Abstract
The interfacial rheology of surfactant mixtures (SBT and Tween® 80) at the oil/water interface is investigated using toluene as a model oil. The surfactant ratio in the mixed system has an important impact on the interfacial properties. After adding Tween® 80, the interfacial tension and modulus of SBT show remarkable changes. Compared with the individual SBT or Tween® 80 systems, the interfacial properties of the mixed surfactant system improve, especially at a 1:1 ratio. At the optimum ratio, synergistic adsorption takes place resulting in improved asphalt emulsion stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cold recycling is a new technique which has become one of the popular methods for rehabilitating existing asphalt pavements because of cost-effectiveness and conservation of paving materials [1]. It is difficult to understand the physical behavior of emulsified asphalts due to the high complexity of the system. Therefore, understanding and predicting the behavior and performance characteristics of emulsified asphalts is becoming an important topic for researchers.
Through interfacial studies, one can determine the fundamental mechanisms and kinetics of surfactant adsorption, film formation and rupture that ultimately govern emulsion behavior. As the interfacial area is larger or surfactants spread more thinly, interfaces with a large elastic modulus present a relatively high energy barrier to coalescence. As a result, a droplet with an elastic film is less likely to deform during a collision with another droplet resulting in a reduced coalescence rate and higher emulsion stability. It is essential to study their interfacial properties to obtain the knowledge of asphalt emulsions.
Attempts to quantify interfacial film compressibility and elasticity have been made via Langmuir film balance techniques [2, 3], shear viscometric measurements [4, 5] and oscillatory drop measurements [6, 7]. Interfacial dilatational rheology has proven itself to be a useful technique for exploring the interfacial adsorption behavior of surfactants, as well as their elasticity at air–liquid or liquid–liquid interfaces [8–11]. It is also suitable to study film elasticity at crude oil–water [12], model oil-air [13] and model oil–water [14] interfaces. It has been found that emulsion stability is highly relevant to rheological properties at the water–oil interface and a film with high elasticity has better stability. Kang et al. studied the stability and mechanism of synthetic w/o crude oil emulsion with the addition of polymer and surfactant. It was found that surfactant decreases oil–water interfacial tension and the oil–water interfacial elasticity is improved by addition of polymer [15]. Ortiz et al. investigated the change in film properties to analyze the effect of additives on asphaltene interfacial films and emulsion stability. Additives were found to have two opposing effects on film properties and emulsion stability: (1) decreasing or eliminating the crumpling ratio which destabilized emulsions and (2) decreasing interfacial tension which enhanced emulsion stability [16].
Based on previous research, we believe that studying interfacial rheological could also provide guidance for optimizing asphalt emulsion formulas. Herein, we investigated the interfacial properties of two surfactants, SBT and Tween® 80, at the oil–water interface to simulate their interactions in asphalt emulsions. The ratio of SBT and Tween® 80 in the mixed surfactant system was taken into account and proved to have a great influence on the interfacial properties. Optimizing the ratios of SBT and Tween® 80 improved the performance of asphalt emulsions. Therefore, this study could offer theoretical foundation for the development of emulsified asphalt.
Experimental Details
Materials
Indulin® SBT (MeadWestvaco, China), a cationic slow-set emulsifier was employed to prepare asphalt emulsions and was used as received. SBT is a complex reaction product specifically formulated for cationic slow setting (CSS) emulsions. Asphalt emulsions prepared using a slow-set emulsifier have a longer demulsification time as it contacts with the aggregates (stones). CSS emulsion sets slowly in contact with reactive aggregates of high surface area. It is engineered and designed to perform in a wide variety of applications including slow set slurry surfacing, tack coat, fog seal, and solventless cold mix applications such as recycling and base stabilization.
Tween® 80 [Polyoxyethylene (20 EO) sorbitan monooleate] was industrial grade with a purity of 99 %, produced by Tianjin DAMAO Chemical Reagent Company, China. Pure toluene, used as a model oil phase, was purchased from Tianjin Kermel Chemical Reagent Company in China with a purity of 99 %. Asphalt (AH-90) was purchased from the Shell Corporation in China.
To relate the interfacial rheology to emulsion stability, the effects of surfactant concentration and ratio are of interest. Four surfactant mixture ratios were selected and individual surfactant SBT as well as Tween® 80 were also investigated for comparison. Total surfactant concentration of individual and mixture systems ranged from 1 to 2000 mg/L.
Interfacial Tension Measurements
Interfacial tension was measured using an automated drop tensiometer (Tracker™, Teclis Instruments). The oil phase was injected into a quartz cuvette filled with the water phase through a syringe with a U-shaped needle. An oil droplet was formed at the tip of the needle in water. The profile of the droplet was captured by a CCD camera. The results were analyzed by a video image profile digitizer board and transferred to a computer.
The dynamic interfacial tension of the surfactant-model oil interface was also investigated using the pendant drop technique. Before the droplet was formed, the image capture software was triggered, collecting images at 2 frames/s for the first 10 min and 1 frame/min thereafter. Experimental runs of 10,000 s were chosen as reported in the literature [17]. Both the concentration and ratio of mixed surfactants were taken into account. The samples with 10 mg/L and variable ratio, and those with 1:1 ratio and variable concentration were measured to obtain their dynamic interfacial tensions.
The temperature was 25.0 ± 0.1 °C for all the interfacial tension measurements.
Interfacial Elasticity Measurements
To obtain the interfacial dilatational rheology of a liquid–liquid interface, a droplet is formed to provide an interface and the interfacial tension (γ) is tracked as a function of time. Controlled oscillatory strain deformations of the interfacial area (A) are applied to recover rheological information of the interfacial films. The interfacial dilatational modulus (ϵ) is defined by the following expression [18]:
In an oscillating system, the interfacial dilatational modulus is a complex quantity. It consists of a real and an imaginary component, which can be expressed as follows:
where \(\in^{\prime}\) is the real component (elastic modulus), and \(\in^{\prime \prime}\) is the imaginary part (viscous modulus). There is a phase shift, ω, between γ(t) and A(t) which is defined as the phase angle. Purely elastic interfaces present complete in-phase behavior between γ(t) and A(t) with a phase angle of 0°, whereas purely viscous interfaces are completely out-of-phase and the phase angle is equal to 90°. The elastic and viscous moduli are functions of the magnitude of the dilatational modulus, \( \left| \in \right| = ( \in^{\prime 2} + \in^{\prime \prime 2} )^{ 1/ 2} \), and they can be calculated from the phase shift:
All dilatational rheology experiments were run on a pendent drop tensiometer Tracker. Finer control of drop volume was afforded by using a 500-μL microsyringe and higher-gauge curved needle. A personal computer analyzes images of the droplet shape to solve for γ from the force balance between Laplace and head pressures on the droplet.
The oscillation amplitude has an effect on the elastic and viscous moduli [14]. It is reported that both moduli do not show a significant change when the amplitude is lower than 2 %. In the current work, the oscillation amplitude was 10 % of the initial area similar to other report research [9, 18, 19].
Elasticity measurements were taken after 4 h to allow equilibrium adsorption of surfactants at the oil/water interface. To perform an elasticity measurement, the drop size was oscillated periodically rather than continuously. The frequency of each set of oscillations was 0.017, 0.033, 0.1, 0.2 and 0.5 s−1 (periods of 60, 30, 10, 5 and 2 s, respectively), and the interval between sets of oscillations was one period.
Preparation of Asphalt Emulsion and Its Storage Stability Measurements
An aqueous phase was prepared by dispersing 2.5 wt% emulsifier (emulsifier mixture of SBT and Tween® 80 with different ratios) in distilled water. Asphalt emulsions were obtained by homogenizing 60 wt% asphalt (temperature 130 °C) with 40 wt% aqueous phase (temperature 55 °C) in a high-speed colloid mill.
Storage stability of the asphalt emulsion was measured according to ASTM D244. This test method determines the difference in percent residue of samples taken from the top and bottom of material placed in undisturbed simulated storage at 25 °C for 24 h. The result is expressed as the average of the two individual values obtained by determining the difference between the percent residue of the top and bottom samples for each storage cylinder. Small values are related to more favorable storage stability.
Results and Discussion
Interfacial Activity
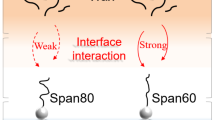
Interfacial tension of SBT/Tween® 80 mixtures at different ratios is shown in Fig. 1. At higher concentrations, more SBT and Tween® 80 molecules adsorb onto the oil/water interface, which decreases the interfacial tension. The interfacial tension of individual Tween® 80 is a little lower than that of individual SBT below 50 mg/L, but much higher than SBT thereafter suggesting that SBT has stronger ability to reduce interfacial tension. Below 300 mg/L, the interfacial tension of the surfactant mixtures is lower than that of individual SBT or Tween® 80. Below this concentration, the interfacial tension is higher as the proportion of SBT in the mixtures is larger. Above this concentration, the mixtures of SBT/Tween® 80 have similar interfacial tension, which is between the individual SBT and Tween® 80 values. These results suggest that Tween® 80 and SBT adsorb collaboratively at the interface, especially at a ratio of 1:1.
The dynamic interfacial tension is shown in Fig. 2. All the target samples show a rapid initial decrease followed by a progressive reduction in the decay rate. For the samples of individual Tween® 80 and SBT in Fig. 2a (ratios of 0:1 and 1:0), the interfacial tension appears to approach 18 and 17 mN/m asymptotically with time, respectively. The ratio of SBT/Tween® 80 mixtures has great influence on the dynamic interfacial tension. For 1:1 and 2:1 ratios, the interfacial tension is much lower than that of the individual surfactants. The interfacial tension is close to that of individual surfactants with increasing concentration of SBT in the mixture. The variation trend of dynamic interfacial tension shows an interesting phenomenon. The shape of the dynamic interfacial tension curve of 1:1 and 2:1 is similar to that of individual Tween® 80 (ratio 0:1). The curves with respect to 3:1 and 4:1 overlap that of individual SBT (ratio 1:0). Based on above results, it infers that Tween® 80 adsorbs at the oil/water interface faster than SBT, and synergistic adsorption is likely to happen in the mixture systems with ratios of 1:1 and 2:1. As a result, the dynamic interfacial tension is lower than that of individual Tween® 80 or SBT.
From Fig. 2b, the concentration of emulsifier also affects the dynamic interfacial tension. The interfacial tension decreases with increasing surfactant concentration. At concentrations greater than 100 mg/L, the decreasing rate with the concentration becomes very small. Moreover, it is clear from the experimental data that interfacial tension does not reach a constant value after 10,000 s for some samples. A possible explanation might be that after completion of adsorption, interfacial tension continues to decrease due to conformational relaxation/reorganization of the surfactants [17].
Interfacial Viscoelasticity
Important information on the properties of adsorption layers can be obtained via measurements of dilatational rheology. The dilatational viscoelasticity of SBT/Tween® 80 is shown in Figure S1 of the Supporting Information. The dilatational modulus increases with frequency at all the different ratios of SBT and Tween® 80. It is believed that the dependence of modulus on frequency is caused by two relaxation processes at the interface [20, 21]. One is the exchange of surfactants between the bulk aqueous solution and the interface, and the other is the conformational change of surfactants at the interface. Under compression conditions, some molecules at the interface can dissolve into the bulk aqueous solution and the equilibrium concentration is restored. At low frequency, there is enough time for each cycle to reach “equilibrium”. This process occurs fully and there is no resistance to compression-expansion. In this case, the surface tension shows little change, thus the modulus is smaller. At high frequency, the exchange rate is much lower than the frequency of the disturbance. The modulus is higher and the adsorbed surfactant layer behaves as an insoluble monolayer. Therefore, the dilatational modulus increases with increasing oscillation frequency.
Both concentration and ratio have influence on the dilatational modulus. Except for the 3:1 ratio, the modulus of the mixture system is higher than that of individual SBT or Tween® 80 system. At a ratio of 1:1, the modulus is the highest among the six target systems. At the optimum ratio, SBT and Tween® 80 molecules cooperate with each leading to synergistic adsorption with an enhancement of the viscoelasticity. It is also clear that the curves corresponding to concentrations higher than 100 mg/L are much lower than the other curves in the mixture systems (Figure S1c to S1f). The maximum of dilatational modulus is at lower concentration for the mixture systems compared with the individual system.
To clearly observe the effect of concentration on the dilatational modulus, the variation of modulus as a function of concentration at a fixed frequency is shown in Fig. 3. At SBT and Tween® 80 ratios of 0:1 and 1:1, the modulus decreases with the increasing concentration. For the other four systems, the modulus first increases and then decreases with maximums at 100, 10, 50, 50 mg/L at ratios of 1:0, 2:1, 3:1, 4:1, respectively. The maximum of systems 0:1 and 1:1 is definitely lower than 1 mg/L, and the modulus of the 1:1 system is the largest. This suggests that SBT/Tween® 80 complex forms at lower concentration than the critical micelle concentration of SBT (CMC = 20 g/L).
There are two effects acting on the surface dilatational viscoelasticity as the surfactant bulk concentration increases. The surfactant concentration at the interface layer becomes higher, and the surfactant molecules are prone to diffuse from the bulk to the subsurface. The dilatational elasticity is larger as the surfactant concentration increases, while it will decrease because of the surfactant diffusion from bulk to interface [22, 23]. When the concentration increases to a certain value, the two effects on the modulus have achieved an equilibrium state, and the modulus reaches the maximum value. A further increase of concentration in the bulk can only induce SBT and Tween® 80 molecules diffusing from the bulk to the interface, thus the modulus becomes much lower.
The variations of elastic and viscous moduli as a function of frequency are shown in Figure S2 of the Supporting Information. The values also increase with frequency, and the elastic modulus is obviously much larger, close to the value of the dilatational modulus. At the same frequency, the elastic modulus is much larger than the viscous modulus and the phase angle is smaller than 45°, which infers that the elastic property is dominant at the oil/water interface. It can be observed that the elastic modulus increases up to a maximum value with the increase of concentration at a given frequency. As has been stated for dilatational modulus, the increasing surface concentration and surfactant molecular exchange at low and high concentrations play a dominant role in determining the dilatational elasticity [24]. The dilatational viscous component is weakly affected by the concentration. Differences among all the curves of viscous moduli are quite small. To clarify the effect of surfactant concentration, one fixed frequency was chosen: the variation curves of elastic and viscous moduli as a function of the concentration are shown in Fig. 4.
The elastic modulus is strongly affected by the concentration. The majority of these curves first increase and then decrease with increasing surfactant concentration. The maximum elastic modulus for each mixture system appears at smaller concentrations compared with the individual SBT system. Additionally, the maximum value of the mixture systems is relatively larger. Different from the elastic modulus, the viscous modulus changes little at low frequency. At higher frequency, it decreases slowly with increasing surfactant concentration. It can be implied that surface deformation is the dominant factor affecting the dilatational viscosity of SBT/Tween® 80 solutions.
The interfacial properties are related to the emulsion stability. Storage stabilities of asphalt emulsions with different ratios of SBT and Tween® 80 are shown in Table 1. The emulsion is composed of 40 wt% aqueous phase and 60 wt% oil phase (asphalt). The concentration of surfactant is 2.5 wt%. The 1:1 system shows the optimum stability. All other mixed systems present relatively bad stability. These results are in good agreement with the interfacial rheology measurements. The mixture of SBT and Tween® 80 with a 1:1 ratio has good interfacial properties and stabilizes the asphalt droplets in water.
Conclusions
Interfacial properties of SBT and Tween® 80 at the oil/water interface were studied by interfacial elasticity measurements. Both SBT and Tween® 80 molecules can be adsorbed onto the oil/water interface. Mixed surfactant systems have more favorable properties compared with individual surfactant systems. The ratio of SBT and Tween® 80 has a great influence in the properties of emulsions. It is confirmed that the emulsion shows better stability at a 1:1 ratio. At this ratio, the interfacial tension is lower and the maximum interfacial modulus appears at lower surfactant concentration. At the optimum ratio, the surfactants cooperate with each other and synergistic adsorption may take place. As a result, the interfacial properties of emulsions are improved. These deductions are further confirmed by the results of the storage stabilities of asphalt emulsions. It seems that the interfacial rheological study could be a favorable way to optimize preparation conditions of emulsified asphalt.
References
Kim Y, Im S, Lee HD (2011) Impacts of curing time and moisture content on engineering properties of cold in-place recycling mixtures using foamed or emulsified asphalt. J Mater Civ Eng 23:542–553
Zhang LY, Lawrence S, Xu Z, Masliyah JH (2003) Studies of Athabasca asphaltene Langmuir films at air-water interface. J Colloid Interface Sci 264:128–140
Zhang LY, Xu Z, Masliyah JH (2003) Langmuir and Langmuir-Blodgett films of mixed asphaltene and a demulsifier. Langmuir 19:9730–9741
Spiecker PM, Kilpatrick PK (2004) Interfacial rheology of petroleum asphaltenes at the oil-water interface. Langmuir 20:4022–4032
Li M, Xu M, Ma Y, Wu Z, Christy A (2002) Interfacial film properties of asphaltenes and resins. Fuel 21:1847–1853
Bouriat P, El Kerri N, Graciaa A, Lachaise A (2004) Properties of a two-dimensional asphaltene network at the water-cyclohexane interface deduced from dynamic tensiometry. Langmuir 20:7459–7464
Aske N, Orr R, Sjöblom J, Kallevik H (2004) Interfacial properties of water-crude oil systems using the oscillating pendant drop. Correlations to asphaltene solubility by near infrared spectroscopy. J Disp Sci Technol 25:263–275
Fainerman VB, Lucassen-Reynders EH (2002) Adsorption of single and mixed ionic surfactants at fluid interfaces. Adv Colloid Interface Sci 96:295–323
Lucassen-Reynders EH, Cagna A, Lucassen J (2001) Gibbs elasticity, surface dilational modulus and diffusional relaxation in nonionic surfactant monolayers. Colloids Surf A 186:63–72
Fan Y, Simon S, Sjöblom J (2010) Influence of nonionic surfactants on the surface and interfacial film properties of asphaltenes investigated by Langmuir balance and brewster angle microscopy. Langmuir 26:10497–10505
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussien IA, Pabon M (2014) Evaluation of rheological and thermal properties of a new fluorocarbon surfactant-polymer system for EOR applications in high-temperature and high-salinity oil reservoirs. J Surf Deterg. doi:10.1007/s11743-014-1600-7
Dicharry C, Arla D, Sinquin A, Graciaa A, Bouriat P (2006) Stability of water/crude oil emulsions based on interfacial dilatational rheology. J Colloid Interface Sci 297:785–791
Bauget F, Langevin D, Lenormand R (2001) Dynamic surface properties of asphaltenes and resins at the oil-air interface. J Colloid Interface Sci 239:501–508
Sztukowski DM, Yarranton HW (2005) Rheology of asphaltene–toluene/water interfaces. Langmuir 21:11651–11658
Kang W, Xu B, Wang Y, Li Y, Shan X, An F, Liu J (2011) Stability mechanism of W/O crude oil emulsion stabilized by polymer and surfactant. Colloids Surf A 384:555–560
Ortiz DP, Baydak EN, Yarranton HW (2010) Effect of surfactants on interfacial films and stability of water-in-oil emulsions stabilized by asphaltenes. J Colloid Interface Sci 351:542–555
Rane JP, Harbottle D, Pauchard V, Couzis A, Banerjee S (2012) Adsorption kinetics of asphaltenes at the oil–water interface and nanoaggregation in the bulk. Langmuir 28:9986–9995
Aske N, Orr R, Sjöblom J (2002) Dilatational elasticity moduli of water/crude oil interfaces using the oscillating pendant drop. J Disp Sci Technol 23:809–825
Freer EM, Svitova T, Radke CJ (2003) The role of interfacial rheology in reservoir mixed wettability. J Petrol Sci Eng 39:137–158
Ravera F, Ferrari M, Santini E, Liggieri L (2005) Influence of surface processes on the dilational viscoelasticity of surfactant solutions. Adv Colloid Interface Sci 117:75–100
Stubenrauch C, Fainerman VB, Aksenenko EV, Miller R (2005) Adsorption behavior and dilational rheology of the cationic alkyl trimethylammonium bromides at the water/air interface. J Phys Chem B 109:1505–1509
He F, Xu G, Pang J, Ao M, Han T, Gong H (2011) Effect of amino acids on aggregation behaviors of sodium deoxycholate at air/water surface: surface tension and oscillating bubble studies. Langmuir 27:538–545
Wu D, Xu GY, Feng YJ, Li YM (2007) Aggregation behaviors of gelatin with cationic gemini surfactant at air/water interface. Int J Biol Macromol 40:345–350
Stubenrauch C, Miller R (2004) Stability of foam films and surface rheology: an oscillating bubble study at low frequencies. J Phys Chem B 108:6412–6421
Acknowledgments
We gratefully acknowledge financial support from the scientific research project of Shanxi province Communication Department (Contract Nos. 2013-1-7).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11743_2015_1715_MOESM1_ESM.doc
Supplementary material 1 (DOC 2459 kb). Figure S1. Variation of dilatational modulus as a function of frequency with different ratios of SBT/Tween® 80 at (a) 0:1, (b) 1:0, (c) 1:1, (d) 2:1, (e) 3:1, (f) 4:1 at 25 °C. Figure S2. Variation of elastic (solid point) and viscous (hollow point) modulus as a function of frequency with different ratios of SBT/Tween® 80 at (a) 0:1, (b) 1:0, (c) 1:1, (d) 2:1, (e) 3:1, (f) 4:1. All measurements performed at 25 °C
About this article
Cite this article
Pang, J., Du, S., Chang, R. et al. Interfacial Rheology of Mixed Surfactants at the Oil/Water Interface. J Surfact Deterg 18, 747–753 (2015). https://doi.org/10.1007/s11743-015-1715-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1715-5