Abstract
Stearic acid (1) was utilized as a new cheap starting material in the manufacture of important biologically active heterocycles as thiadiazole, oxadiazole, triazole and triazinone derivatives (2–11) by treating with different nucleophiles. These heterocycles bears active hydrogen atom (SH, NH and NH2) which could be propoxylated by different moles of propylene oxide (5, 10, 15 mol) to produce nonionic compounds (12–21)a–c having functions as surface and antimicrobial activities. The structural elucidation of these compounds is based on IR, 1H NMR, 13C NMR and elemental analysis. The surface properties (surface and interfacial tension, cloud point, witting time, foaming properties and emulsion stability) and the biodegradability were screened beside these compounds have been tested for their antimicrobial activity.
Graphical Abstract
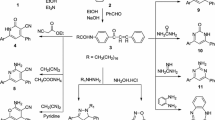
Stearic acid 1 was utilized as a new cheap starting material in the manufacture of important biologically active heterocycles as thiadiazole, oxadiazole, triazole and triazinone derivatives by treating with different nucleophile at different reaction conditions.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Our work on the synthesis of heterocyclic fatty compounds is closely related to Sharpless’s click chemistry. Our idea was to achieve access to pharmacologically interesting compounds based on renewable raw materials that serve as important feedstocks for the chemical industry with regard to a sustainable development [1] through simple transformations starting from easily available fatty compounds. Many fatty acids and their derivatives are known for their antimicrobial [2] and antifungal activities [3]. Fatty acid hydrazide is an important starting material for a wide range of derivatives used as pharmaceutical products and surfactants [4]. The synthesis of interesting heterocycles, such as 1,3,4-oxadiazoles are used as biologically active compounds in medical science and agriculture [5]. Various 1,3,4-oxadiazoles show herbicidal effects, especially against broad leafed weeds and grasses in crops such as rice and corn [6]. Also, 1,3,4-thiadiazoles were reported as highly anti-inflammatory [7], anti-microbial [8], pesticidal [9], antiparasitic property [10], anticancer [11], anticonvulsant agents [12–14]. We were interested in the synthesis of triazole, oxazole, thiadiazole, oxadiazole and further N and O/S-containing heterocyclic fatty acid derivatives in order to enlarge the variety of interesting fatty compounds and to open up a new potential for renewable raw materials as possible biologically active compounds. In view of the above mentioned facts and in continuation of our work on the syntheses of biologically important heterocyclic compounds [15–18]. It was interesting to prepare some biologically active heterocycles (triazole, thiadiazole and oxadiazole) which constitute an important class of organic compounds with diverse biological activities.
Experimental Protocols
All melting points are uncorrected and determined by the open capillary method using a Gallenkamp melting point apparatus. IR spectra (KBr disk) of the synthesized compounds were recorded on an FT/IR-BRUKER, Vector 22 (Germany), JASCO FT/IR-4100 (Japan), and JASCO FT/IR-460+ (Japan). 1H- and 13C-NMR spectra were recorded in deuterated chloroform (CDCl3) or dimethyl sulfoxide (DMSO-d6) as a solvent on a Varian Mercury VXR-300 spectrometer (300 MHz for 1H NMR and 75 MHz for 13C NMR) using TMS as internal reference and chemical shifts are expressed in δ (ppm). All the synthesized compounds gave satisfactory elemental analyses. Surface active properties were carried out at the Chemistry Department of the Faculty of Applied Science, Umm Al-Qura University, Saudi Arabia. Antibacterial and antifungal activity was carried out in Micro Analytical Center, Faculty of Science, Cairo University, Egypt.
2-Stearoylhydrazinecarbothioamide (2)
A solution of stearic acid hydrazide 1 (2 g, 0.05 mol) in ethanol (50 mL) to which mixed potassium thiocyanate (0.1 mol) and hydrochloric acid 3 mL were added with constant stirring for 2 h, the mixture was immediately evaporated to dryness on a steam bath. On cooling, the separated solid was filtered, washed with cold water, dried and recrystallized from ethanol as white needles. (1.37 g; 73 %), mp 122–124 °C.; IR: 3,433, 3,279, 3,134 for primary and secondary amino group, 2,919, 2,850 (CH aliphatic), 1,696 (CO) and 1,290 (C=S) cm−1. 1H NMR (CDCl3): δ 0.89 (t, 3H, terminal CH3), 1.24–2.15 (m, 32H, CH aliphatic), 7.40 (s, 1H, CONHNH), 8.98 (s, 2H, NH2), 10.29 (s, 1H, CONH). Anal. calc. for C19H39N3OS (357.60): C, 63.82; H, 10.99; N, 11.75; S, 8.97. Found C, 63.87; H, 11.04; N, 11.71; S, 9.02.
3-Heptadecyl-1H-1,2,4-triazole-5(4H)-thione (3)
A solution of thiosemicarbazide 2 (3 g, 0.01 mol) in ethanol (15 mL) and potassium hydroxide (10 %, 10 mL) was refluxed for 7–8 h on a steam bath. It was cooled and acidified with dilute HCl. The resulting solid was filtered, dried and recrystallized from ethanol to yield 3 (2.15 g; 71 %), mp 91–93 °C.; IR: 3,421, 3,196 (NH), 2,919, 2,849 (CH aliphatic), 1,617 (C=N), 1,309 (C=S) cm−1; 1H NMR (CDCl3): δ 0.86 (t, 3H, terminal CH3), 1.26–1.86 (m, 32H, CH aliphatic), 10.97 (s, 1H, NH), 13.98 (s, 1H, NH). 13C NMR (DMSO-d6): δ 13.70, 16.57, 22.31, 25.25, 28.45, 28.47, 28.52, 28.53, 28.58, 28.75, 28.84, 28.88, 29.02, 29.09, 31.31, 33.68, 39.07, 154.79, 175.22. Anal. Calc. for C19H37N3S (339.58): C, 67.20; H, 10.98; N, 12.37; S, 9.44. Found C, 67.27; H, 11.01; N, 12.35; S, 9.40.
5-Heptadecyl-1,3,4-oxadiazol-2-amine (4)
A solution of thiosemicarbazide 2 (1.4 g, 0.01 mol) in ethanol (15 mL) was added to a solution of sodium hydroxide (5 N, 5 mL) with cooling and stirring. To this clear solution, a solution of I2/KI was added till permanent tinge of iodine persisted at room temperature. The mixture was immediately refluxed and more I2/KI was added till a permanent tinge was obtained. The mixture was then cooled and poured into ice-cold water; the solid that separated was collected by filtration, washed with water and with dilute thiosulfate solution and again with water. The solid was dried and recrystallized from ethanol to give 4 (1.25 g; 62 %), mp 65–67 °C.; IR: 3,316, 3,144 (NH2), 1,612 (C=N), 1,166 and 715 (oxadiazole ring) cm−1. 1H NMR (CDCl3): δ 0.89 (t, 3H, terminal CH3), 1.24–2.15 (m, 32H, CH aliphatic), 6.67 (s, 2H, NH2 which disappeared on addition of D2O). 13C NMR (CDCl3): δ 14.15, 20.95, 22.70, 24.12, 24.84, 25.57, 29.16, 29.37, 29.44, 29.47, 29.51, 29.59, 29.63, 29.66, 29.67, 29.70, 31.92, 153.48, 178.64. Anal. Calc. for C19H37N3O (323.52): C, 70.54; H, 11.53; N, 12.99. Found C, 70.51; H, 11.50; N, 12.96.
5-Heptadecyl-1,3,4-thiadiazol-2-amine (5)
Sulfuric acid (98 %; 10 mL) was added to the thiosemicarbazide 2 (1.5 g, 0.05 mol), and the mixture was stirred for 24 h at room temperature. The mixture was then poured onto crushed ice (200 g), neutralized with concentrated ammonium hydroxide solution and stirred for 20 min. The separated crude product was filtered, washed with water, dried and recrystallized from aqueous ethanol to afford 5 (079 g; 52 %), mp 108–110 °C.; IR: 3,319, 3,188 (NH2), 1,603 (C=N) cm−1. 1H NMR (CDCl3): δ 0.88 (t, 3H, terminal CH3), 1.25–2.59 (m, 32H, CH aliphatic), 7.82 (S, 2H, NH2 which disappeared on addition of D2O). Anal. Calc. for C19H37N3S (339.58): C; 67.20, H; 10.98, N; 12.37, S; 9.44. Found: C; 66.91, H; 10.67, N; 12.18, S; 9.23.
5-Heptadecyl-1,3,4-thiadiazol-2(3H)-one (6)
Sodium nitrite solution (10 %; 10 mL) was added dropwise to an ice cooled suspension of 5 (0.95 g, 0.01 mol) and hydrochloric acid (5 mL) in cold water (20 mL), with continuous stirring over a period of 20 min. The temperature was then allowed to rise to room temperature and the mixture was heated to boiling for 10 min, cooled and allowed to stand overnight. The separated crude product was filtered, washed with water, dried and recrystallized from ethanol to yield 6 (0.55 g; 58 %), mp 65–67 °C.; IR: 3,271 (NH), 1,684 (CO), 1,611 (C=N) cm−1. 13C NMR (CDCl3): δ 14.15, 25.25, 28.45, 28.47, 28.52, 28.53, 28.58, 28.75, 28.84, 28.88, 29.02, 29.04, 29.09, 31.31, 33.68, 39.07, 31.92, 154.74, 175.22. Anal. Calc. for C19H36N2OS (340.57): C, 67.01; H, 10.65; N, 8.23; S, 9.42. Found C, 67.23; H, 10.75; N, 8.03; S, 9.22.
N-phenyl-2-stearoylhydrazinecarbothioamide (7)
Equimolar quantities (1.7 g, 0.01 mol) of acid hydrazide 1 and phenyl isothiocyanate (0.01 mol) were dissolved in absolute ethanol (25 mL) in a round-bottom flask. The solution was refluxed for 3 h on a water bath. The solution was concentrated under reduced pressure and the solid separated was collected and recrystallized from ethanol to give 7 (1.2 g; 70 %), mp 139–141 °C.; IR: 3,396, 3,221 (NH), 1,676 (CO), 1,209 (C=S) cm−1. 1H NMR (CDCl3): δ 0.86 (t, 3H, terminal CH3), 1.19–2.47 (m, 32H, CH aliphatic), 4.59 (s, 1H, NH which disappeared on addition of D2O), 7.31–7.33 (m, 5H, ArH), 7.59 (S, 1H, NH), 11.36 (S, 1H, NH). Anal. Calc. for C25H43N3OS (433.69): C, 69.23; H, 9.99; N, 9.69; S, 7.39. Found C, 69.38; H, 10.05; N, 9.49; S, 7.43 %.
3-Heptadecyl-4-phenyl-1H-1,2,4-triazole-5(4H)-thione (8)
A solution of 7 (1.2 g, 0.01 mol) in ethanol (15 mL) was added to a solution of sodium hydroxide (5 N, 5 mL) with cooling and stirring. The reaction mixture was refluxed for 6–8 h. After completion, the reaction mixture was allowed to cool, filtered and acidified with hydrochloric acid (2 M). The precipitate obtained was filtered washed with water and dried and recrystallized from ethanol to obtain 8 (0.82 g; 68 %), mp 85–87 °C.; IR: 3,228 (NH), 3,043 (CH aromatic), 1,601 (C=N), 1,186 (C=S) cm−1. 1H NMR (CDCl3): δ 0.86 (t, 3H, terminal CH3), 1.23–2.76 (m, 32H, CH aliphatic), 11.99 (s, 1H, NH which disappeared on addition of D2O), 7.29–7.57 (m, 5H, ArH). 13C NMR (CDCl3): δ 13.75, 24.66, 28.37, 28.48, 28.58, 28.66, 28.74, 28.84, 28.86, 28.86, 28.89, 28.91, 28.96, 28.99, 29.07, 31.30, 33.65, 33.70, 39.08, 116.69, 121.22, 128.42, 129.47, 154.74, 173.10. Anal. Calc. for C25H41N3S (415.68): C, 72.24; H, 9.94; N, 10.11; S, 7.71. Found C, 72.33; H, 10.02; N, 10.25; S, 7.83.
5-Heptadecyl-N-phenyl-1,3,4-thiadiazol-2-amine (9)
A solution of 7 (0.95 g, 0.005 mol) was added portionwise to (25 mL) of concentrated sulfuric acid at 0 °C with continuous stirring. The reaction mixture was stirred further for 3 h at room temperature and then allowed to stand overnight. Neutralization with diluted sodium hydroxide precipitated a crude solid, which was filtered, and washed with water. The crude product was then recrystallized from DMF to furnish 9 (0.51 g; 53 %), mp 89–91 °C.; IR: 3,221 (NH), 1,601 (C=N) cm−1. 1H NMR (CDCl3): δ 0.95 (t, 3H, terminal CH3), 1.43–2.96 (m, 32H, CH aliphatic), 6.50–7.08 (m, 5H, ArH), 8.01 (s, 1H, NH which disappeared on addition of D2O). Anal. Calc. for C25H41N3S (415.68): C, 72.24; H, 9.94; N, 10.11; S, 7.71. Found C, 72.41; H, 10.11; N, 10.18; S, 7.88.
5-Heptadecyl-N 3-phenyl-4H-1,2,4-triazole-3,4-diamine (10)
A mixture of 7 (1.3 g, 0.005 mol) and hydrazine hydrate (0.025 mol) was refluxed in ethanol (20 mL) for 3 h. The reaction mixture was cooled and poured over crushed ice. The solid separated was filtered and recrystallized from ethanol/water to get 10 (0.85 g; 65 %), mp 118–120 °C.; IR: 3,241, 3,160 (NH), 3,052 (CH aromatic), 1,601 (C=N) cm−1. 1H NMR (CDCl3): δ 0.87 (t, 3H, terminal CH3), 1.21–2.97 (m, 32H, CH aliphatic), 5.02 (s, 1H, NH2 which disappeared on addition of D2O). 7.17–7.63 (m, 5H, ArH), 10.08 (s, 1H, NH). Anal. Calc. for C25H43N5 (413.64): C, 72.59; H, 10.48; N, 16.93. Found C, 72.72; H, 10.64; N, 17.15.
3-Heptadecyl-1,2-dihydro-1,2,4-triazin-5(6H)-one (11)
Acid hydrazide 1 (1.6 g, 0.01 mol) and chloroacetamide (0.41 g, 0.01 mol) were refluxed in DMF (30 mL) for 26–30 h. After the completion of the reaction (monitored by TLC), the reaction mixture was concentrated, cooled and poured into ice-cold water (100 g). The desired triazinone which separated out was filtered and dried. Further purification by column chromatography over silica gel using a petroleum ether-diethyl ether mixture as the eluent afforded 11 (1.2 g; 75 %), mp, 144–146 °C.; IR: 3,454, 3,231 (NH), 1,687 (CO), 1,566 (C=N) cm−1. 1H NMR (CDCl3): δ 0.85 (t, 3H, terminal CH3), 1.24–1.65 (m, 32H, CH aliphatic), 2.15 (s, 2H, CH2 of triazinone ring), 3.89 (s, 1H, NH2), 6.67 (s, 1H, NH which disappeared on addition of D2O). Anal. Calc. for C20H39N3O (337.54): C, 71.17; H, 11.65; N, 12.45. Found C, 71.25; H, 11.74; N, 12.28.
Preparation of Nonionic Surfactants (12–21a–c) from the Synthesized Compounds (2–11)
The terms of nonionic surfactants refers chiefly to polyoxypropylene derivatives, they are usually prepared by the addition of different moles (n) of propylene oxide (n ≈ 5, 10, 15 mol) to synthesized products (2–11) at any active hydrogen atoms (NH, NH2, SH) using KOH as the catalyst. The processes were completed as described in [19]. The accurate amount of propylene oxide taken up and average degree of propenoxylation (n) was determined from the increased mass of the reaction mixture and confirmed by spectroscopy. The structures of the synthesized nonionic surfactants were confirmed via IR and 1H-NMR spectra. All IR spectra after the addition of the propylene oxide, showed, two broad bands at 1,070 and 960 cm−1 characteristic for the νC–O–C ether linkage of polypropenoxy chain, beside the original bands of the compound and 1H-NMR spectra after the addition of propylene oxide, showed, the protons of propenoxy group were assigned as a broad multiple signals in the region (3.1–3.9) ppm, beside the other protons of the compound.
Biological Activity
Antimicrobial activity of the prepared compounds was tested via a modification of the cup-plate method [20].
The Surface Active Properties
Surface and Interfacial Tensions
Surface tension and interfacial tension were measured using a Du Nouy tensiometer (KRUSS type 8451), at various concentration of the synthesized surfactants (0.05–10−6 mol/L) and at 25 °C [21].
Cloud Point
The cloud point, measure as inverse solubility characteristic of nonionic surface active agents, was determined by gradual heating 1.0 % wt solution in a controlled temperature bath recording the temperature at which the clear or nearly clear solutions become definitely turbid. Cooling the solutions until they become clear again confirmed the reproducibility of this temperature [22].
Wetting Time
The wetting powers of the tested surfactants were determined by immersing a sample of cotton fabric in 1.0 wt aqueous solution of the surfactants and measuring the sinking time in seconds [23].
Foaming Properties
The foam production for 1.0 wt solution was measured by the foam height initially produced [24].
Emulsion Stability
The emulsion was prepared from 10 mL of a 20 mmol/L aqueous solution of surfactant and 5 mL of toluene at 40 °C. The emulsifying properties was determined by the time it took for an aqueous volume separating from the layer to reach 9 mL counting from the moment of the cession shaking [24].
Biodegradability
Samples which were taken daily or more frequently were filtered through filter paper before measuring the surface tension. Surface tension measurements were made periodically (each day) on each sample during the degradation test [25] Biodegradation percent (D) for each sample was calculated using the following relation. D = [(γ t − γ0)/(γ bt − γ0)] where γt = Surface tension at time t. γ0 = Surface tension at time zero (initial S. T.). γ bt = Surface tension of the blank experiment at time t (without sample).
Chemistry
Stearohydrazide (1) used as a starting material, was conveniently prepared from stearic acid following the previously reported method [26]. The behavior of 1 toward some nitrogen nucleophiles was investigated. Thus, the reaction of 1 with potassium thiocyanate in methanol and hydrochloric acid afforded thiosemicarbazide derivative 2. The latter compound proved to be a useful key intermediate in the synthesis of several heterocyclic nuclei. Thus, when 2 was refluxed with potassium hydroxide in ethanol yielded 3-heptadecyl-1H-1,2,4-triazole-5(4H)-thione (3). Treatment of 2 with potassium iodide and iodine in the presence of sodium hydroxide furnished 5-heptadecyl-1,3,4-oxadiazol-2-amine (4). Also, cyclization of 2 in the presence of conc. H2SO4 produced 5-heptadecyl-1,3,4-thiadiazol-2-amine (5) which was treated with sodium nitrite in the presence of hydrochloric acid to give 5-heptadecyl-1,3,4-thiadiazol-2(3H)-one (6) (Scheme 1).
On the other hand, the reactivity of 1 towards phenyl isothiocyanate under different reaction conditions was investigated. Thus, the reaction of 1 with phenyl isothiocyanate in dry benzene under reflux gave the thiosemicarbazide derivative 7 which was subjected to intermolecular cyclization in alkaline medium (2 M NaOH) followed by acidification with HCl to give 1,2,4-triazole derivative 8. Also, cyclization of 7 in the presence of conc. H2SO4 gave thiadiazole derivative 9. Moreover, treatment of 7 with hydrazine hydrate afforded 1,2,4-triazole derivative 10. Of particular interest, a cyclocondensation reaction of 1 with chloroacetamide in DMF under reflux conditions gave 3-heptadecyl-1,2-dihydro-1,2,4-triazine-5(6H)-one (11).
Conversion of the Prepared Compounds (2–11) to Nonionic Surfactants (12–21)a–c
The built up surfactant molecules containing a heterocyclic moiety provide us with a most important class of surface active agents due to their dual characters, one due to conflict between the affinity of the hydrophobic and hydrophilic structure shows surface active properties and another one that is due to the heterocyclic moiety confirmed with aid of a hydrophilic moiety (propylene oxide) give its biological activity. Propoxylation of the new compounds (2–11) with various quantities of propylene oxide (5, 10, and 15 mol) produced nonionic surfactants (12–21)a–c, respectively. The surface active properties of the prepared propoxylated compounds (12–21)a–c were measured in a neutral medium by traditional procedures to evaluate the possible utilization of these compounds in various industrial fields. Scheme (2 ) shows the propenoxylation of compounds 5 and 10 as examples. The structures of the synthesized chemicals are listed in (Table 4).
Biological Activity
Some of the synthesized compounds were screened in vitro against some bacteria such as Escherichia coli, Staphylococcus aureus and some fungi such as Aspergillus flavus and Candida albicans. Gentamycin was taken as a positive reference for antibacterial activity. The results are tabulated in (Table 1), which shows that the samples have high antibacterial and moderate antifungal activities on the tested microorganisms. The results revealed that compounds 3, 8, 21c were found to have an excellent antibacterial activity against E. coli and S. aureus, while compounds 6, 15b, 19c, 20b exhibited a moderate antibacterial activity and compounds 4, 5 showed only a slight antibacterial activity. Furthermore, compounds 3 and 8 were found to be excellent antifungals. Moreover, the biological activities of some tested compounds after propoxylation showed a greater variation than those without propylene oxide.
Surface Active Properties
Nonionic surfactants are used in diverse applications, both in industry and at home. Their moderate foaming and good detergency are employed in a variety of ways in the leather industry [27]. It is used to accelerate soaking, and liming is improved by the addition of wetting agents [28]. In addition, nonionic surfactants are used extensively because of their good detergency, easy rinsing and low foaming in the cleaning of milk and beer bottles. The surface active and related properties of the synthesized compounds including, surface and interfacial tension, cloud point, wetting time, foaming, and emulsification properties are given in (Table 2 ).
The data in Table 2 show that the surface and interfacial tensions increased upon increasing the number of propylene oxide units added to the molecule [29]. All these compounds show high cloud points, when in hot water, which increased with an increasing number of moles of propylene oxide [30]. Also, the synthesized compounds exhibited efficient wetting properties that wetting time decreased with increasing numbers of propylene oxide units. Emulsion stability is found to decrease with increasing numbers of propylene oxide units [31], while the foam height is found to increase [32]. The surface active properties were independent of the heterocyclic moiety but dependent on the hydrophobic (C18) and hydrophilic (propylene oxide) units; however, the heterocyclic moiety reveals biological activities of the synthesized molecules, i.e. these compounds are used as effective emulsifying agents in many fields, such as cosmetics, formulations, pesticides, dyes, textiles, etc.
Biodegradability
For keeping the environment free from pollution, the biodegradability of the synthesized compounds was evaluated, which was determined by the die-away test, followed by surface tension measurements [33]. The biodegradability data are given (Table 3) within the experimental accuracy; all the prepared nonionic surfactants seem to degrade easily. Biodegradation of these compounds was found to depend mainly on the propylene oxide chain length when they had the same hydrophobic part. Also, the results showed that on the first day 40–50 % of the surfactants was biodegradable, and after that they disappeared completely after 6 days, which means that these compounds are safe for human beings as well as for the environment (Table 4).
References
Sandra F, Jurgen OM (2003) Synthesis of new heterocyclic fatty compounds. Eur J Org Chem 2003:885–893
Rauf A, Bandary MR, Matto RM (2008) Synthesis, characterization and antimicrobial activity of long-chain hydrazones. Acta Chim Solv 55:448
Mujeebur-Rahman VP, Mukhtar S, Ansari WH, Lemiere G (2005) Synthesis, stereo-chemistry and biological activity of some novel long alkyl chain substituted. Eur J Med Chem 40:173
Ansari FA, Quraishi MA (2010) Oleo-chemicals triazoles as effective corrosion inhibitors for mild steel in acetic acid media. PetroMin PipeLiner 36–42
Toliwal SD, Jadav K, Scientefic J (2009) Inhibition of corrosion of mild steel by phenyl thiosemicarbazides of nontraditional oils. Ind Res 68:235–241
Eissen M, Metzger JO, Schmidt E, Schneidewind U (2002) 10 Years after Rio—Concepts on the contribution of chemistry to a sustainable development. Angew Chem Int Ed 41:414–436
Aamir M, Kumar S (2007) Synthesis and evaluation of anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation properties of ibuprofen derivatives. Acta Pharm 57:31–45
Radwan MA, Ragal EA, Sabry NM, El-Shenawy SM (2007) Synthesis and biological evaluation of new 3-substituted Indole derivatives as potential anti-inflammatory and analgesic agents. Bioorg Med Chem 15:3832–3841
Ahmed SM, Ahmed F, Osman SM (1985) Preparation and characterization of derivatives of isoricinoleic acid and their antimicrobial activity. J Am Oil Chem Soc 62:1578–1580
Al-Qahtani A, Siddiqui YM (2009) Inhibition of growth of Leishmania donovani promastigotes by newly synthesized 1,3,4-thiadiazole analogs. J Saudi Pharm Soc 16:227–232
Bayrak H, Demirbas A, Karaoglu SA, Demirbas N (2009) Synthesis of some new 1,2,4-triazoles, their mannich and schiff bases and evaluation of their antimicrobial activities. Eur J Med Chem 44:1057–1066
Almasirad A, Tabatabai SA, Faizi M (2004) Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg Med Chem 14:6057–6059
Rauf A, Sharma S, Gangal S (2008) One-pot synthesis, antibacterial and antifungal activities of novel 2,5-disubstituted-1,3,4-oxadiazoles. Chin Chem Lett 19:5–8
Oruc EE, Rallas S (2004) 1,3,4-Thiadiazole derivatives. Synthesis, structure elucidation, and structure-antituberculosis activity relationship investigation. J Med Chem 47:6760–6767
Biermann U, Friedt W, Lang S, Lürs W, Machmüller G, Metzger JO, Rüschgen KM, Schäfer HJ, Schneider MP (2000) New syntheses with oils and fats as renewable feedstock for the chemical industry. Angew Chem Int Ed 39:2206–2224
Amine MS, Aly AA, El-Sayed R (2006) Synthesis and surface active properties of condensed and non-condensed quinazoline derivatives of industrial application. Ind J Chem 45B:1020–1027
Eissa AMF, El-Sayed R (2006) Synthesis and evaluation of condensed and noncondensed heterocyclic compounds of industrial application. J Heterocycl Chem 43:1161–1168
El-Sayed R (2008) Surface active properties and biological activity of novel nonionic surfactants containing pyrimidines and related nitrogen heterocyclic ring systems. Grasas Aceites 59:110–420
El-Sawy AA, Mahmoud AA, Shaker NO (1990) Preparation and surface active properties of oxypropylated diol monoesters of fatty acids. J Serb Chem Soc 55(7):395
Findly A (1963) Practical physical chemistry, 6th edn. Longmans, London, p 1040
Eissa AMF (2007) Synthesis and evaluation of some surface active agents from long chain fatty amine. Grasas Aceites 58:379–389
Durham K (1961) Properties of detergent solutions-amphipathy and adsorption, surface activity and detergency, vol 1. MacMillan, London, pp 1–28
Draves CZ, Clarkso R (1931) Surface activity and detergency. J Am Dye Stuff Rep 20:201
Ross J, Milles GD (1941) Apparatus for comparison of foaming properties of soaps and detergents. Oil Soap 18:99–102
Falbe J (1986) Surfactants for consumer. Springer, Heidelberg, vol 4, pp 139–141
Amine MS, Eissa AMF, Elsawy AA, Shaaban AF, El Sayed R (2004) New heterocyclic having double characters as antimicrobial and surface active agents. Part 3: nonionic compounds from fatty acid hydrazide. OLAJ Szappan Kozmetika 53:124–128
Eissa AMF, Ahmed MHM (2003) Nonionic surface active agents containing heterocyclic moieties. J Olaj Szappan Kozmetika 52:11–17
Rosen MJ (1989) Surfactants and interfacial phenomena, 2nd edn. Wiley, New York, p 286
Chlebicki J, Sokołowski A (1991) Adsorption of n-alkylthiooligooxypropylene glycols at aqueous solution-air interface. Colloids Surf 56:251–261
Ahmed MHM (2004) Preparation and surface active properties of novel succinic acid based surfactants. J Olaj Szappan Kozmetika 53:23–28
Takeshi H (1970) Studies of ester containing surfactant: preparation and properties of sodium sulphalkanoates. Bull Chem Soc 43:2236–2239
Seong KT, Toshiyaki K, Toshikozu NH, Ikeda JI (1996) Surface-active properties of novel cationic surfactants with two alkyl chains and two ammonio groups. J Am Oil Chem Soc 73:907–911
Eter ET, Richard RE, David A (1974) Biodegradable surfactants derived from corn starch. J Am Oil Chem Soc 51:486–494
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
El-Sayed, R. Substituted Thiadiazole, Oxadiazole, Triazole and Triazinone as Antimicrobial and Surface Activity Compounds. J Surfact Deterg 16, 39–47 (2013). https://doi.org/10.1007/s11743-012-1368-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1368-6