:C12TAB mixtures and the relation to foam film formation and stability. J Phys Chem B. 2015;119(40):12877–86.
Jiang XM, Zhang L, Zhang WQ, Zhao S. Dilational properties of an anionic gemini surfactant with a hydrophobic spacer. J Surfactants Deterg. 2015;18:41–5.
Feng J, Liu XP, Zhang L, Zhao S, Yu JY. Dilational properties of anionic gemini surfactants with polyoxyethylene spacers at water–air and water–decane interfaces. Langmuir. 2010;26:11907–14.
Wang C, Cao XL, Guo LL, et al. Effect of molecular structure of catanionic surfactant mixtures on their interfacial properties. Colloids Surf A Physicochem Eng Asp. 2016;509:601–12.
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (grant nos. 21403017, 51404039), National Science Fund for Distinguished Young Scholars of China (grant no. 21225313), PetroChina Innovation Foundation (grant no. 2014D-5006-0309).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lai, L., Mei, P., Wu, XM. et al. Interfacial Dynamic Properties and Dilational Rheology of Sulfonate Gemini Surfactant and its Mixtures with Quaternary Ammonium Bromides at the Air–Water Interface. J Surfact Deterg 20, 565–576 (2017). https://doi.org/10.1007/s11743-017-1954-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-1954-8