Abstract
Surfactant adsorption films at the air/water interface, which usually exert a significant influence over system behaviour, are closely related to the surfactant molecular structure. In this research, a series of Gemini surfactants containing four hydroxyl groups at the head groups, namely: alkanediyl-α,ω-bis[di(2-hydroxylethyl) dodecylammonium bromide], abbreviated to 12(2OH)-s-12(2OH), where s = 3, 6, 8 and 10, respectively, were investigated by using interfacial dilatational rheological measurements. The effects of oscillating frequency, bulk concentration and spacer length on the dilatational behaviour of 12(2OH)-s-12(2OH) were investigated. The variation of dilatational modulus of 12(2OH)-8-12(2OH) with concentrations showed two peaks which were attributed to different configurations adopted by the spacer, revealing the effects of dynamic configuration of the spacer on surfactant interfacial adsorption. The fit to the results, with that data available, using the Lucassen/van den Tempel (LVT) model showed that the presence of hydroxyl groups near the head groups of Gemini surfactants enhanced the interfacial elasticity of the adsorption films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants can adsorb at fluid interfaces to form adsorption monolayers, which are important for the stabilisation of emulsions or foams. The resistance to the disturbance of the films arises from the dynamic equilibrium between surfactant molecules in the bulk solution and surfaces. With increasing focus on the application of emulsions [1–3] and foams [4–7], it is essential to enrich the knowledge regarding the structure and dynamic behaviour of allied adsorption films. Since the adsorption, dispersion and even conformation of surfactant molecules can be revealed by the interfacial rheological properties of adsorption films [8, 9], the surface dilatational measurements thus become useful and important techniques for investigating surfactant film structures and their dynamic behaviour.
Gemini surfactants, which refer to a new type of surfactants following work by Menger [10], have received considerable research attention during recent years. Compared with traditional surfactants, Gemini surfactants are made up of two hydrophilic head groups, two hydrophobic tails and a spacer linked at, or near, the head groups. They display unique solution behaviour and rich aggregation morphologies and offer new areas of application, which expand the conception of surfactants [11, 12]. In a Gemini surfactant molecule, the spacer is the key structural element. The length [13], rigidity [10, 14, 15] and stereochemistry [16, 17] of the spacer affect the self-assembly of Gemini surfactants. Once an adsorption film has been formed by a Gemini surfactant, the spacer is expected to affect the interfacial dilatational behaviour because the position of the spacer is close to the head group of the surfactant and is situated within the adsorption layer. Although Gemini surfactants have been applied in many foam and emulsion systems [18–22], research into the adsorption films formed by Gemini surfactants using surface dilatational measurements has been reported recently. Xu et al. [23] investigated the dilatational rheological properties of a Gemini surfactant 1,2-ethane bis(dimethyl dodecyl ammonium bromide). The results showed that the strength of the interfacial layer is greater than that of the dodecyltrimethyl ammonium bromide. They also revealed that Gemini surfactant has a stronger binding ability with gelatin than that of DTAB [24]. Zhang et al. [25] studied anionic Gemini surfactants with polyoxyethylene spacers. The number of ethylene oxide groups was found to influence the nature of the interfacial film. This phenomenon indicates that the adsorption films formed by Gemini surfactants have properties that differ from those of traditional surfactants. Due to the rather complex molecular structure of Gemini surfactants, the intermolecular interactions among multiple groups are thus involved, which resulted in sophisticated adsorption film structures and new film properties.
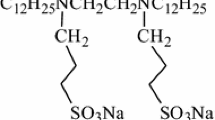
Hydrogen bonds are important molecular interactions involved in the self-assembly of surfactants. When hydroxyl groups are introduced into the molecular structure of a Gemini surfactant [26–30], the resultant hydrogen bonding between molecules can affect their aggregation behaviour. The stronger adsorption at the air/water interface [31] and a stronger ability to form wormlike micelles [32] have been verified for Gemini surfactants containing hydroxyl groups. Since the adsorption film properties have a close relationship to foam stability, Zhao et al. [33] have investigated three Gemini surfactants containing hydroxyl groups at the spacer. The results obtained by surface dilatational measurements showed that the presence of hydrogen bonds indeed improved the foam stability formed by these surfactants. However, the dilatational properties of the adsorption films formed by Gemini surfactants containing hydroxyl groups have been little researched, to date. A deeper understanding of the self-assembly and dynamic behaviour of such surfactants needs more sample systems. In the present work, we investigated a series of Gemini surfactants containing four hydroxyl groups at the head groups (Fig. 1), namely alkanediyl-α,ω-bis[di(2-hydroxylethyl) dodecylammonium bromide], abbreviated as 12(2OH)-s-12(2OH), where s = 3, 6, 8 and 10, respectively, using surface dilatational measurements. Gemini surfactants with such a structure provided an opportunity to investigate the effect of spacer length and hydroxyl groups on the properties of those adsorption films formed.
Experimental
Materials
1-bromododecane, diethanolamine, 1,3-dibromopropane, 1,6-dibromopropane, 1, 8-dibromopropane and 1,10-dibromodecane were all purchased from Aladdin Reagent Co., Ltd, China. The 12(2OH)-s-12(2OH), where s = 3, 6, 8 and 10, respectively, was synthesised in our laboratory.
Synthesis
An improved method of surfactant synthesis, differing from that reported by Wang et al. [30], was used. The general procedure may be described as follows: 1-bromododecane and excessive diethanolamine were reacted at 60 °C for 48 h. After addition of alkaline water, the raw product was extracted with ether. The extracts were combined and the ether was removed. The residue was distilled under vacuum to get the N-dodecyldiethanolamine as a viscous liquid. The corresponding dibromoalkane was mixed with N-dodecyldiethanolamine at a ratio of 1:2.5 in an autoclave. After addition of small amounts of ethanol, the reaction was carried out at 110 °C for 120 h. After cooling, the resulting mixture was recrystallised from ethanol/ethyl acetate or ethyl acetate three times to obtain the final product. The molecular structure and purity were confirmed by 1HNMR and elemental analysis provided in Supporting Information.
Methods
The dilatational properties of 12(2OH)-s-12(2OH) were measured by using an optical angle meter OCA-20 with oscillating drop accessory ODG-20. The variation of the relative area (A) was set at about 6 %, and the accessible frequency ranged from 0.01 to 1 Hz. The photo was taken at a speed of 250 frame per second by a CCD camera, from which the changes in interface tension (dγ) were calculated. The interfacial dilatational modulus was then obtained from ε * = dγ/AdA. The phase angle (θ) was acquired by Fourier transform analysis. The dilatational elasticity and dilatational viscosity were calculated based on Eqs. (4) and (5), respectively, where ω = 2πv, and v is the frequency of sinusoidal oscillation.
Theory
The Gibbs interfacial dilatational modulus, ε *, is defined as follows:
where γ represents the interfacial tension, and A indicates the interfacial area. Actually, ε * contains two components and can then be presented as a complex number,
where ε is an elastic component, accounting for dilatational elasticity stored in the interface, η represents a loss modulus accounting for the energy dissipated in the relaxation process and is also called dilatational viscosity. ω is the oscillating frequency. θ is the phase angle which relates to the rate of dilatational elasticity and dilatational viscosity and can be calculated as follows:
The dilatational elasticity ε and dilatational viscosity η can be calculated from the absolute value of dilatational modulus |ε*| and phase angle θ, respectively.
Results and discussion
Influence of oscillating frequency
It is seen that all surfactants investigated showed similar dilatational behaviours with frequency. In brief, only the variations of dilatational parameters with oscillation frequency of 12(2OH)-3-12(2OH) were discussed.
The surfactant molecules in the interfacial adsorption layers are in a dynamic equilibrium with the ones in bulk. When an external force changes the surface area, a surface tension gradient is produced, and the surfactant molecules in the adsorption layer have either to diffuse from the bulk to the interface, or vice versa, to decrease the gradient. At low frequencies, the surfactant molecules have enough time to respond to the disturbance by the diffusion between the surface and bulk or by the rearrangement in the monolayer. As a result, the interfacial elasticity, which is mainly related to the energy deviation of the surfactants from their equilibrium state, gives a rather low value. While at high frequencies, it is difficult for the surfactant molecules to restore themselves to the equilibrium state due to the correspondingly short time intervals. The monolayer thus behaves in a more elastic fashion. This can be seen from in Fig. 2b and in other interfacial systems [34], where the interfacial elasticity increases with frequency over the range examined; however, interfacial viscosity decreases with increasing frequency for those concentrations shown (Fig. 2c). According to reported results, the effect of dilatational frequency on the interfacial viscosity is rather complex. With an increase in the frequency, the interfacial viscosity may decrease, increase or yield an extreme value [35, 36] because the interfacial viscosity originates from multiple relaxation processes such as diffusion or molecular rearrangement within the monolayer. The viscosity behaviour of 12(2OH)-3-12(2OH) shown here indicates that the relaxation of interfacial films is dominated by some slow processes. The characteristic frequency of the relaxation may be smaller than the examined frequencies [36]. Due to the action with a rather complex molecular structure, the behaviour of Gemini surfactant 12(2OH)-3-12(2OH) in an adsorbed monolayer is more complicated than that of single tailed surfactants. The relaxation processes thus become slower. The complex modulus behaves in a fashion almost the same as the interfacial elasticity, indicating that the adsorption films are elastic. Besides, all phase angles swept during the examined frequency range are positive, revealing that the surface tension change leads to the surface area change.
Experimental plots of complex modulus (ε *, a), interfacial elasticity (ε, b), interfacial viscosity (η, c) and phase angle (θ, d) as a function of frequency (v), respectively, for 12(2OH)-3-12(2OH) aqueous solutions at 25 °C. The symbols represent different surfactant concentrations: log(C/mmol L−1) = 1.60(square), 1.40(circle), 1.20(triangle), 1.00(inverted triangle), 0.80(diamond), 0.60(left-pointing triangle) and 0.40(right-pointing triangle)
Influence of concentrations
The variation of interfacial elasticity and viscosity of 12(2OH)-3-12(2OH) with concentration is shown in Fig. 3. Both 12(2OH)-6-12(2OH) and 12(2OH)-10-12(2OH) show similar behaviour (Fig. S4 and Fig. S7: Supporting Information). A maximum appears for both interfacial elasticity and viscosity with the increase of the concentration for all examined frequencies. The increase of concentration in bulk solution usually exerts two effects on the dilatational properties of an adsorption film: one is to cause an increase of surfactant concentration on the interface, which shortens the distance between surfactant molecules at the interface and makes molecular interactions stronger, contributing to the dilatational elasticity; the other is to accelerate the diffusion rate of the surfactant molecules between the bulk and the surface so that the local surface tension gradient caused by the extra disturbance is quickly reduced. This will lead to a decrease in surface elasticity. The two contrary effects in combination produce a maximum elasticity with increasing surfactant concentration.
Semi-logarithmic plots of dilational interfacial elasticity (ε, a) and interfacial viscosity (η, b) of 12(2OH)-3-12(2OH) as a function of the concentration C for different frequencies. The symbols represent v/Hz = 0.015(square), 0.046(circle), 0.100(triangle), 0.464(inverted triangle) and 1.000(diamond)
The interfacial viscosity is also affected by increasing surfactant concentration. As stated above, the interfacial viscosity is related to multiple relaxation processes involving diffusion and rearrangement of the molecules. The accelerated diffusion rate and increased concentration on the surface can enhance these relaxation processes, which promotes interfacial viscosity at low concentrations, while the decreased surface tension gradient may cause a decrease in the dilatational modulus and in the dilatational viscous component [36]. As a result, a maximum appeared in the interfacial viscosity with increasing concentration.
Noticeably, the dilatational behaviour of 12(2OH)-8-12(2OH) with increasing concentration differs somewhat from that of other surfactants (Fig. 4). The dilatational modulus is resolved into elastic and viscous moduli. For both curves, there were two maxima at lower frequencies, which admit the occurrence of multiple relaxation processes owing to the longer time intervals between external disturbances. In fact, such dilatational behaviour has been discovered for some nonionic surfactants [37] and a Gemini surfactant with an ethylene oxide chain as the spacer [25]. The reason is attributed to the reorientation and compression of ethylene oxide chains in the interfacial layer [25]; however, the Gemini surfactant 12(2OH)-8-12(2OH) does not have a long ethylene oxide chain as either a hydrophilic head group or spacer; the explanation above may not be suitable. Zana et al. investigated the adsorption behaviour of alkanediyl-α,ω-bis(dimethylalkylammonium bromide) Gemini surfactants at an air/water interface [38]. The results show that the molecular occupation area reaches a maximum when the number of carbon atoms in the spacer (s) is between 10 and 12. The reason for this is attributed to the different configurations adopted by the spacer at low and high s values. At low s values, the spacer is more or less shown to stretch at the air/water interface. While when s ≥ 10, the spacer adopts a folded, wicket-like conformation. This reveals that, besides chain length and chemical structure, the configuration adopted by the spacer in different conditions is also an important factor affecting the adsorption behaviour of surfactants. As a result, the unique dilatational properties of 12(2OH)-8-12(2OH), which has a spacer composed of a polymethylene chain, may be explained by the configuration variation of the spacers.
Semi-logarithmic plots of dilational interfacial elasticity (ε, a) and interfacial viscosity (η, b) of 12(2OH)-8-12(2OH) as a function of the concentration C for different frequencies. The symbols represent v/Hz = 0.015(square), 0.046(circle), 0.100(triangle), 0.464(inverted triangle) and 1.000(diamond)
The first maximum in the interfacial elasticity-concentration curves (Fig. 4a) is caused by increasing concentration. Compared with that at s = 3 or 6, the maximum of 12(2OH)-8-12(2OH) shifts to lower concentrations. Similar behaviour is observed for 12(2OH)-10-12(2OH) (Supporting Information, Fig. S7), indicating a stronger adsorption at the air/water interface. It means that 12(2OH)-s-12(2OH) becomes more hydrophobic with the increase of spacer length. This is in good agreement with Zana et al. [38], in which the spacer becomes sufficiently hydrophobic when s ≥ 10. Obviously, the hydrophobicity of 12(2OH)-8-12(2OH) is in an intermediate state. The spacer configuration adopted by 12(2OH)-8-12(2OH) is thus sensitive to external stimulus, which is the origin of the second maximum.
At equilibrium, the spacer of 12(2OH)-8-12(2OH) generally maintains a stretched configuration at the air/water interface. However, once an oscillation is applied to the interface, the molecules are forced to change their configurations and the distances between them to respond to the periodical area changes. The information on molecular interactions can thus be obtained from the oscillation measurements. It is suggested that in such a dynamic processes, the stretched spacer of 12(2OH)-8-12(2OH) has opportunities to overcome the energy barrier to be curved towards the air side of the interface due to its hydrophobicity, and the two alkyl chains are drawn close because of the curved spacer. The interactions between molecules are thus enhanced, which contributes to the elasticity of the interfacial film. This additional process is superimposed on the original processes to produce the second maximum. For 12(2OH)-10-12(OH), the spacer has already been curved at its equilibrium state. Furthermore, the configuration of the spacer is not sensitive to the oscillation caused areal changes to the interface. In other words, the configuration adopted by the sufficiently long spacers has no strong need to change in response to oscillation because they have already been curved. As a result, there is no obvious second maximum in the elasticity-concentration curves, and only an increasing tendency of elasticity is observed at higher concentrations (Supporting Information, Fig. S7).
The configuration adopted by the spacer is relative to the distance between the two head groups of a Gemini surfactant, which is dependent on the electrostatic repulsion. Under normal equilibrium conditions, the electrostatic repulsion is fixed. While in the presence of extra electrolyte, the repulsion can be significantly decreased. Thus, with the distance between the head groups being shortened, the originally stretched spacer of 12(2OH)-8-12(OH) at the air/water interface should have already become curved in its equilibrium state, and the second maximum that originated from the spacer configuration change will be weakened. This assumption has been identified by measuring the dilatational properties of 12(2OH)-8-12(OH) in the presence of 50 mmol L−1 NaBr (Fig. 5), where the second maximum vanishes upon the addition of NaBr. Besides, the first maximum shifts to much lower concentrations because the adsorption of surfactants is enhanced by the increased ionic strength of such solutions.
Semi-logarithmic plots of dilational interfacial elasticity (ε, a) and interfacial viscosity (η, b) of 12(2OH)-8-12(2OH) with 50 mmol L–1 NaBr as a function of the concentration C for different frequencies. The symbols represent v/Hz = 0.015(square), 0.046(circle), 0.100(triangle), 0.464(inverted triangle) and 1.000(diamond)
Effects of spacer length and hydrogen bonds on the high frequency limit of the elasticity
The interfacial elasticity is closely related to the stability of emulsions and bubbles [19, 39–42]. A tight packing of surfactant molecules at interfaces facilitates the generation of high interfacial elasticity. Compared with traditional surfactants [41], Gemini surfactants can form more compact films at interfaces, which usually exhibit high interfacial elasticity [19, 21], and the interfacial elasticity may be affected by the spacer. Since the interfacial elasticity depends on both the frequency and the concentration, the relationship between the spacer length and the interfacial elasticity appears to be more complex. To exclude the effect of frequency, the elasticity at high frequency may be a suitable parameter for comparison. However, the elasticity at high frequency usually cannot be obtained under normal experimental conditions. One feasible method is to obtain the elasticity at high frequency based on the data at low frequencies using the LVT model [8, 43], which is based on the assumptions that the adsorption is governed only by diffusion processes, and has no energy barriers. The model describes the interfacial moduli as follows:
where ε 0 is the theoretically fitted interfacial elasticity at high frequency, and ω 0 is the molecular exchange parameter.
The variations of ε 0 with concentration for 12(2OH)-s-12(2OH) are shown in Fig. 6. It was observed that the high frequency limit of the elasticity ε 0 increases initially, reaches a maximum and then decreases with increasing concentration. Similar to the interfacial elasticity at low frequencies, the appearance of the maximum is caused jointly by increased interfacial concentrations and the decreased surface tension gradients. The value of ε 0 is 91.0 and 97.0 mN m−1 for 12(2OH)-3-12(2OH) and 12(2OH)-6-12(2OH) systems, respectively, both of which are almost equal. While for a 12(2OH)-8-12(2OH) system, the value of ε 0 decreases to 65.1 mN m−1. Further increases in spacer length resulted in an increase in ε 0. A ε 0 value of 113.0 mN m−1 was found for the 12(2OH)-10-12(2OH) system. This indicated that Gemini surfactants with both short (s = 3 or 6) and long (s = 10), spacers can produce a high interfacial elasticity. The reason for this is attributed to the effect of spacer length on the packing of molecules at interfaces. A Gemini surfactant with a short spacer usually has a smaller occupation area at interfaces than that with longer ones [38], which then forms dense films with enhanced molecular interactions between molecules in the films. As the spacer length increases, the packing density of alkyl chains at the interface decreases, and the molecular interactions are thus weakened. However, when the spacer is long enough to curve towards the air side, such as in the Gemini surfactant 12(2OH)-10-12(2OH), the curved spacer can interact with the alkyl chains of the surfactant and serve as the hydrophobic part. The molecular interaction in the films is enhanced again and thus results in an increased interfacial elasticity ε 0.
As a directive molecular interaction, hydrogen bonds facilitate the aggregation of surfactants [26] in solutions and dense packing at the air/water interfaces [31]. Such additional molecular interactions are expected to promote the increase of interfacial elasticity. This fact has been proven by comparing the behaviours of 12-3-12 and 12(2OH)-3-12(2OH), as shown in Fig. 7. Although both have the same spacer, the limited interfacial elasticity of the later (with four hydroxyl groups) is generally higher than that of the former (without hydroxyl group). Similar behaviour was observed by Zhao et al. [33], where a Gemini surfactant 12-3(OH)-12 with one hydroxyl group in spacer shows stronger limiting interfacial elasticity than that of 12-3-12 without a hydroxyl group. By comparing the head group structure of 12-3-12, 12-3(OH)-12 and 12(2OH)-3-12(2OH), it is clear that the presence of hydroxyl groups near the surfactant head groups is a main factor for promoting the interfacial elasticity of surfactant adsorption films. This observation is helpful for designing surfactants as stabilisers of emulsions and foams.
Conclusions
The dilatational properties of a series of Gemini surfactants containing four hydroxyl groups at the head groups, namely alkanediyl-α,ω-bis[di(2-hydroxylethyl) dodecylammonium bromide], were investigated. The spacer length exerts a significant effect on the properties of the interfacial films because of its involvement in the molecular interactions at the air/water interface. The variation of dilatational modulus of 12(2OH)-8-12(2OH) with concentration presents two peaks, as caused by the configuration changes in the spacer. This indicates that, besides the molecular structure, the dynamic configuration adopted by any specific part of surfactants is also a key factor affecting interfacial viscoelasticity. By comparing the limited interfacial elasticity of 12-3-12 without hydroxyl group and 12(2OH)-3-12(2OH) with four hydroxyl groups, it is obvious that the presence of hydroxyl groups enhances the molecular interactions at the interface and promotes the interfacial elasticity. The results explored are helpful for improving our understanding of the dilatational behaviour of Gemini surfactants. The aforementioned surfactants are also expected to have potential applications in the preparation of stable emulsion and foam systems.
References
Zhai JL, Day L, Aguilar MI, Wooster TJ (2013) Curr Opin Colloid Interface Sci 18:257
Alam MM, Aramaki K (2014) J Oleo Sci 63:97
Schmitt V, Ravaine V (2013) Curr Opin Colloid Interface Sci 18:532
Denkov ND, Tcholakova S, Golemanov K, Ananthpadmanabhan KP, Lips A (2009) Soft Matter 5:3389
Kristen N, Klitzing RV (2010) Soft Matter 6:849
Wilde PJ (2000) Curr Opin Colloid Interface Sci 5:176
Huerre A, Miralles V, Jullien MC (2014) Soft Matter 10:6888
Lucassen J, Van Den Tempel M (1972) Chem Eng Sci 27:1283
Monroy F, Rivillon S, Ortega F, Rubio RG (2001) J Chem Phys 115:530
Menger FM, Littau CA (1991) J Am Chem Soc 113:1451
Han SH, Xu J, Hou WG, Yu XM, Wang YS (2004) J Phys Chem B 108:15043
Guo X, Szoka FC (2003) Acc Chem Res 36:335
Zana R (2002) J Colloid Interface Sci 248:203
Menger FM, Littau CA (1993) J Am Chem Soc 115:10083
Song BL, Hu YF, Zhao JX (2009) J Colloid Interface Sci 333:820
Bello C, Bombelli C, Borocc S, Profio PD, Mancini G (2006) Langmuir 22:9333
Shankar BV, Patnaik A (2007) J Phys Chem B 111:11419
Kim TS, Kida T, Nakatsuji Y, Hirao T, Ikeda I (1996) J Am Oil Chem Soc 73:907
Espert A, Klitzing RV, Poulin P, Colin A, Zana R, Langevin D (1998) Langmuir 14:4251
Pinazo A, Perez L, Infante MR, Franses EI (2001) Colloid Surf A 89:225
Acharya DP, Gutierrez JM, Aramaki K, Aratani K, Kunieda H (2005) J Colloid Interface Sci 291:236
Tehrani-Bagha AR, Holmberg K (2010) Langmuir 26:9276
Wu D, Feng YJ, Xu GY, Chen YJ, Cao XR, Li YM (2007) Colloid Surf A 299:117
Wu D, Xu GY, Feng YJ, Li YM (2007) Int J Biol Macromol 40:345
Feng J, Liu XP, Zhang L, Zhao S, Yu JY (2010) Langmuir 26:11907
Borse MS, Devi S (2004) Colloid Surf A 245:1
Borse M, Sharma V, Aswal VK, Pokhriyal NK, Joshi JV, Goyal PS, Devi S (2004) PCCP 006:3508
Sharma V, Borse M, Aswal VK, Pokhriyal NK, Joshi JV, Goyal PS, Devi S (2004) J Colloid Interface Sci 277:450
Borse M, Sharma V, Aswal VK, Goyal PS, Devi S (2005) J Colloid Interface Sci 284:282
Huang X, Han YC, Wang YX, Cao MW, Wang YL (2008) Colloid Surf A 325:26
Song BL, Shang SB, Song ZQ (2012) J Colloid Interface Sci 382:53
Pei XM, Zhao JX, Ye YZ, You Y, Wei XL (2011) Soft Matter 7:2953
Wu XN, Zhao JX, Li EJ, Zou WS (2011) Colloid Polym Sci 289:1025
Zhang HX, Xu GY, Wu D, Wang SW (2008) Colloid Surf A 317:289
Huang YP, Zhang L, Zhang L, Luo L, Zhao S, Yu JY (2007) J Phys Chem B 111:5640
Liu K, Zhang L, Cao XL, Song XW, Luo L, Zhang L, Zhao S (2011) J Chem Eng Data 56:3925
Fainerman VB, Aksenenko EV, Lylyk SV, Makievski AV, Ravera F, Petkov JT, Yorke J, Miller R (2009) Colloid Surf A 334:16
Alami E, Beinert G, Marie P, Zana R (1993) Langmuir 9:1465
Stubenrauch C, Miller R (2004) J Phys Chem B 108:6412
Sonin AA, Bonfillon A, Langevin D (1994) J Colloid Interface Sci 162:323
Bergeron V (1997) Langmuir 13:3474
Santini E, Ravera F, Ferrari M, Stubenrauch C, Makievski A, Kragel J (2007) Colloid Surf A 298:12
Lucassen J, Van Den Tempel M (1972) J Colloid Interface Sci 41:491
Acknowledgments
Support from the National Natural Science Foundation of China (31300486, 21203078), the Natural Science Foundation of Jiangsu Province (BK20130128) and the Fundamental Research Funds for the Central Universities (JUSRP1017) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1527 kb)
Rights and permissions
About this article
Cite this article
Pei, X., Zhang, Q., Liu, Z. et al. Surface dilatational properties of Gemini surfactants containing multiple hydroxyl groups. Colloid Polym Sci 294, 1405–1412 (2016). https://doi.org/10.1007/s00396-016-3892-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3892-9