Abstract
Lauric acid and propylene oxide were used to modify four biologically active heterocycles (thiazole, pyrazole, triazole, and pyrrole) to synthesize 17 new surfactants. The chemical structures of these surfactants were confirmed using infrared and 1H, and 13C nuclear magnetic resonance (NMR) spectroscopy. The surfactants all show good surface activity, low critical micelle concentration (CMC) values, high cloud points, and tight interfacial packing. All showed antimicrobial activity on both bacteria and fungi. In addition, biodegradation testing demonstrated significant breakdown within seven days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fatty acid hydrazides containing an amide group attached to a primary amine group have wide application in pharmaceutical studies [1, 2]. Hydrazides have demonstrated effectiveness against tuberculosis [3]. 1,3,4-Oxadiazoles are biologically active materials used in medical science and agriculture [4]. 1,3,4-Thiadiazoles are reported to have anti-inflammatory, antimicrobial, and anticonvulsant efficacy [5–7]. The triazole nucleus has a reported antitumor effect [8–10]. Finally, the thiadiazole nucleus has demonstrated antibacterial, antiviral, and antioxidant results [11–13].
In view of these applications of fatty acid heterocyclic derivatives as biologically active agents [14], we developed a new synthetic method to modify these derivatives to increase surface activity and, perhaps, antimicrobial potency [15]. This builds on our previous synthetic work on biologically important heterocyclic compounds. Oleochemicals are essential to a variety of industries such as coatings, surfactants, plasticizers, lubricant additives, cosmetics, pharmaceuticals, soaps, detergents, textiles, plastics, and as mild surfactants in cosmetic formulations [16, 17].
In view of the above mentioned facts and in continuation of our work on the syntheses of biologically important heterocyclic compounds [18–21], we investigate an efficient synthesis with the goal of obtaining more potent surface activity and pharmacologically active compounds.
Experimental Section
Materials and Methods
The chemicals, with 98 % purity. used in the synthesis of all compounds were obtained from the Sigma-Aldrich Chemical Company. The solvents used were of spectroscopic grade and used without further purification.
Melting points were determined by the open capillary method using a Gallen Kamp melting point apparatus and are uncorrected. The infrared spectra were recorded using potassium bromide (KBr) discs on a FTIR 8300 Shimadzu spectrophotometer and the results are expressed in wave number (cm−1). 1H, and 13C nuclear magnetic resonance (NMR) spectra were recorded with a Bruker AC300 spectrometer (Fällanden, Switzerland) operating at 300 MHz for 1H and 75 MHz for 13C, using deuterochloroform (CDCl3) as a solvent. Chemical shifts are expressed in δ (ppm) using tetramethylsilane (TMS) as the internal standard. Elemental microanalysis was carried out using a CHNS (carbon, hydrogen, nitrogen, sulfur) elemental analyzer model EA3000 EURO VECTOR instruments. Biological activity was screened at the Microbiology Department, Faculty of Applied Science, Umm Al-Qura University, Saudi Arabia.
Synthesis of Hetrocyclic Compounds (Schemes 1, 2)
Synthesis of Ethyl Dodecanoate (2)
A mixture of lauric acid (1) (2 g, 0.01 mol), absolute ethanol (50 mL) and conc. H2SO4 (0.5 ml) was refluxed for 8–10 h in a round bottom flask and then cooled to 5 °C. The solvent was removed by distillation and the reaction mixture washed with 1 % aqueous sodium bicarbonate solution. The reaction mixture was extracted with ethyl acetate and the solid separated was dried over magnesium sulfate, filtered, and collected to obtain compound 2.
Synthesis of Dodecane Hydrazide (3)
To a solution of 2 (2.28 g, 0.01 mmol) in absolute absolute ethanol (30 mL), hydrazine hydrate (0.64 g, 0.02 mol) was added and the reaction mixture was heated under reflux for 6 h and then left to cool. The solid product was collected by filtration and purified by crystallization form ethanol. White powder, (1.93 g, 85 %), mp. 115–117 °C. IR (ν/cm−1): 3342–3225 (NH, NH2), 2953, 2848 (CH in alkyl chain) and 1676 (C=O). 1H NMR: δ 6.83 (s, 1H, NH), 3.92 (s, 2H, NH2), 0.95 (t, 3H, terminal CH3), 1.40–1.25 (m, 20H, CH2 in alkyl chain). Anal. Calc. for C12H26N2O (214.35): C, 67.24; H, 12.23; N, 13.07 %. Found: C, 66.98; H, 11.97; N, 12.78 %.
Synthesis of N-Phenyl-5-undecyl-1,3,4-oxadiazol-2-amine (4)
A mixture of hydrazide 3 (2.14 g, 0.01 mol) and phenyl isothiocyanate (1.35 g, 0.01 mol) in dimethylformamide (20 mL) was heated under reflux for 8 h. The reaction was cooled and the solvent evaporated under reduced pressure. The solid product obtained was crystallized from a mixed solvent ethanol and dimethylformamide (10:10). Yellow solid, (1.39 g, 65 %), mp. 96–98 °C. IR (ν/cm−1): 3221 (NH), 3091 (CH aromatic), 2950, 2848 (CH aliphatic) and 1618, 1597 (C=N). 1H NMR: δ 8.00 (s, 2H, NH), 7.38–7.47 (m, 5H, ArH), 1.36–1.26 (m, 20H, CH2 in alkyl chain), 0.89 (t, 3H, terminal CH3). Anal. Calc. for C19H29N3O (315.45): C, 72.34; H, 9.27; N, 13.32 %. Found: C, 72.61; H, 9.57; N, 13.11 %.
Synthesis of 3-Undecyl-1H-1,2,4-triazole-5(4H)-thione (5)
Equimolar amounts of hydrazide 3 (2.14 g, 0.01 mol) and ammonium thiocyanate (0.76 g, 0.01 mol) in (50 mL) ethanol with (5 mL) hydrocholoric acid was heated under reflux for 5 h. The solid that appeared on cooling was filtered and recrystallized from ethanol. White powder, (1.32 g, 62 %, mp. 97–99 °C. IR (ν/cm−1): 3314–3289 (NH), 2954, 2848 (CH aliphatic) and 1597 (C=N). 1H NMR: δ 8.40, 12.14 (2 s, 2H, 2NH), 1.23–1.30 (m, 20H, CH2 in alkyl chain), 0.91 (t, 3H, terminal CH3). 13C NMR: δ 175.63, 153.19, 35.96, 31.32, 29.99, 29.54, 29.24, 29.16, 28.93, 26.84, 25.82, 24.78, 22.57. Anal. Calc. for C13H25N3S (255.42): C, 61.13; H, 9.87; N, 16.45; S, 12.55 %. Found: C, 60.90; H, 9.68; N, 16.12; S, 12.19 %.
Synthesis of 5-Amino-1-dodecanoyl-1H-pyrazol-3(2H)-one (6)
A solution of potassium hydroxide (1.12 g, 0.02 mol) in ethanol (20 mL) was added to hydrazide 3 (2.14 g, 0.01 mol). After stirring for 30 min, ethyl cyanoacetate (1.12 g, 0.01 mol) was added dropwise and the mixture was heated under reflux for 10 h. The reaction mixture was then cooled, diluted with water and acidified with conc. HCl. The resulting solid was washed with water, filtered, dried and crystallized from ethanol. White powder crystals, (1.51 g, 71 %), mp. 155–157 °C. IR (ν/cm−1): 3318–3141 (NH, NH2), 2951, 2848 (CH aliphatic), 1712 (C=O ester), 1678 (C=O), 1618 (C=C). 1H NMR: δ 10.35 (s, 1H, NH), 6.75 (s, 2H, NH2), 1.23–1.30 (m, 20H, CH2 in alkyl chain), 0.91 (t, 3H, terminal CH3). Anal. Calc. for C15H27N3O2 (281.39): C, 64.02; H, 9.67; N, 14.93 %. Found: C, 63.83; H, 9.89; N, 15.15 %.
Synthesis of N-(1,3-Dioxoisoindolin-2-yl)dodecanamide (7)
A mixture of hydrazide 3 (2.14 g, 0.01 mol) and phthalic anhydride (1.48 g, 0.01 mol) in (10 mL) glacial acetic acid was heated under reflux for 6 h. The mixture was poured into crushed ice (≈30 g) after cooling. The separated residue was washed with water, filtered, dried and crystallized from acetic acid. Colorless crystals, (1.73 g, 81 %), mp. 125–127 °C. IR (ν/cm−1): 3208 (NH), 2918, 2849 (CH aliphatic), 1676 broad (C=O). 1H NMR: δ 9.11 (s, 1H, NH), 7.58–7.90 (m, 4H, ArH), 1.30–1.40 (m, 20H, CH2 in alkyl chain), 0.91 (t, 3H, terminal CH3). Anal. Calc. for C20H28N2O3 (344.45): C, 69.74; H, 8.19; N, 8.13 %. Found: C, 70.01; H, 8.48; N, 8.45 %.
Synthesis of 2-Dodecanoyl-N-phenylhydrazinecarbothioamide (8)
A mixture of hydrazide 3 (2.14 g, 0.01 mol), phenyl isothiocyanate (1.35 g, 0.01 mol) in dry benzene (25 mL) was heated under reflux for 6 h. After cooling the solvent was evaporated under reduced pressure. The solid product obtained was filtered, washed with ethanol and finally crystallized from ethanol. Colorless crystals (1.79 g, 84 %), mp. 137–139 °C. IR (ν/cm−1): 3411–3127 (NH), 2918, 2850 (CH aliphatic), 1666 (C=O). 1H NMR: δ 8.75, 4.66, 4.21 (3 s, 3H, 3NH), 1.45–1.30 (m, 20H, CH2 in alkyl chain), 0.88 (t, 3H, terminal CH3). Anal. Calc. for C19H31N3OS (349.53): C, 65.29; H, 8.94; N, 12.02; S, 9.17 %. Found: C, 65.01; H, 8.63; N, 11.77; S, 9.36 %.
Synthesis of 4-Phenyl-5-undecyl-4H-1,2,4-triazole-3-thiol (9)
A mixture of thiosemicarbazide 8 (3.49 g, 0.05 mol) and sodium hydroxide solution (1.0 g in 5 mL H2O) in ethanol (30 mL) was heated under reflux for 4 h, then allowed to cool. The mixture was acidified with concentrated hydrochloric acid and the formed solid product was filtered, washed with water and recrystallized from ethanol. White solid, (2.65 g, 76 %), mp. 112–114 °C. IR (ν/cm−1): 3046 (CH aromatic), 2918, 2849 (CH aliphatic), 1597, 1574 (2C=N). 1H NMR: δ 12.25 (s, 1H, SH), 7.58–7.32 (m, 5H, ArH), 1.29–1.18 (m, 20H, CH2 in alkyl chain), 0.90 (t, 3H, terminal CH3). 13C NMR: δ 168.30, 152.72, 129.97, 129.02, 128.13, 127.72, 34.20, 31.76, 30.40, 29.44, 28.86, 26.10, 25.45, 22.75, 14.22. Anal. Calc. for C19H29N3S (331.52): C, 68.84; H, 8.82; N, 12.68; S, 9.67 %. Found: C, 68.56; H, 8.66; N, 12.43; S, 9.28 %.
Synthesis of N-Phenyl-5-undecyl-1,3,4-thiadiazol-2-amine (10)
Thiosemicarbazides 8 (3.49 g, 0.01 mol) was treated with concentrated sulphuric acid (5 ml) at 0 °C in an ice bath with constant stirring for 12 h. The reaction mixture was poured into ice water (50 mL). The product was precipitated, filtered and washed with excess cold water. Pale yellow solid, (1.91 g, 55 %), mp. 100–102 °C. IR (ν/cm−1): 3193 (NH), 2850, 2919 (CH aliphatic), 1618, 1550 (C=N). 1H NMR: δ 7.92 (s, 1H, NH), 7.41–7.26 (m, 5H, ArH), 1.40–1.23 (m, 20H, CH2 in alkyl chain), 0.88 (t, 3H, terminal CH3). 13C NMR: δ 168.33, 153.04, 133.59, 129.89, 129.06, 128.42, 127.94, 34.43, 31.79, 29.54, 29.28, 28.84, 26.03, 25.66, 22.80, 14.73. Anal. Calc. for C19H29N3S (331.52): C, 68.84; H, 8.82; N, 12.68; S, 9.67 %. Found: C, 69.08; H, 9.11; N, 12.46; S, 9.32 %.
Synthesis of (Z)-N′-(4-(4-Bromophenyl)-3-phenylthiazol-2(3H)-ylidene)dodecanehydrazide (11)
A solution of hydrazide 3 (2.14 g, 0.01 mol) in ethanol (40 mL), and p-bromophenacyl bromide (2.77 g, 0.01 mol) was heated under reflux for 5 h. The reaction mixture was poured on an ice-water mixture and the pH adjusted to 7 using sodium hydroxide. The solid product was collected by filtration and crystallized from ethanol. Pale yellow crystals, (1.56 g, 73 %), mp. 110–112 °C. IR (ν/cm−1): 3332 (NH), 2851, 2919 (CH aliphatic), 1670 (CO), 1610 (C=C), 1597 (C=N). 1H NMR: δ 12.41 (s, 1H, NH), 7.58–7.29 (m, 4H, ArH), 6.58 (s, 1H, CH of thiazole ring), 1.42–1.14 (m, 20H, CH2 in alkyl chain), 0.98 (t, 3H, terminal CH3). 13C NMR: δ 172.82, 168.09, 160.07, 142.93, 136.81, 130.14, 129.92, 128.95, 125.66, 122.61, 117.70, 99.83, 31.81, 29.64, 29.35, 28.72, 26.45, 25.33, 22.68, 14.12. Anal. Calc. for C27H34BrN3OS (528.55): C, 61.35; H, 6.48; N, 7.95; S, 6.07 %. Found: C, 61.53; H, 6.69; N, 7.64; S, 6.31 %.
Synthesis of N′-Benzylidenedodecanehydrazide (12)
A solution of hydrazide 3 (2.14 g, 0.01 mol) in methanol (10 mL) and aromatic aldehydes, e.g., benzaldehyde (1.06 g, 0.01 mol), was heated under reflux for 4 h, using a few drops of acetic acid. After cooling, the formed solid product was filtered, dried and recrystallized from ethanol. Yellow solid, (1.66 g, 78 %), mp. 90–92 °C. IR (ν/cm−1): 3187 (NH), 2915, 2847 (CH aliphatic), 1669 (C=O), 1602 (C=N). 1H NMR: δ 8.83 (s, 1H, NH), 8.20 (s, 1H, CH=N), 7.85–7.29 (m, 5H, ArH), 1.46–1.28 (m, 20H, CH2 in alkyl chain), 0.93 (t, 3H, terminal CH3). Anal. Calc. for C19H30N2O (302.45): C, 75.45; H, 10.00; N, 9.26 %. Found: C, 75.69; H, 10.27; N, 9.02 %.
Synthesis of N-(4-Oxo-2-phenylthiazolidin-3-yl)dodecanamide (13)
To a stirred solution of compound 12 (3.02 g, 0.01 mol) in dry benzene (30 mL), thioglycollic acid (0.92 g, 0.01 mol) in dry benzene (5 mL) was added. The reaction mixture was heated under reflux for 10 h and the excess solvent evaporated under pressure. The obtained solid was filtered, dried and crystallized from ethanol. Pale yellow, (2.05 g, 68 %), mp. 75–77 °C. IR (ν/cm−1): 3260 (NH), 3026 (CH aromatic), 2918, 2850 (CH aliphatic), 1716, 1665 (C=O). 1H NMR: δ 7.79 (s, 1H, NH), 7.40–7.26 (m, 5H, ArH), 4.68 (s, 1H, CH of thiazole ring), 3.66, 3.81 (2 s, 2H, CH2 of thiazole ring), 1.46–1.28 (m, 20H, CH2 in alkyl chain), 0.89 (t, 3H, terminal CH3). 13C NMR: δ 176.48, 167.12, 133.83, 130.47, 129.26, 128.85, 127.73, 126.00, 63.17, 41.71, 31.79, 30.77, 29.46, 29.14, 25.61, 24.86, 22.80, 18.43, 14.23. Anal. Calc. for C21H32N2O2S (376.56): C, 66.98; H, 8.57; N, 7.44; S, 8.52 %. Found: C, 67.22; H, 8.78; N, 7.71; S, 8.81 %.
Synthesis of 1-(3,5-Diamino-4,5-dihydro-1H-pyrazol-1-yl)dodecan-1-one (14)
A mixture of hydrazide 3 (2.14 g, 0.01 mol) and malononitrile (0.66 g, 0.01 mol) was heated under reflux in ethanol (30 mL) with a few drops of pipredine for 5 h. After cooling, the separated product was filtered, dried and recrystallized from ethanol. White yellow powder, (1.62 g, 76 %), mp. 99–101 °C. IR (ν/cm−1): 3314–3200 (NH2), 2919, 2849 (CH aliphatic), 1660 (C=O), 1599 (C=N). 1H NMR: δ 7.79 (s, 1H, NH), 6.78 (s, 1H, CH of diazole ring), 3.90 (s, 2H, NH2), 2.52 (s, 2H, NH2), 1.71 (s, 2H, CH2 of diazole ring), 1.30–1.27 (m, 20H, CH2 in alkyl chain), 0.99 (t, 3H, terminal CH3). 13C NMR: δ 174.06, 152.82, 51.48, 34.61, 31.91, 29.60, 29.47, 29.33, 29.31, 29.29, 25.51, 22.69, 18.43, 14.12. Anal. Calc. for C15H30N4O (282.25): C, 63.79; H, 10.71; N, 19.84 %. Found: C, 64.00; H, 10.93; N, 19.60 %.
Synthesis of N-(2,5-Dimethyl-1H-pyrrol-1-yl)dodecanamide (15)
A solution of hydrazide 3 (2.14 g, 0.01 mol) in ethanol (20 mL) and acetonyl acetone (2.28 g, 0.02 mol) with a few drops of glacial acetic acid was heated on a water bath for 5 h. After cooling, the mixture was poured into cold water and the obtained solid was filtered, washed with water, dried and crystallized from ethanol. Brown solid, (1.84 g, 86 % yield, mp. 65–67 °C. IR (ν/cm−1): 3272 (NH), 2915, 2848 (CH aliphatic), 1665 (C=O), 1590 (C=C). 1H NMR: δ 7.82 (s, 1H, NH), 5.85 (2 s, 2H, 2CH of pyrrole ring), 2.20 (s, 6H, 2CH3), 1.40–1.26 (m, 20H, CH2 in alkyl chain), 0.94 (t, 3H, terminal CH3). Anal. Calc. for C18H32N2O (292.46): C, 73.92; H, 11.03; N, 9.58 %. Found: C, 73.67; H, 10.81; N, 9.79 %.
Synthesis of 2-Undecyl-4H-1,3,4-oxadiazin-6(5H)-one (16)
A solution of hydrazide 3 (2.14 g, 0.01 mol) in acetic anhydride (20 mL) and chloroacetic acid (0.94 g, 0.01 mol) in the presence of sodium acetate (1.64 g, 0.02 mol) was heated under reflux for 4 h, then poured into water. A solid product was obtained by filtration and recrystallization from toluene. White solid, (1.43 g, 67 %), mp. 94–96 °C. IR (ν/cm−1): 3216 (NH), 2918, 2849 (CH aliphatic), 1698 (C=O), 1598 (C=N). 1H NMR: δ 8.88 (s, 1H, NH), 3.66 (s, 2H, CH2 of oxadiazine ring), 1.32–1.23 (m, 20H, CH2 in alkyl chain), 0.89 (t, 3H, terminal CH3). 13C NMR: δ 177.41, 155.72, 51.48, 31.79, 29.22, 29.16, 29.12, 29.07, 26.32, 25.19, 24.97, 22.80, 20.89. Anal. Calc. for C14H26N2O2 (254.37): C, 66.10; H, 10.30; N, 11.01 %. Found: C, 65.88; H, 10.08; N, 11.28 %.
Synthesis of 5-Undecyl-1,3,4-oxadiazole-2(3H)-thione (17)
Finely powdered KOH (1.12 g, 0.02 mol) was dissolved in ethanol (30 mL), and hydrazide 3 (2.14 g, 0.01 mol) was added. (0.76 g, 0.01 mol) of carbon disulfide was then slowly added and the reaction mixture was heated under reflux on a steam bath for 10 h. The concentrated solution was cooled and acidified with hydrochloric acid. The precipitated solid was filtered, washed with ethanol, dried and crystallized from ethanol. White yellow solid, (1.47 g, 69 %), mp. 81–83 °C. IR (ν/cm−1): 3221 (NH), 2918, 2848 (CH aliphatic), 2640 (C=S), 1618 (C=N). 1H NMR: δ 11.5 (s, 1H, NH), 1.42–1.41 (m, 20H, CH2 in alkyl chain), 0.86 (t, 3H, terminal CH3). Anal. Calc. for C13H24N2OS (256.41): C, 60.89; H, 9.43; N, 10.93; S, 12.51 %. Found: C, 61.15; H, 9.66; N, 11.16; S, 12.77 %.
Synthesis of 4-Amino-5-undecyl-4H-1,2,4-triazole-3-thiol (18)
An ethanol solution (30 mL) of 1,3,4-oxadiazole 17 (0.01 mol) and hydrazine hydrate (0.38 g, 0.012 mol) was heated under reflux for 3 h. After cooling, the solution was poured into ice water. The resulting product was filtered, washed with water and crystallized from ethanol. White solid, (1.58 g, 62 %), mp. 94–96 °C. IR (ν/cm−1): 3318, 3141 (NH2), 2919, 2848 (CH aliphatic), 1618, 1570 (C=N), 1176 (SH). 1H NMR: δ 11.61 (s, 1H, SH), 6.86 (s, 2H, NH2), 1.41–1.30 (m, 20H, CH2 in alkyl chain), 0.919 (t, 3H, terminal CH3). 13C NMR: δ 166.85, 156.43, 34.46, 31.92, 30.72, 29.64, 29.57, 29.35, 29.21, 28.95, 28.51, 26.17, 25.70, 25.64. Anal. Calc. for C13H26N4S (270.44): C, 57.74; H, 9.69; N, 20.72; S, 11.86 %. Found: C, 57.55; H, 9.42; N, 20.48; S, 11.63 %.
Synthesis of 5-Undecyl-1,3,4-thiadiazole-2(3H)-thione (19)
Finely powdered KOH (1.12 g, 0.02 mol) was dissolved in ethanol (30 mL), and hydrazide 3 (2.14 g, 0.01 mol) was added. (0.76 g, 0.01 mol) of carbon disulfide was then slowly added over a period of 10 min and the reaction mixture stirred for an additional 1 h. The reaction mixture was then heated under reflux on a steam bath for 12 h, concentrated, cooled and acidified with diluted H2SO4. The solid mass that precipitated was filtered, washed with ethanol, dried and recrystallized from ethanol. Pale yellow solid, (1.51 g, 71 %), mp. 125–127 °C. IR (ν/cm−1): 3225 (NH), 2919, 2848 (CH aliphatic), 2635 (C=S), 1603 (C=N). 1H NMR: δ 10.75 (s, 1H, NH), 1.39–1.24 (m, 20H, CH2 in alkyl chain), 0.89 (t, 3H, terminal CH3). Anal. Calc. for C13H24N2S2 (272.47): C, 57.30; H, 8.88; N, 10.28; S, 23.54 %. Found: C, 57.02; H, 8.56; N, 10.43; S, 23.79 %.
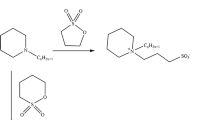
Preparation of Nonionic Surface Active Agents (Schemes 3, 4)
Nonionic surface active agents were prepared by addition of propylene oxide (5 mol) to synthesized compounds 3–19. Propylene oxide was added above the melting point of each compound using KOH as catalyst [22]. The quantity of propylene oxide which reacted and the average degree of propoxylation were measured through the change in mass of the reaction mixture (increase of mixture weight after addition of propylene oxide is the average amount of propoxylation). The based catalyst was neutralized by adding HCl to a pH of 7. Addition of propylene oxide produced mixtures of propoxylated products, and their structures were confirmed on the basis of IR and 1H NMR spectra. IR spectra revealed a broad band in the region of (3500–2500) cm−1 (OH) and two other bands in the regions of (1100–1000) and (950–900) cm−1 for (C–O–C ether linkage of polypropoxy chain) besides the original bands of the starting compounds. 1H NMR spectra showed the protons of the propoxy groups, which appeared as broad multiple signals in the region of (3.2–3.8), in addition to the other signals of the starting compounds.
Biological Activity
Antimicrobial activity of the prepared compounds was tested via a modified Kirby-Bauer disc diffusion method [23]. The compounds were tested against Escherichia coli and Staphylococcus aureus bacteria. The compounds were also tested against Aspergillus flavus and Candida albicans fungus. A filter-paper disk, impregnated with the compound to be tested, was placed on an agar surface. The compound diffused from the filter paper into the agar. The concentration of the compound is highest next to the disk, and decreases with increased distance from the disk. If the compound is effective against bacteria at a certain concentration, no colonies will grow where the concentration in the agar is greater than or equal to the effective concentration. This is the zone of inhibition. This, along with the rate of antibiotic diffusion, is used to estimate the bacteria’s sensitivity to that particular antibiotic. In general, larger zones correlate with smaller minimum inhibitory concentration (MIC) of an antibiotic for that bacterium. Inhibition produced by the test is compared with that produced by a known concentration of a reference compound. This information can be used to choose appropriate antibiotics to combat a particular infection.
Surface and Interfacial Tension Measurements
Surface and interfacial tension measurements of the prepared surfactants (γ) were made at 25 °C with a Du Nouy tensiometer (Kruss K6) [24] with a platinum ring for various concentrations of the synthesized surfactants. Paraffin oil was used for the interfacial tension measurements using aqueous surfactant solutions (0.1 wt%) at room temperature (25 °C).
Cloud Point Measurements
In a temperature-controlled bath, a 1 wt% solution of the tested compound was gradually heated until the clear or nearly clear solutions became definitively turbid [25]. The temperature was then recorded and the solution was allowed to cool down until it became clear again. The process was repeated to check the reproducibility of the recorded temperature.
Wetting Time Measurements
Wetting time was measured by immersing a cotton skein (1 g) in a 0.1 wt% solution of the prepared surfactants in distilled water at 25 °C according to the Draves technique [26]. The sinking time was measured in seconds.
Foam Measurements
A typical method in which 25 mL of the solution (1.0 wt%) was shaken vigorously for 10 s in a 100 ml graduated cylinder with glass stopper at 25 °C was used. The foam height was measured [27].
Emulsion Stability Measurements
In a 100-mL graduated, stoppered tube, an aqueous solution of the surfactant (10 mL, 20 mol) was mixed with light paraffin oil (6 mL). The mixture was shaken vigorously by magnetic stirring for 2 min at 25 °C. The formed emulsion was separated and observed. The time taken for the separation of the aqueous layer indicates the emulsion stability of the surfactant [28].
Interfacial Properties
The critical micelle concentration (CMC) is the minimum concentration at which surfactant molecules begin to form micelles [29]. CMC values were obtained through a conventional plot of the surface tension versus the concentration logarithm of the surfactant. The CMC concentration corresponds to the point where the surfactant first shows the lowest surface tension, and after which the surface tension remains nearly constant.
The effectiveness of a certain surfactant (π cmc) is expressed in terms of the decrease in the surface tension that is induced by this surfactant at the CMC [30]. The effectiveness was calculated from Eq. (1):
where γ o is the surface tension measured for the pure water at the appropriate temperature and γ cmc is the surface tension at the CMC.
Efficiency (pC20)
The efficiency of a surfactant (pC20) is the negative log of the surfactant concentration required to lower the surface tension of water by 20 mN/m [31].
Maximum Surface Excess Гmax
The maximum surface excess can be attained from the Gibbs Equation shown in Eq. (2):
where R is the ideal gas constant, T is the absolute temperature and dγ/dlogc is the slope of the surface tension versus logc plot at 25 °C [32].
Minimum Surface Area (A min)
Minimum surface area (A min) was calculated from Eq. (3) [33]:
where N is Avogadro’s number and Γmax is the maximum surface excess calculated from Eq. 2.
Biodegradability of the Synthesized Surfactants
The biodegradation tests of the synthesized nonionic surfactants were performed according to the river water Die-Away method [34] using Nile River water. In this test, a stirred solution containing the tested surfactant (1000 ppm) was incubated at 25 °C. Samples were withdrawn daily, filtered using Whatman filter paper (GE Healthcare Life Sciences) and the surface tension was measured using a Du Nouy tensiometer (Kruss type K6). The process was repeated for seven days. The biodegradation percentage (D) was calculated in terms of the measured surface tension according to Eq (4):
where γ0 is the surface tension at time zero (initial surface tension), γt is the surface tension at time t, and γbt is the surface tension of the blank experiment at time t (without surfactant).
Results and Discussion
Biological Activity
Investigation of the antimicrobial screening of some of the synthesized compounds (20–36) revealed that all the tested compounds showed antibacterial activity against Escherichia coli and Staphylococcus aureus, and also showed fungicidal activity on one or more of Aspergillus flavus and Candida albicans as shown in Table 1. Tetracycline and Amphotericin B were taken as a reference for antibacterial and antifungal agents, respectively. Formation of thiazole 33 and thiadiazole 36 showed an excellent activity against Aspergillus flavus. Thiazole 27 and thiadiazole derivative 28 exhibited good antibacterial activity. Moreover, pyrazole derivatives 23 and 30 exhibited moderate antifungal activity against Candida albicans. In addition, oxdiazine derivative 31 showed antibacterial and antifungal activities similar to the oxdiazole derivatives 21 and 35. The presence of the heterocyclic moiety in the prepared nonionic surfactant molecules appears to enhance antibacterial activity.
Surface Activity Properties
Moderate foaming and good detergency are surfactant characteristics employed in a variety of ways in the leather industry [35]. The surface activity and related properties of the synthesized compounds are given in Table 2. The surface activity properties were independent of the heterocyclic moiety but depended on the hydrophobicity (C12) and hydrophilicity (propylene oxide units), i.e., these compounds are used as effective emulsifying agents in many fields, such as cosmetics, formulations, pesticides, textiles, etc.
Surfactants are used in a large number of applications due to their ability to alter the energy relationships at interfaces and to lower surface and interfacial tension [36]. The surface tension results indicate surfactants 23 and 30 have the maximum ability while 21 shows the minimum ability to reduce surface tension of an aqueous system in the series of an amphiphile being reported. In addition, comparing structurally related triazole derivatives, 32 is slightly more effective in decreasing the surface tension than triazole derivatives 22 and 26. The oxazolidine derivatives 21 and 35 induced the lowest reduction in surface and interfacial tension of the surfactants tested.
Cloud Point
For nonionic surfactants, a common and characteristic observation is that they exhibit reverse solubility versus temperature behavior in water. The cloud point is a critical factor in the performance of nonionic surfactants; generally, nonionic surfactants show optimal effectiveness when used near or below their cloud point. The cloud point depends on the chemical structure [37, 38]. The results (Table 2) show that surfactants 26 and 32 have the maximum cloud point while amphiphile 21 has the minimum cloud point in the series of amphiphiles being reported. These results suggest that these surface-active compounds can be used over a wide range of temperatures.
Wetting Time
In the present work, the efficiency of the synthesized surfactants as wetting agents was measured according to the Draves technique [26], which measures the ability of a surfactant solution to displace air from a weighted skein of cotton by spread wetting. Shorter wetting times indicate more efficient wetting. Surfactants 20–36 demonstrated varying wetting abilities depending on the chemical structure, as indicated in Table 2. Surfactants 26 and 32 exhibited the shortest sinking time, and, consequently, are the most efficient wetting agents among the studied group.
Foaming Properties
The foaming ability of the synthesized surfactants was examined using the Ross Miles method [27]. As indicated in Table 2, surfactants 29 and 32 showed the least foaming. In generally, all of the synthesized surfactants have relatively low foam. Addition of PO groups to a surfactant usually lowers the foam due to a decrease in the cloud point. Branching in the headgroup also limits close packing of molecules at the air/water interface necessary to stabilize the foam lamella.
Emulsion Stability
Studies are still being carried out on the utilization of surfactants in emulsion formulation, which is of immense importance to technological development. In many textile processes, such as scouring and dyeing, it is necessary to add surfactants to the bath in order to remove oily impurities from the fibers. The emulsifying power of the prepared surfactants in terms of time needed for the separation of 9 mL of the solution is presented in Table 2. Generally, the measured time ranged between 102 and 165 min, thus, indicating moderate emulsifying properties for the synthesized surfactants. The moderate emulsion stability may mean a co-surfactant may be required to stabilize emulsions with these surfactants.
Critical Micelle Concentration
The CMC is a measure of surfactant efficiency as it indicates the amount of a surfactant required to reach maximum surface tension reduction. A surfactant having a low CMC value enjoys excellent wetting, emulsifying, solubilizing, and detergency properties [39]. Within a given series of structurally related surfactants, factors that lead to a decrease in the surface tension are expected to decrease the CMC value. As shown in Table 3, the synthesized surfactants 23 and 33 have the lowest CMC values, which can be attributed to lower solubility of the surfactant molecules.
Effectiveness (π cmc)
The maximum reduction in surface tension caused by the dissolution of amphiphilic molecules is a measure of its effectiveness (π cmc) [40, 41]. π cmc of the synthesized surfactants is the difference between the surface tension of distilled water and that at the CMC values The synthesized surfactants 31 and 35 have the greatest ability to reduce surface tension of the aqueous system, as shown in Table 3. Logically, the effectiveness of the prepared surfactants decreases as γ cmc increases.
Efficiency (pC20)
Efficiency of adsorption (pC20) is defined as the negative log of the surfactant concentration required to lower the surface tension of water by 20 mN/m. pC20 values are useful for comparing the efficiency of adsorption of a surfactant at the air/water interface [42]. The larger the pC20, the more efficiently the surfactant is adsorbed at the interface and the more efficiently it reduces the surface tension. pC20 values for the investigated surfactants are given in Table 3. In general, all of the synthesized compounds show good efficiency. The results indicate that surfactant 29 has the highest adsorption efficiency. The lowest pC20 value was observed for 23.
Maximum Surface Excess (Γmax)
The extent of surfactant adsorption at a liquid surface is expressed in terms of its surface excess concentration (Γ max ) which is defined as the excess surfactant present per unit area of surface over the bulk concentration. The surface excess depends on the molecular structure. A larger area per molecule indicates that the molecules are less tightly packed at the air/water interface [43]. The results given in Table 3 indicate that the consequential increase of Γmax leads to a decrease in A min values.
Minimum Surface Area (A min)
The minimum area per surfactant molecule A min at the air/water interface at surface saturation provides information about the degree of packing and the orientation of the adsorbed surfactant molecule [44]. The calculated average areas A min are given in Table 3. In general, most of the synthesized surfactants have low A min values, indicating tight packing at the interface. Surfactants 32 and 35 have the lowest A min values.
Biodegradability
Biodegradation is the destruction of a chemical by the metabolic activity of microorganisms. Since surfactants are susceptible to biodegradation and, in order to examine their effect on water pollution, the biodegradability of the synthesized compounds was evaluated. Biodegradation was evaluated by the conventional River Die-Away test employing the surface-tension technique as an analytical tool [45, 46]. The biodegradability data are presented in Table 4. Within experimental accuracy, all the prepared nonionic surfactants seem to be easily degraded. In general, all of the surfactants show 40–50 % biodegradation within the first day. With 7 days, all of the surfactants showed greater than 90 % degradation.
Conclusion
A new class of nonionic surface active agents containing heterocyclic moieties were synthesized. All the new nonionic surfactants displayed good surface activity properties that are affected by the size of the hydrophilic group. In general, all of the surfactants show low foaming potential and relatively short wetting times. The lower the CMC values and the higher the Гmax, the higher the emulsion stability for light paraffin oil. In addition, the synthesized surfactants showed good biodegradability within seven days.
References
Toliwal S, Jadav K, Patel K (2009) Synthesis and biological evaluation of fatty hydrazides of by-products of oil processing industry. Ind J Pharm Sci 71(2):144–148
Himani V, Aiman A, Abdul R, Fohad MH, Iqbal A (2014) Synthesis and antimicrobial evaluation of fatty chain substituted 2,5-dimethyl pyrrole and 1,3-benzoxazin-4-one derivatives (2014). J Saudi Chem Soc. doi:10.1016/j.jscs.2014.04.008
Kuldip U, Atul M, Ravi C, Chetna R, Kena R, Hardevsinh Vand Anamik S (2013) Synthesis and antitubercular activity of 1,3,4-oxadiazoles clubbed with pyrroles. Chem Biol Interface 3(2):107–115
Toliwal SD, Jadav K, Scientefic J (2009) Inhibition of corrosion of mild steel by phenyl thiosemicarbazides of nontraditional oils. Ind Res 68:235–241
Aamir M, Kumar S (2007) Synthesis and evaluation of antiinflammatory, analgesic, ulcerogenic and lipid peroxidation properties of ibuprofen derivatives. Acta Pharm 57:31–45
Radwan MA, Ragal EA, Sabry NM, El-Shenawy SM (2007) Synthesis and biological evaluation of new 3-substituted Indole derivatives as potential anti-inflammatory and analgesic agents. Bioorg Med Chem 15:3832–3841
Rauf A, Sharma S, Gangal S (2008) One-pot synthesis, antibacterial and antifungal activities of novel 2,5-disubstituted-1,3,4-oxadiazoles. Chin Chem Lett 19:5–8
Desai NC, Shihora PN, Moradia DL (2007) Synthesis and characterization of new quinazolines as potential antimicrobial agents. Ind J Chem B 46:550–553
Aamir M, Kumar S (2007) Synthesis and evaluation of anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation properties of ibuprofen derivatives. Acta Pharm 57:31–45
Aurangzeb H, Noel FT, Shelly G (2011) Synthesis, characterization and antifungal evaluation of 5-substituted-4-amino-1,2,4-triazole-3-thioesters. Molecules 16:1297–1309
Mustafa MA, Alaa HJ, Jawad KS (2012) Synthesis, Characterization and evaluation of biological activity of new heterocyclic compounds containing 1,2,4-triazoleand 1,3,4-thiadiazole rings. Int J Appl Sci Technol 2(10):155–164
Stefania-Felicia B, Gabriel S, Gabriela LA, Constantin D, Florica B, Gabriela B (2012) New heterocyclic compounds from 1,2,4-triazole and 1,3,4-thiadiazole class bearing diphenyl-sulfone moieties. Synthesis, characterization and antimicrobial activity evaluation. Eur J Med Chem 49:417–423
Priyanka R, Aastha P, Kishore D (2013) Microwave-assisted synthesis of [1, 2, 4]triazoles: a short review. Int J Chem Pharm Sci 4(3):13–18
Khairujjaman L, Aiman A, Rauf A (2014) Synthesis and spectral characterization of novel fatty acid chain substituted pyrazoline derivatives. Rasayan J Chem 7(3):276–280
Aiman A, Himani V, Rauf A, Fohad MH, Iqbal A (2014) Synthesis, biological screening of novel long chain derivatives of 1,3-disubstituted-1H-pyrazol-5(4H)-one and 2-substituted-3H-1,4-phthalazin-1,4-dione: structure-activity relationship studies. J King Saud Univ Sci 26:290–299
Yildirim A, Cetin M (2012) Synthesis and characterization of some novel higher c, n-diphenyl nitrones, isoxazolines, and mercaptobenzimidazoles as oleochemicals. Phosphorus Sulfur Silicon 187:952–964
Yildirim A, Ozturk S, Cetin M (2013) Long-chain alkylthia-benzimidazoles as corrosion inhibitors for carbon steel in H2SO4 solution. Phosphorus Sulfur Silicon 188:855–863
El-Sayed R, Asghar BH (2014) Synthesis of various nitrogen heterocycles as antimicrobial surface agents. J Heterocycl Chem 51:1245–1251
El-Sayed R (2013) Surface pharmaceutical application of pyrazole isoxazole, pyrimidine and pyridine derivatives. Afinidad 70(562):142–148
Sobhy M, El-Sayed R, Abdallah M (2013) The effect of non ionic surfactants containing triazole, thiadiazole and oxadiazole as inhibitors of the corrosion of carbon steel in 1 M hydrochloric acid. J Surf Deter 16(6):937–946
El-Sayed R (2013) Substituted Thiadiazole, Oxadiazole, Triazole and Triazinone as Antimicrobial and Surface Activity Compounds. J. Surf Deter 16(1):39–47
Morgós J, Sallay P, Farkas L, Rusznák I (1986) A new approach of ethoxylation catalyzed by bridge head nitrogen containing compounds. J Am Oil Chem Soc 63:1209–1210
Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (2007) Manual of clinical microbiology, 9th edn. ASM Press, Washington, pp 1152–1172
Weil JK, Stirton AJ, Nunez-Ponzoa MV (1966) Ether alcohol sulfates. The effect of oxypropylation and oxybutylation on surface active properties. J Am Oil Soc 43:603–609
Durham K (1961) Properties of detergent solutions-amphipathy and adsorption. In: Surf Activity Deterg, vol 1. MacMillan, London, pp 1–28
Draves CZ, Clarkson R (1931) A new method for the evaluation of wetting agents. J Am Dye Stuff Report 20:201
El-Sukkary MA, El-Sawy AA, El-Dib F (1987) Synthetic detergents from crude rice bran oil. Hung J Ind Chem 15:317–322
Lu JR, Lee EM, Thomas RK, Penfold J, Flitsch SL (1993) Direct determination by neutron reflection of the structure of triethylene oxide monododecyl ether layers at the air/water interface. Langmuir 9:1352–1358
Matsuoka K, Moroi Y (2003) Micellization of fluorinated amphiphiles. Curr Opin Coll Interface Sci 8:227–234
Zhigang Xu, Pengfei Li, Weihong Qi, Zongshi Li, Lubo Ch (2006) Effect of aromatic ring in the alkyl chain on surface properties of aryl alkyl surfactant solution. J Surf Deter 9:245–248
Karakashev SI, Nguyen AV, Miller JD (2008) Equilibrium adsorption of surfactants at the gas-liquid interface. Adv Polym Sci 218:25–55
Rosen MJ (2004) Surfactant and interfacial phenomena, 3rd edn. Willey, New York, p 63
Falbe J (1986) Surfactants for consumer, chap 4. Springer, Heidelberg
Pfaller MA, Burmeister L, Bartlett MA, Rinaldi MG (1988) Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol 26:1437–1441
Somaya AR, Eissa AMF, Nadia A, Ahmad MN (1998) Synthesis and characterization of some peptides having surface activity using polyethylene glycol. J Pharm Sci 7:27–32
Miller P, Westra P (1998) How surfactants work. Colorado State Univ. Coop. Ex., Prod. Crop Ser. No. 0.564
Chen ML, Wang ZW, Zhang GX, Gu J, Cun Z, Tao FM (2007) Studies on the cloud points of nonionic surfactants with QSPR. Chem Res Chin U 23:715–719
Ahmed MHM (2004) Preparation and surface active properties of novel succinic acid based surfactants. OLAJ Szappan Kozmet 53:23–28
Eissa AMF (2007) Synthesis and evaluation of some surface active agents from long chain fatty amine. Grasas Aceites 58(4):379–389
El-Sukkary MMA, Syed NA, Aiad I, El-Azab WIM (2008) Synthesis and characterization of some alkyl polyglycosides surfactants. J Surf Deter 11:129–137
Zhou L, Jiang X, Li Y, Chen Z, Hu X (2007) Synthesis and properties of a novel class of gemini pyridinium surfactants. Langmuir 23:11404–11408
Rosen MJ (1978) Surfactants and interfacial phenomena. Willey, New York
Lu JR, Lee EM, Thomas RK, Penfold J, Flitsch SL (1993) Direct determination by neutron reflection of the structure of triethylene oxide monododecyl ether layers at the air/water interface. Langmuir 9:1352–1358
Verma SK, Gosh KK (2011) Micellar and surface properties of some monomeric surfactants and a cationic gemini surfactant. J Surf Deter 14:347–352
Bhadani A, Singh S (2011) Synthesis and properties of thioether spacer containing gemini imidazolium surfactants. Langmuir 27:14033–14044
Karakashev SI, Nguyen AV, Miller JD (2008) Equilibrium adsorption of surfactants at the gas-liquid interface. Adv Polym Sci 218:25–55
Acknowledgments
The authors would like to thank the Institute of Scientific Research & Revival of Islamic Culture for providing facilities to carry out the research. The authors are also thankful to the staff of the Microbiology Department, Faculty of Science, Umm Al-Qura University for biological activity screening of the tested compounds.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
El-Sayed, R., Khairou, K.S. Propoxylated Fatty Thiazole, Pyrazole, Triazole, and Pyrrole Derivatives with Antimicrobial and Surface Activity. J Surfact Deterg 18, 661–673 (2015). https://doi.org/10.1007/s11743-015-1684-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1684-8