Abstract
New pyridinium Gemini surfactants have been synthesized by esterification of renewable fatty acids with halogenated alcohols furnishing respective esters (2-chloroethyl hexadecanoate, 2-chloroethyl tetradecanoate, 2-chloroethyl dodecanoate, 2-bromoethyl hexadecanoate, 2-bromoethyl tetradecanoate and 2-bromoethyl dodecanoate) followed by their subsequent treatment with 4,4′-trimethylenedipyridine resulting into the formation of title Gemini surfactants: (4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecanoyl oxy) ethyl) dipyridinium chloride(7), (4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecanoyl oxy) ethyl) dipyridinium chloride (8), 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecanoyl oxy) ethyl) dipyridinium chloride (9), (4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecanoyl oxy) ethyl) dipyridinium bromide (10), (4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecanoyl oxy) ethyl) dipyridinium bromide (11), 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecanoyl oxy) ethyl) dipyridinium bromide (12). Their identifications are based on IR, 1H-, 13C-NMR, DEPT, COSY and mass spectral studies. Their surface active properties are also evaluated on the basis of surface tension and conductivity measurements and thermal stability of these long chain cationics Gemini surfactants have been measured by thermal gravimetric analysis under nitrogen atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis of surfactants based on natural renewable raw material is carried out extensively in the cosmetic and detergent industries. In order to achieve these objectives, it is necessary to use renewable low-cost materials that are available in large quantities and to design molecular structures that shows improved performance, favorable properties and reduced environmental impact. The search for novel surfactants with higher efficiency and effectiveness gave birth to the concept of Gemini surfactants. The new class of Gemini cationic surfactants is a response to the increasing consumer demand for the products. Gemini surfactants are a new generation of surfactants composed of two monomeric surfactant molecules chemically bonded together by a spacer at or near their head groups. Thus, Gemini surfactants possess two hydrophilic and two hydrophobic groups. They are more surface-active and have much lower critical micelle concentration (CMC) values than their monomeric counterparts [1–3]. Because of their unique physical–chemical properties, Gemini surfactants continue to gain widespread interest for various applications [4, 5]. They possess special and unusual aggregation properties, etc. [6–9]. So they were widely used as effective emulsifiers, bactericidal agents, dispersants, anti-foaming agents, detergents, etc. These compounds are also applicable in the solubilization of dyes and pigments in the textile industry [10–13], gene therapy [14–17], the synthesis of highly mesoporous materials [18, 19], etc. A wide range of original surfactants derived from renewable resources have been developed with potential applications notably, in detergent and cosmetic industries. The production of these entirely natural molecules may substitute the surfactants conventionally used. If they have been designed using environmentally friendly processes.
Environmental concern has become one of the major driving forces for the development of new surfactants. Nowadays the scientific community trying to synthesize the surfactants which are not harmful to the environment and are easily biodegradable. The surfactants containing ester bonds are readily biodegradable [2]. Gemini surfactants with an ester group have high surface activity too.
Keeping in view the past work and perception on cationic Gemini surfactants we have attempted to synthesize them from materials like fatty acids and halo-alcohols. The purpose of this work was to prepare and characterize the cationic Gemini surfactants with ester bonds inserted between the hydrocarbon tails and the positively charged head groups and to evaluate their surface active properties.
Experimental Section
Materials
Chloroethanol, bromoethanol and 4,4′-trimethylenedipyridine were purchased from Sigma–Aldrich Chemical Co. USA. Lauric acid, Myristic acid, Palmitic acid and silica gel for T.L.C were purchased from S. D. Fine Chemicals Ltd; Mumbai, India. Sulfuric acid was purchased from Merck, Germany.
Instrumentation
IR spectra were recorded as a thin film on KBr Pellet on a Shimadzu 8400s FT-IR (Kyoto, Japan) instrument. Mass spectra were recorded on Waters Q-T of Micro mass using ESI as an ion source at sophisticated analytical instrumentation facility (SAIF), Panjab University, Chandigarh. 1H-, DEPT and 13C-NMR spectra were recorded on a JEOL AL-300 (JEOL, Japan) system as a solution in CDCl3, using tetramethylsilane (TMS) as an internal standard.
Preparation of 2-Chloro/bromoethyl (Dodecanoate, Tetradecanoate, Hexadecanoate)
The synthesis of compounds (1–6) was done by stirring fatty acids (palmitic, myristic and lauric 0.01 mol; 2.56, 2.28 and 2 g) with halogenated alcohols (chloroethanol and bromoethanol 0.01 mol; 0.080 and 1.23 g) followed by the addition of a catalytic amount of sulfuric acid [20]. All the reactants were then stirred for 2–3 h at 60 °C. The progress of reaction was monitored by thin layer chromatography [silica gel G coated (0.25 mm thick) glass plates using hexane: ethyl acetate (98:2) as mobile phase, the spots were visualized in iodine]. The reaction got completed in 3 h. The method of purification of the resulting esters was reported in our previous work [21]. The yields of resulting esters are reported in parenthesis [2-chloroethyl hexadecanoate (1, 92 %), 2-chloroethyl tetradecanoate (2, 92 %), 2-chloroethyl dodecanoate (3, 91 %), 2-bromoethyl hexadecanoate (4, 90.1 %), 2-bromoethyl tetradecanoate (5, 94.4 %) and 2-bromoethyl dodecanoate (6, 92 %)].
Synthesis of Gemini Surfactants
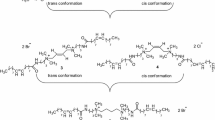
Each resulting ester (1–6) was then reacted with 4,4′-trimethylenedipyridine in 2:1 molar ratio (0.02 mol for esters 1–6 and 0.01 mol for 4,4′-trimethylenedipyridine) at 60 °C for 2 h (for chloro esters) and 30 min (for bromo esters) i.e. for 1, 6.364 g; for 2, 5.816 g; for 3, 5.241 g; for 4, 7.244 g; for 5, 6.682 g; for 6, 612.24 g; and 4,4′-trimethylenedipyridine (1.982 g) were taken. In each case, the resulting crude product was crystallized with ether and subsequently recrystallized in cold acetone to get the pure compounds (7–12) which were characterized on the basis of IR, 1H-, 13C-NMR, DEPT, COSY experiments and mass spectral analysis as (4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecanoyl oxy) ethyl) dipyridinium chloride(7), (4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecanoyl oxy) ethyl) dipyridinium chloride (8), 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecanoyl oxy) ethyl) dipyridinium chloride (9), (4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecanoyl oxy) ethyl) dipyridinium bromide (10), (4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecanoyl oxy) ethyl) dipyridinium bromide (11), 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecanoyl oxy) ethyl) dipyridinium bromide (12) (Scheme 1).
Conductivity Measurements [21, 22]
The CMC of these surfactants (7–12) were determined by the conductivity method. The conductivity as a function of surfactant concentration was measured at 25 °C. Measurements were performed with an Equiptronics Conductometer (Auto temperature conductivity meter model E.Q.661) with stirring to control the temperature. The solutions were thermostated in the cell at 25 °C. The curve of conductivity versus surfactant concentration was taken as the CMC. The degree of counterion binding (β) was calculated as (1−α), where α = smicellar/spremicellar, i.e., ratio of the slope before and after CMC.
Surface Tension Measurements
Surface tension values were used to calculate CMC using a CSC Du Nouy interfacial tensiometer (Central scientific Co., Inc.) equipped with a platinum-iridium ring (circumference 5.992 cm) at 25 °C. The tensiometer was calibrated using triple distilled water. For the determination of CMC and surface tension, adequate quantities of a concentrated stock solution were used. The data of this determination is presented in Table 1.
Thermal Stability Measurements
The thermal stability of the present Gemini surfactants were measured with an SDT Q600 thermal gravimetric analyzer (TGA), using a nitrogen atmosphere. All samples were measured in aluminum pans under a nitrogen atmosphere at a heating rate of 10 °C/min.
Analysis of Products
The structures of the ester-based Gemini pyridinium surfactants (7–12) have been established by IR, 1H-, 13C-NMR, DEPT and mass spectral data. The IR spectra of the pyridinium Gemini surfactants (7–12) showed the absorption bands in the region at 2,915–2,849 cm−1 indicating the presence of methylene groups. The absorptions at 1,741–1,730 cm−1 indicate the presence of ester carbonyl group whereas other absorptions at 1,641–1,620 cm−1 indicate the presence of C=N. The band at 1,570–1,540 cm−1 very well established, the presence of aromatic C=C of all products (7–12). The two terminal methyl protons of these Gemini surfactants (7–12) were observed as a distorted triplet at δ 0.87–0.88 in their 1H-NMR spectra. Broad singlets in (7–12) were observed at δ 1.25–1.26 accountable for methylene protons of chain. Triplet signals were observed at δ 1.65–1.67 due to presence of methylene protons next to terminal methyl groups. Multiplets were observed at δ 1.92–2.17 due to sandwiched methylene protons of spacer (PyCH2 CH 2 CH2Py). Other multiplets were observed at δ 2.16–2.79 due to two methylene protons next to ester methylene. Other multiplets were observed at 2.97–0.04 due to α methylene protons of spacer (PyCH 2 CH2 CH 2 Py). A third type of triplets was observed at δ 4.17–4.21 due to α methylene protons. A fourth type of triplets was observed at δ 6.01–6.04 due to methylene protons attached to nitrogen of pyridine. The two sets of ring protons of pyridine methine were observed as a doublet at δ 7.81–8.04 and δ 9.07–9.32. 13C/DEPT NMR spectra displayed sp3 carbon of terminal methyl group at δ 14.00–14.08. The carbons next to terminal methyl groups were observed in the range of δ 22.58–22.66. The carbons (COOCH2CH2CH2) were observed at 25.63–25.69. The middle carbon of the spacer, i.e., (PyCH2 CH2CH2Py) was observed at 28.31–28.35. The chain carbons were observed at 29.10–29.90. The methylene carbons, i.e., (CH2CH2CH3) were observed at 31.83–31.89. The α methylene carbons of spacer i.e. (PyCH2CH2 CH2Py) were observed at 34.89–35.00. The α methylene carbons attached to pyridine nitrogen were observed at δ 60.36–60.58. Other signals were observed at δ 67.26–67.35 due to methylene carbons α to carbonyl groups. Other structure revealing signals were observed at δ 127.78–127.90 due to ring carbons located β to nitrogen of pyridine. Other structures revealing signals were observed at δ 145.96–146.15 due to quaternary carbon joined to methylene group of spacer. More significant signals were observed at δ 162.74–162.85 due to ring carbons attached α to nitrogen of pyridine. The carbonyl carbons were observed at δ 166.61–166.78. All these data were almost comparable with the previous report [23]. On all these accounts the structures of (7–12) were deduced as being (4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecanoyl oxy) ethyl) dipyridinium chloride (7), (4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecanoyl oxy) ethyl) dipyridinium chloride (8), 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecanoyl oxy) ethyl) dipyridinium chloride (9), (4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecanoyl oxy) ethyl) dipyridinium bromide (10), (4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecanoyl oxy) ethyl) dipyridinium bromide (11), 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecanoyl oxy) ethyl) dipyridinium bromide (12). The structures of these Gemini surfactants (7–12) are further consolidated by ESI–MS (positive ion) mass spectral data. Important peaks in these spectra are found at m/z 763.3, 764.4, 765.5, 707.4, 708.4, 651, 652.4, 763.3, 764.4, 765.5, 707.4, 708.4, 651, 652.4. These ion peaks account for the loss of proton and two chloride/bromide ions from the molecule leading to the formation of positively charged parent ion {M−2Cl)−1)}+/{M−2Br)−1)}+ and direct loss of two chloride/bromide ions from the molecule leading to formation of (M−2Cl)+/(M−2Br)+ positively charged ions. All the spectral results of the synthesized compounds are provided in the supplementary file.
Surface Active Properties of Gemini Surfactants (7–12)
Critical Micelle Concentration
Gemini surfactants have low CMC values [24, 25]. The CMC values and degrees of counter ion binding of these new pyridinium amphiphiles have been determined by conductivity method. These new Gemini pyridinium amphiphiles have low CMC values. It has been found that the CMC of these Gemini amphiphiles decreases with increases in chain length. The values of CMC and degrees of counter ion binding are given in Table 1. The graphs of the concentration vs. conductivity have been plotted in Fig. 1a. It is found that the pyridinium Gemini surfactants with bromine as a counter ion have low CMC values as compared to the pyridinium Gemini surfactants with chlorine as a counter ion [26].
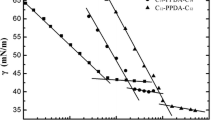
a Specific conductivity vs. concentration plot of Gemini surfactant 7. b Plot of surface tension vs. log of surfactant concentration of Gemini surfactant 7. c Representing the thermal decomposition curve of surfactant 11 determined by TGA, indicating the start (T start) and onset (T onset) temperature
The Degree of Counterion Binding (β)
The ratio of the slopes of the conductivity vs. the concentration curve above and below the CMC gives the degree of counterion dissociation α (i.e., α = smicellar/spremicellar) and (1−α) gives the degree of counterion binding, β. It is an important parameter because it manifests the counterions that are contained in the stern layer to counterbalance the electrostatic force that opposes micelle formation. Quagliotto et al. [22] determined the β value for a series of Gemini bispyridinium bromides having different spacers where they had shown that a different spacer is responsible for a different β value. The β value signifies the ability of the counter ion to bind micelles. It was found that the β value decreases with increases in chain length (Table 1).
Surface Tension Measurements
The CMC of the new pyridinium Gemini surfactants were calculated by using surface tension measurements Fig. 1b. The important parameters of Gemini surfactants, i.e., the effectiveness of surface tension reduction (ΠCMC) was obtained from the surface tension plots. The parameter, ΠCMC is the surface pressure at the CMC and is defined as:
where γ 0 is the surface tension of pure solvent and γ CMC is the measured surface tension at the CMC. The maximum reduction in surface tension caused by the dissolution of amphiphilic molecules has been indicated by ΠCMC and as a result ΠCMC becomes a measure for the effectiveness of the amphiphile to lower the surface tension of the water [27]. Pyridinium Gemini surfactants synthesized in present work (7, 8, 9, 10, 11 and 12) have a greater ability to reduce surface tension of aqueous systems. The maximum surface excess concentration (Γmax) was estimated by applying Gibbs adsorption isotherm [28] to the surface tension data:
where R is the gas constant (8.314 J/mol K), T is the absolute temperature, and C is the surfactant concentration. The value of n is taken as 2 as there is one counter ion associated with each cationic head group. The minimum area occupied by a single amphiphilic molecule at the air–water interface (A min) was also obtained by applying the Gibbs adsorption isotherm to the surface tension data:
where N A is the Avogadro constant. All pyridinium Gemini surfactants have lower A min values (Table 1). The lowest A min values of pyridinium Gemini surfactants (7 and 10) can be attributed to tighter packing of the longer chains at the interface [28, 29]. The Gibbs energy of the micellization (ΔG 0mic) was calculated by use of the following equation [30].
where X CMC is the mole fraction at the CMC and α is the extent of counter ion dissociation.
The micellization free energy indicates negative sign because thermodynamically stable micelles are formed spontaneously. The results from Table 1 indicate that the driving force for micellization becomes large as ΔG 0mic becomes more negative. The standard Gibbs energy of adsorption (ΔG 0ads) was obtained from the following relationship [31].
Here, ΠCMC denotes the surface pressure at the CMC (ΠCMC = γ 0−γ CMC, where γ 0 and γ CMC are the surface tensions of water and the surfactant solution at the CMC, respectively). The free energy of adsorption (ΔG 0ads) represents the free energy of transfer of 1 mol of surfactant in solution to the surface, and the free energy of micellization (ΔG 0mic) represents the work done to transfer the surfactant molecules from the monomeric form at the surface to the micellar phase [32]. The standard free energy of micellization (ΔG 0mic) and adsorption (ΔG 0ads) is always negative, indicating tendencies to form micelles in solution and to adsorb at the air/water interface [33]. If the value of (ΔG 0ads) is more negative and greater than the difference between (ΔG 0ads) and (ΔG 0mic), then the adsorption of surfactant molecules at the interface becomes more favorable because of the greater freedom of motion of hydrocarbon chains at the planar air/aqueous solution interface than in the interior of the micelle. However, if the energy difference is small, then less work has to be done to transfer surfactant molecules from the monomeric form at the surface to the micellar phase. When the difference in the free energies is small, the surfactant undergoes aggregation more readily than when the difference in the free energies is large. This is evident from the results obtained by Yeshimua et al. [34]. The (ΔG 0mic) and (ΔG 0ads) values of Gemini pyridinium surfactants are summarized in Table 1. The difference in the free energy gap is small for Gemini pyridinium surfactants therefore; these surfactants have a greater tendency to aggregate in solution as compared to other surfactants.
The graphs of the surface tension vs. concentration are shown for Gemini surfactants (7–12). A clear break is observed in all the Pyridinium Gemini surfactants Fig. 1b. It is observed from the graphs that Pyridinium Gemini surfactants having bromine as a counter ion have low CMC values as compared to the pyridinium Gemini surfactants having chlorine as a counter ion. The CMC values are reported in Table 1 for all the Gemini surfactants. The values for both the conductivity methods and surface tension method correspond well with each other.
Thermal Stability Measurements
Thermal stability measurement shows that these long chain Gemini surfactants are stable up to 370 °C. Figure 1c shows a characteristic curve for the decomposition of the Gemini surfactants as measured by TGA. The onset temperature (T onset) is the intersection of the baseline weight, either from the beginning of the experiment and the tangent of the weight vs. temperature curve as decomposition occurs [35]. The start temperature (T start) is the temperature at which the decomposition of the sample begins. The example of the onset and start temperatures is shown in Fig. 1c. The onset and start temperatures for present pyridinium Gemini surfactants are listed in Table 2. Thermal stability measurements designated that these surfactants have better thermal stability. Thermal stability of these Gemini surfactants increases as chain length increases. Also from Table 2 it is found that Gemini pyridinium surfactants having bromine as a counter ion is more thermally stable than surfactants having chlorine as a counter ion.
Conclusion
In the present study we have synthesized fatty acid ester-based pyridinium Gemini surfactants through an environmentally friendly process. The Gemini cationic surfactants (7–12) synthesized in the present work are produced in excellent yields and these surfactants have been examined and are found to have good surface active properties. The results show that Gemini pyridinium surfactants with longer hydrophobic chains have lower CMC values. It is found that Gemini surfactants having bromide as a counter ion have low CMC values as compared to Gemini surfactants having chloride as a counter ion. Further, results shows that these Gemini surfactants have good thermal properties. The thermal properties of these Gemini surfactants increase with increases in chain length. Also from Table 2 it is found that Gemini pyridinium surfactants having bromine as a counter ion are more thermally stable than surfactants having chlorine as a counter ion. In addition, these pyridinium cationic Gemini surfactants may show good antimicrobial properties, and a DNA binding capability if tested properly.
References
Zana R (2002) Preparation and properties of new ester-linked cleavable Gemini surfactants. Adv Colloid Interface Sci 97:203–207
Tehrani-Bagha AR, Holmberg K (2007) Reactions and synthesis in surfactant system. J Colloid Interface Sci 12:81–84
Zhang J, Zheng Y, Yu P, He L, Wang R (2010) Synthesis, characterization and surface activity of a polyoxyethylene ether trimeric quaternary ammonium surfactant. J Surf Deterg 13:155–158
Cross J, Singer EJ (1994) Cationic surfactants: analytical and biological evaluation. Marcel Dekker, New York
Huber L, Nitschke L, Holmberg K (2002) Environmental aspects of surfactants. In: Holmberg K, Shah DO, Schwuger MJ (eds) Handbook of applied surface and colloid chemistry, Wiley Chichester, UK 1:509–516
Nowicki J (2010) Emulsion properties and phase equilibrium of new asymmetric Gemini surfactants consisting of fatty acids ester of polyethoxylated alcohol or phenol. J Surf Deterg 13:195–199
Rosen MJ (1991) Geminis: a new generation of surfactants. Chem Tech 21:23–27
Menger FM, Littau CA (1993) Gemini surfactants, a new class of self-assembling molecule. J Am Chem Soc 115:1083–1090
Azzam EMS (2001) Effect of alkyl groups in anionic surfactants on solution properties of anionic-nonionic surfactant system. J Surf Deterg 4:293–296
Zana R (2002) Dimeric and oligomeric surfactants, behaviour at interfaces and in aqueous solution a review. J Colloid Interface Sci 97:205–253
Al-Sabagh AM, Azzam EMS, Mahmoud SA, Sulah NEA (2007) Synthesis of ethoxylated alkyl sulfosuccinate surfactant and investigation of mixed solutions. J Surf Deterg 10:3–8
Bunton CA, Robinson L, Schaak J, Stam MF (1971) Catalysis of nucleophilic substitution by micelles of dicationic detergents. J Org Chem 36:2346–2350
Devinsky F, Masarova L, Lacko I (1985) Surface activity and micelle formation of some new bis quaternary ammonium salts. J Colloid Interface Sci 105:235–239
Devinsky F, Lacko I, Bitterova F, Tomeckova L (1986) Relationship between structure, surface activity and micelle formation of some new bis quaternary isoesters of 1,5-pentane diammonium dibromides. J Colloid Interface Sci 114:314–322
Zana R, Xia J (2004) Gemini surfactants. Marcel Dekker, New York, pp 301–302
Torregrosa R, Pastor MI, Yus M (2007) Solvent free direct regioselective ring opening of epoxides with imidazoles. Tetrahedron 63:469–473
Rodrguez A, Graciani MM, Moya ML (2007) Effects of ethylene glycol addition on the aggregation and micellar growth of cationic dimeric surfactants. Langmuir 23:11496–15505
Barreleiro PA, Lindman B (2003) The kinetics of DNA–cationic vesicle complex formation. J Phys Chem B 107:6208–6213
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. John Wiley, New Jersey 21
(1995) Bailey’s industrial oil and fat products, vol 5, 5th edn. John Wiley, New York
Patial P, Shaheen A, Ahmad I (2013) Synthesis of ester based cationic gemini surfactants and appraisal of their surface active properties. J Surf Deterg 16:49–56
Khan AAP, Mohd A, Bano S, Siddiqi KS (2011) Kinetics and mechanism of oxidation of d-penicillamine by potassium hexacyanoferrate(III) ions in aqueous solution in the presence of sodium dodecyl sulphate and cetyltrimethylammonium bromide. J Dispers Sci Technol 32:717–723
Viscardi G, Quagliotto P, Barolo C, Savarino P, Barni E, Fisicaro E (2000) Gemini pyridinium surfactants synthesis and conductometric study of novel class of amphiphiles. J Org Chem 65:8197–8203
Rodrguez A, Graciani MM, Moya ML (2007) Effects of ethylene glycol addition on the aggregation and micellar growth of cationic dimeric surfactants. Langmuir 23:11496–15505
Rosen MJ, Mathias JH, Davenport L (1999) Aberrant aggregation behavior in cationic Gemini surfactants investigated by surface tension, interfacial tension, and fluorescence methods. Langmuir 15:7340–7346
Quagliotto P, Viscardi G, Barolo C, Barni E, Bellinvia S, Fisicaro E, Compari C (2003) Gemini pyridinium surfactants synthesis and conductometric study of novel class of amphiphiles. J Org Chem 68:7651–7660
Rosen MJ (2004) Surfactants and interfacial phenomena, 2nd edn. Wiley, New Jersey 25
Rosen MJ (1989) Surfactants and interfacial phenomena, 2nd edn. Wiley, New York 16
Anouti M, Jones J, Boisset A, Jacquemin J, Caillon-Caravanier M, Lemordant D (2009) Aggregation behavior in water of new imidazolium and pyrrolidinium alky carboxylates protic ionic liquids. J Colloid Interface Sci 340:104–111
Song LD, Rosen MJ (1996) Surface properties, micellization and premicellar aggregation of Gemini surfactants with rigid and flexible spacer. Langmuir 12:1149–1153
Verma SK, Gosh KK (2011) Micellar and surface properties of some monomeric surfactants and a cationic Gemini surfactant. J Surf Deterg 14:347–352
Bhadani A, Singh S (2011) Synthesis and properties of thioether spacer containing Gemini imidazolium surfactants. Langmuir 27:14033–14044
Tsubone K, Arakawa Y, Rosen MJ (2003) Structural effects on surface and micellar properties of alkanediyl-α,ω-bis(sodium N-acyl-β-alaninate) Gemini surfactants. J Colloid Interface Sci 262:516–524
Yeshimua T, Bong M, Matsuoka K, Honda C, Endo K (2009) Surface properties and aggregate morphology of partially fluorinated carboxylate-type anionic Gemini surfactants. J Colloid Interface Sci 339:230–235
Fredlake CP, Crosthwaite JP, Hert DG, Aki SNVK, Brennecke JF (2004) Thermophysical properties of imidazolium-based ionic liquids. J Chem Eng Data 49:954–964
Acknowledgments
We are thankful to the UGC (University grant commission India) for providing the research grant for this work and the Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh for the mass spectral analysis of the compounds.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Patial, P., Shaheen, A. & Ahmad, I. Gemini Pyridinium Surfactants: Synthesis and Their Surface Active Properties. J Surfact Deterg 17, 929–935 (2014). https://doi.org/10.1007/s11743-014-1563-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1563-8