Abstract
A series of 4-substituted N-(2-mercaptophenyl)salicylideneimine Schiff bases were synthesized and investigated for corrosion inhibition of mild steel in hydrochloric acid medium. Inhibition through adsorption mechanism is proposed for these inhibitors, which is well supported by electrochemical impedance spectroscopy, the Langmuir adsorption isotherm and Scanning Electron Microscope morphologies of inhibited and uninhibited mild steel specimens. The negative ∆G ads indicates the spontaneous adsorption of the inhibitor on a mild steel surface. Among all the examined inhibitors, 5-bromo-N-(2-mercaptophenyl)salicylideneimine showed a higher inhibition efficiency. In order to reveal the usefulness of these Schiff bases as corrosion inhibitors under various circumstances, weight loss measurements were performed at various temperatures, acid concentrations and immersion times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ever since the report of using hydrochloric acid that contains an organic inhibitor for cleaning scaled water pipes [1], several organic compounds have been studied with great interest for corrosion inhibition. The corrosion of mild steel in acid solutions can be readily inhibited by the use of chemical inhibitors. Organic compounds that contain hetero atoms such as nitrogen, oxygen and sulfur are usually effective inhibitors [2].
An assessment of the literature reveals that most of the commercial inhibitor formulations include carbonyls and amines as essential ingredients [3–5]. It has been observed that condensation products of carbonyls and amines, which are known as anils or Schiff bases, give higher inhibition efficiency than that of constituent carbonyls and amines [6–10]. The inhibition nature of Schiff bases is attributed to the presence of the C=N group, electronegative nitrogen, sulphur and/or oxygen atoms. The action of such inhibitors depends on the specific interaction between the functional groups and the metal surface. The greatest advantage of many Schiff base compounds is that they can be conveniently and easily synthesized from relatively cost effective materials.
In view of the high performance of mercaptans [7–10] as an efficient corrosion inhibitors, we synthesized Schiff base corrosion inhibitors namely, 5-chloro-N-(2-mercaptophenyl)salicylideneimine (A), 5-bromo-N-(2-mercaptophenyl)salicylideneimine (B), 5-methoxy-N-(2-mercaptophenyl)salicylideneimine (C), 5-nitro-N-(2-mercaptophenyl) salicylideneimine (D) and N-(2-mercaptophenyl)naphthylideneimine (E). The inhibiting nature of Schiff bases A–E was investigated by means of weight loss measurements, polarization studies, electrochemical impedance spectroscopy, adsorption isotherms and surface analysis through a Scanning Electron Microscope (SEM).
Experimental
Materials
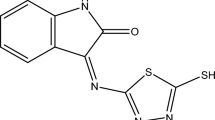
Mild steel specimens having compositions 0.10% C, 0.34% Mn, 0.08% P and the remainder being Fe, were cut up into pieces having the dimensions 3 cm × 2 cm × 0.03 cm and used for corrosion inhibition studies. The Schiff base inhibitors (Fig. 1) were prepared by condensation reaction between salicylaldehyde or its derivatives or 2-hydroxy-1-naphthaldehyde and o-aminothiophenol in ethanol [11, 12].
Anal. calc. for 5-chloro-N-(2-mercaptophenyl)salicylideneimine (A): C13H10NSOCl: C, 59.20; H, 3.82; N, 5.31; S, 12.16%. Found: C, 59.21; H, 3.83; N, 5.31; S, 12.17%. IR (KBr): νC=N = 1,621, νC–O = 1,321, νC–S = 753 cm−1. Anal. Cald for 5-bromo-N-(2-mercaptophenyl)salicylideneimine (B): C13H10NSOBr: C, 50.66; H, 3.27; N, 4.54; S, 10.40%. Found: C, 50.68; H, 3.27; N, 4.54; S, 10.41%. IR (KBr): νC=N = 1,612, νC–O = 1,317, νC–S = 757 cm−1. Anal. Cald for 5-methoxy-N-(2-mercaptophenyl)salicylideneimine (C): C13H13NSO2: C, 64.84; H, 5.05; N, 5.40; S, 12.36%. Found: C, 64.84; H, 5.07; N, 5.40; S, 12.36%. IR (KBr): νC=N = 1,623, νC–O = 1,324, νC–S = 758 cm−1. Anal. Cald for 5-nitro-N-(2-mercaptophenyl)salicylideneimine (D): C13H10N2SO3: C, 56.92; H, 3.67; N, 10.21; S, 11.69%. Found: C, 56.92; H, 3.68; N, 10.20; S, 11.69%. IR (KBr): νC=N = 1,618, νC–O = 1,305, νC–S = 754 cm−1. Anal. calc. for and N-(2-mercaptophenyl)naphthylimine (E): C17H13NSO: C, 73.09; H, 4.69; N, 5.01; S, 11.48%. Found: C, 73.09; H, 4.68; N, 5.02; S, 11.48%. IR (KBr): νC=N = 1,610, νC–O = 1,304, νC–S = 757 cm−1.
Weight Loss Studies
The weight loss experiments were performed as described in our previous reports [13–15]. Double distilled water was used to prepare solutions of 1 M hydrochloric acid and various concentrations of inhibitor. Weight loss measurements were performed at temperatures of 300, 310, 320 and 330 K (±1 K) for 2 h in 1 M hydrochloric acid solution. The inhibition efficiency (IE%) and surface coverage (Ө) were calculated using the following equations.
where W 0 and W are the weight loss of the mild steel in the absence and presence of inhibitors, respectively.
Electrochemical Studies
A CH electrochemical analyzer (model 604B) was used to record Tafel polarization curves and Nyquist impedance curves. A conventional three-electrode system was used for this purpose. A mild steel specimen with an exposed area of 1 cm2 was used as a working electrode. Pt and saturated calomel electrodes (SCE) were used as auxiliary and reference electrodes respectively. The working electrode was polished with 1/0, 2/0, 3/0 and 4/0 grade emery papers and degreased with double distilled water and acetone before use. The anodic and cathodic polarization curves were recorded by a constant sweep rate of 1 mVs−1. The linear Tafel segments of the anodic and cathodic curves were extrapolated to the corrosion potential to obtain the corrosion current densities. Inhibition efficiency (IE%) was calculated from corrosion current density values by using Eq. 3.
where \( I_{\text{corr}}^{\prime } \) and I corr are corrosion current densities in the absence and presence of inhibitor.
AC impedance measurements were carried out in the atmosphere of air in the range from 1 to 10,000 Hz at an amplitude of 0.01 V. The impedance diagrams are given by Nyquist representation. The electrical equivalent circuit for the system is shown in Fig. 2. In the given electrical equivalent circuit, R s is the solution resistance, R ct is the charge transfer resistance, and C dl is the double layer capacitance. The inhibition efficiency (IE%) was calculated from charge transfer resistance values with the help of Eq. 4.
where R ct and \( R_{\text{ct}}^{\prime } \) are charge transfer resistances in the absence and presence of inhibitor.
Surface Analysis
The specimens for surface morphological examination were immersed in an acid containing optimum concentration (200 ppm) of inhibitors and blank for 2 h. Then, they were removed from the solution, rinsed quickly with acetone, and then dried. The analyses were performed using a Hitachi 3000H Scanning Electron Microscope.
Results and Discussion
Weight loss Measurements
The weight loss experiments were performed with varying concentrations of inhibitors, acid concentration, temperature and the reaction time or immersion time. The higher the concentration of the inhibitor, the higher is the IE%. Inhibition of the mild steel corrosion was observed at all concentrations of Schiff bases used in the study (i.e. 50, 100, 200 ppm) (Table 1). The variation of IE% with increase in inhibitor concentrations suggests that the increase in the inhibitor concentration increases the number of molecules adsorbed over the mild steel surface. The adsorbed inhibitor molecules block the active sites on the metal in which direct acid attack proceeds, and protect the metal from corrosion. It is implicit that the inhibitors show the highest inhibition efficiency (around 90%) at 200 ppm in 1 M hydrochloric acid. Despite the fact that there is no much difference in the IE% of the inhibitors, Schiff bases B and E have shown an as much as 90% under the given conditions.
Table 2 represents the inhibition efficiency of the inhibitors A–E at different acid concentrations, temperatures and immersion periods. It is evident from the values that increases in the concentration of the acid decreases the inhibition efficiency of the inhibitor. This is due to the increased aggressiveness of the acid solution. All the inhibitors showed at least 55% of IE against 4 M hydrochloric acid.
Increasing the temperature of the corrosion environment increases the corrosion rate that leads to the decrease in the inhibition efficiency of the inhibitors. The inhibitors (A–E) showed at least 55% of IE at 330 K (Table 2). The decrease in the IE% is attributed to the increased effect of temperature on the dissolution process of steel and partial desorption of the inhibitor from the metal surface [15]. In the case of a prolonged incubation time of mild steel in 1 M hydrochloric acid solution showed a decrease in IE%. This suggests that the mechanism of inhibition may be adsorption and possibly due to physisorption. The physisorption is attributed to weak van der Waal’s forces, which disappear at elevated temperatures. These weight loss measurement results are in accordance with the previous reports [8, 16–18]. Under all corrosive circumstances, inhibitor E showed comparatively higher IE% than other inhibitors. Though there is not much difference between the IE% of inhibitors, the observed trend among these inhibitors follows this order: E > B > C > D > A.
Potentiodynamic Polarization Studies
The potentiodynamic polarization studies were carried out for mild steel specimens, both in the presence and absence of the Schiff base inhibitors (A–E) in 1 M hydrochloric acid. The result of Tafel polarization for E is presented in Fig. 3. Various electrochemical parameters such as corrosion potential (E corr), corrosion current density (I corr), and tafel constants (b a and b c) obtained from cathodic and anodic curves are given in Table 3.
The I corr values obtained for solutions with inhibitors are lower than the acid solution. The presence of inhibitors resulted in a slight shift of the corrosion potential in the active direction in comparison to the result obtained in the absence of the inhibitor. Both the anodic and cathodic current densities were lower indicating that imines suppressed both the anodic and cathodic reactions, although mainly the cathodic one. The values of cathodic Tafel slopes (b c) were found to increase with increasing concentrations of the inhibitors, which suggests that the inhibitors affect the hydrogen evolution reaction [19]. The anodic slope values (b a) of inhibitors B–E were mostly constant, which indicates that these inhibitors did not change the mechanism of iron dissolution. But inhibitor A showed an increase in the value of b a, which is attributed to the effect of inhibitor on the anodic reaction [20]. According to Riggs et al., [21] if the displacement in corrosion potential is more than ±85 mV with respect to the corrosion potential of the blank, the inhibitor can be considered as a distinctive cathodic or anodic type. In the present study, though a small deviation in the corrosion potential is observed, the average displacement was not more than 85 mV, which suggests that the studied Schiff bases act like mixed-type inhibitors [22–24]. These observations suggest that the Schiff base inhibitors A–E inhibit both anodic and cathodic reactions but mainly inhibit the cathodic reaction.
Electrochemical Impedance Spectra Studies
Electrochemical impedance spectra for the mild steel/1 M hydrochloric acid interface in the absence and presence of inhibitors were recorded in the frequency range 1–10 kHz. The representative impedance data obtained for inhibitor E is shown in Fig. 4. The Nyquist plots contain depressed semi-circle with the centre under the real axis, whose size increases with the inhibitor indicating a charge transfer (R ct) process mainly controlling the corrosion of mild steel. Such a behavior, characteristic for solid electrodes and often referred to as a frequency dispersion, has been attributed to roughness and other inhomogeneities of the solid surface [25]. It is apparent from these plots that the impedance response of mild steel in uninhibited acid solution has significantly changed after the addition of the inhibitors in the corrosion solutions. This indicated that the impedance of the inhibited substrate has increased with increasing concentration of inhibitors [26].
As seen from Table 4, the value of charge transfer resistance (R ct) increases with an increasing inhibitor concentration indicating the considerable surface coverage by the inhibitor molecules via a strong bonding to the surface [27, 28]. On increasing the concentration of the inhibitors the value of R ct increases and C dl decreases (Table 4). A decrease in the capacitance, which can result from a decrease in the local dielectric constant and/or an increase in the thickness of the electrical double layer, suggests that the inhibitor molecules act by adsorption at the metal/solution interface [29].
Bode plots for inhibitor E are also presented in Fig. 5. A new phase angle shift at a higher frequency range and a continuous increase in the phase angle shift with increasing concentration of inhibitors was observed. The continuous increase in the phase angle shift is obviously correlated with the progress of surface coverage by the inhibitor molecules [30].
Adsorption Isotherms
The mechanism of corrosion inhibition by the inhibitors A–E may be explained on the basis of the adsorption behavior. Using the degree of surface coverage (θ), which is obtained from weight loss measurements, Tafel polarization and electrochemical impedance spectra (EIS), the data were fitted to various isotherms such as the Langmuir adsorption isotherm, Temkin, Freundlich and Frumkin. It can observed that the plot obeys only the Langmuir adsorption isotherm. Therefore the mechanism of inhibition could be through surface coverage of adsorbed inhibitor on mild steel surface and consequently there is no interaction between the molecules adsorbed at the metal surface [31]. The higher inhibitive property of the inhibitor is attributed to the presence of π electrons, heteroatoms and the larger molecular size, which ensures greater coverage of the metallic surface.
Figure 6 shows Langmuir isotherm plots using weight loss measurements. The expected linear relationship is well approximated. The values of correlation coefficient and slopes were determined from the Langmuir adsorption isotherms. The values of correlation coefficient are 1.0 for all the studied inhibitors (Table 5), where as the values of slopes are more than 1.0. Since the values of slope are more than unity, it could be concluded that each Schiff base unit occupies more than one adsorption site on the steel surface.
The values of activation energy (E a) were calculated using the Arrhenius equation. The free energy of adsorption (∆G ads) at different temperatures was calculated using the following equation [32].
where θ is the degree of coverage on the metal surface, C is the concentration in mol/L and K ads is equilibrium constant. The degree of surface coverage (θ) for optimum concentration of inhibitor in 1 M hydrochloric acid at 300–330 K for 2 h immersion time has been evaluated from the weight loss values. A plot of log [θ/(1 − θ)] versus 1/T gave a straight line. The value of heat of adsorption (Q ads) was obtained from the slope of this plot. The values of enthalpy of activation (∆H) and entropy of activation (∆S) were calculated using the following equation:
where ∆H ads and ∆S ads are the enthalpy and entropy of adsorption, respectively.
The values of the enthalpy of activation (∆H ads) and the entropy of activation (∆S ads), E a, ∆G ads, and Q ads are listed in Table 5. It was found that the value of activation energy for an inhibited system is lower than that for an uninhibited system, which is attributed to the chemisorption of the Schiff base on the mild steel surface [33]. The value of ∆H ads is higher in the presence of an inhibitor indicating that the high-energy barrier for the reaction in presence of an inhibitor is attained and hence exhibits comparatively low inhibition efficiency at elevated temperature [34]. The ∆S ads values in the absence and presence of inhibitor are negative. This indicates that the activated complex in the rate determining step represents an association rather than a dissociation step, meaning that a decrease in disordering occurs on going from reactants to the activated complex [35]. As it can be seen from the Table 5, the addition of inhibitors causes negative values of ∆G ads, it indicates that adsorption of the studied Schiff bases is a spontaneous process [36, 37]. In general, the values of ∆G ads up to −20 kJ mol−1 are consistent with the electrostatic interaction between the charged molecules and the charged metal (physisorption) while those between −80 and −400 kJ mol−1 are associated with chemisorption as a result of sharing or the transfer of electrons from the inhibitor molecules to the metal surface to form a coordinate type of bond. The calculated ∆G ads values in the range of 50–120 kJ mol−1 indicate, therefore, that the adsorption mechanism of the Schiff bases on mild steel in 1 M hydrochloric acid were both electrostatic-adsorption and chemisorption [38].
The negative value of the heat of adsorption process indicates that the adsorption process is exothermic in nature. These observations of E a and Q ads confirm a physical adsorption of the inhibitor on the mild steel surface in hydrochloric acid. This is in accordance with other workers [39, 40]. The unshared electron pairs in sulfur, nitrogen as well as in oxygen may interact with d-orbitals of iron in steel to provide a protective chemisorbed film [41].
SEM Analysis
The scanning electron microscope images (Fig. 7) were recorded to establish the interaction of organic molecules with the metal surface. The SEM images show the features of mild steel surface after being immersed for 2 h in 1 M hydrochloric acid and hydrochloric acid containing the inhibitor E. The SEM images reveal that the specimens immersed in the inhibitor solutions have a smooth surface while the metal surface immersed in 1 M hydrochloric acid is rough and covered with corrosion products and appears to be full of pits and cavities. This indicated that the inhibitor molecules hinder the dissolution of iron by the adsorption of Schiff base molecules on the steel surface and thereby reduce the rate of corrosion. Based on the SEM images and polarization profiles, it is indicated that the number of anodic sites is reduced with simultaneous reduction in the rate of cathodic reaction.
Mechanism of Inhibition
The studied Schiff base inhibitors have free electron pairs on N, O and S capable of forming a coordination bond with Fe [40, 41] and the mode of interaction between inhibitor molecules and the mild steel surface is shown in Fig. 8. The chemical interactions of inhibitor with the mild steel are indicated with solid lines, whereas, dotted lines are designated as physical interactions. The higher inhibitory efficiency of the Schiff bases can be attributed to the fact that they possess an azomethine group (C=N) in the molecular structure as reactive center. The results obtained are in agreement with the previous reports [42, 43]. And also π electrons from the aromatic rings may interact with the metal surface, and finally, especially in acidic media, electrostatic interaction is possible between the negatively charged iron surface and the positively charged inhibitor, following protonation of its basic functionalities. Furthermore, the size, orientation, shape and electric charge on the molecule determine the degree of adsorption and hence the effectiveness of the inhibitor.
It is observed from Tafel plots that the addition of all the (A–E) inhibitors increases the inhibition efficiency by acting as a mixed-type inhibitor. A potential explanation for the decrease of IE% during 2–5 h of exposure is the likely depletion of actively available inhibitor from the solution, as dissolving iron generates ions that readily coordinate with the inhibitor molecules [44].
The effect of the substituents can be explained on the basis of structure of the inhibitor molecules and inductive effect. In general, inhibitors having more electronegative atoms as well as bulky substituents show a higher IE%. In support of this fact, inhibitor E shows a surface coverage of 0.90. As shown in Fig. 8, it is assumed that there is no direct interaction between the substituents and the metal surface. Therefore, inductive effects (I) of substituents are considered. It is evident from the IE% of inhibitors that a decrease in the −I effect increases the inhibition efficiency. Though all the substituents differ in terms of electronegativity, the reason behind the close values of the IE% is unclear. One of the considerations for explaining this fact is the size of the substituents. However, it has been reported that any attempt to correlate the structure of substituents and IE% for this class of inhibitors is unsuccessful due to the high complexity of the inhibition mechanisms [8, 42, 43].
It is obvious from the structure of the Schiff bases that it includes two closely spaced –OH and –SH groups that can be used in forming an intramolecular hydrogen bond with hydrogen linked to a C=N group, in which case only the C=N group would be available to link the molecule to the surface, therefore it cannot cover the electrode surface effectively at lower concentrations and needs an optimum concentration for effective inhibition of mild steel corrosion [45–47].
Conclusions
Five Schiff base derivatives were studied against the acid corrosion of mild steel by means of weight loss measurements, Tafel polarization and electrochemical impedance spectroscopic techniques. The inhibitors showed a significant inhibition efficiency against high concentrated acid solutions and at high temperatures. Prolonged incubation of mild steel in acid solutions containing inhibitors led to a decrease in the inhibition efficiencies. All the inhibitors showed a maximum efficiency of about 90%. Adsorption of the Schiff bases on the steel surface obeys the Langmuir’s isotherm. The Schiff bases act through both electrostatic-attraction and chemisorption, whereas electrostatic-attraction occurs at low temperatures and chemisorption takes place at higher temperatures.
The electrochemical studies revealed that the studied Schiff bases are mixed-type inhibitors predominantly inhibiting the cathodic reaction. The SEM morphology of the inhibited and uninhibited mild steel specimens gave additional evidence for the adsorption mechanism of the inhibitors. The negative values of ∆G ads provide evidence for the spontaneous adsorption of the Schiff bases onto the surface of mild steel during the inhibition process. The results obtained from different methods shows the studied Schiff bases inhibit the mild steel corrosion in the following order: E > B > C > D > A.
References
Speller FN, Chappell EL, Russell RP (1927) Practical applications of inhibitors for pickling operations. Trans Am Inst Chem Eng 49:165–167
Roberge PR (2000) Handbook of corrosion engineering. McGraw-Hill, New York, p 839
James EM (1935) Corrosion inhibition. US Patent 2,018,682
Negm NA, Zaki MF (2009) Synthesis and evaluation of 4-diethyl amino benzaldehyde Schiff base cationic amphiphiles as corrosion inhibitors for carbon steel in different acidic media. J Surf Deterg 12:321–329
Negm NA, El-Farragry AF, Abdelrahman NR (2011) New Schiff base cationic surfactants: surface and thermodynamic properties and applicability in bacterial growth and metal corrosion prevention. J Surf Deterg 14:505–514
Negm NA, Zaki MF (2008) Corrosion inhibition efficiency of nonionic Schiff base amphiphiles of p-aminobenzoic acid for aluminum in 4 N HCl. Colloids Surf A 322:97–102
Aiad IA, Negm NA (2009) Some Schiff base surfactants as steel-corrosion inhibitors. J Surf Deterg 12:313–319
Behpour M, Ghoreishi SM, Soltani N, Salavati-Niasari M, Hamadanian M, Gandomi A (2008) Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corrosion Sci 50:2172–2181
Negm NA, Aiad IA (2007) Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surf Deterg 10:87–92
Negm NA, Mohammed AS (2004) Surface and thermodynamic properties of diquaternary bola-form amphiphiles containing an aromatic spacer. J Surf Deterg 7:23–30
Tisato F, Refosco F, Mazzi U, Bandoli G, Nicolini MJ (1989) Technetium(V) and rhenium(V) complexes with N-(2-mercaptophenyl)salicylideneiminate. Crystal structure of chloro(oxo)[N-(2-sulphidophenyl)salicylideneiminato-NOS]technetium(V). Chem Soc Dalton Trans 164:1693–1699
Albin LD, Jacobson M, Olson DB (1995) Yellow color-formers. US Patent 5,426,085
Gopiraman M, Selvakumaran N, Kesavan D, Karvembu R (2012) Adsorption and corrosion inhibition behaviour of N-(phenylcarbamothioyl)benzamide on mild steel in acidic medium. Prog Org Coat 73:104–111
Gopiraman M, Sathya C, Vivekananthan S, Kesavan D, Sulochana N (2012) Influence of 2,3-dihydroxyflavanone on corrosion inhibition of mild steel in acidic medium. J Mater Eng Perform 16:240–246
Gopiraman M, Sakunthala P, Kesavan D, Alexramani V, Kim IS, Sulochana N (2011) An investigation of mild carbon steel corrosion inhibition in hydrochloric acid medium by environment friendly green inhibitors. J Coat Technol Res (in press). doi:10.1007/s11998-011-9374-6
Negm NA, Morsy SMI, Said MM (2005) Corrosion inhibition of some novel hydrazone derivatives. J Surf Deterg 8:95–98
Abiola OK, James AO (2010) The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros Sci 52:661–664
Wang H, Fan H, Zheng J (2002) Corrosion inhibition of mild steel in hydrochloric acid solution by a mercapto-triazole compound. Mater Chem Phys 77:655–661
Quartarone G, Bonaldo L, Tortato C (2006) Inhibitive action of indole-5-carboxylic acid towards corrosion of mild steel in deaerated 0.5 M sulfuric acid solutions. Appl Surf Sci 252:8251–8257
Abdallah M (2002) Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros Sci 44:717–728
Riggs OL (1973) Corrosion inhibitors, 2nd edn. Nathan, C. C, Houston, p 43
Mohammad M, Ali RTB, Krister H (2011) Comparison of a cationic Gemini surfactant and the corresponding monomeric surfactant for corrosion protection of mild steel in hydrochloric acid. J Surf Deterg 14:605–613
Manuel EP, Crescencio OO-X, Natalya VL, Jonathan-Boanerge P-N (2011) Imidazolium, pyridinium and dimethyl-ethylbenzyl ammonium derived compounds as mixed corrosion inhibitors in acidic medium. J Surf Deterg 14:211–220
Quraishi MA, Jamal D (2000) Dianils: new and effective corrosion inhibitors for oil-well steel (N-80) and mild steel in boiling hydrochloric acid. Corros Sci 56:156–161
Saliyan VR, Adhikari AV (2008) Inhibition of corrosion of mild steel in acid media by propanohydrazide. Bull Mater Sci 31:699–711
McCafferty E, Hackerman N (1972) Kinetics of iron corrosion in concentrated acidic chloride solutions. J Electrochem Soc 119:999–1009
Mernari B, Elattari H, Traisnel M, Bentiss F (1998) Inhibiting effects of 3,5-bis(n- pyridyl)-4-amino-1,2,4-triazoles on the corrosion for mild steel in 1 M HCl medium. Corros Sci 40:391–399
Saliyan VR, Adhikari AV (2009) N-[4-(diethylamino)benzylidine]-3-{[8-(trifluoro methyl) quinolin-4-yl]thio}propano hydrazide) as an effective inhibitor of mild steel corrosion in acid media. Mater Chem Phys 115:618–627
Ashok Kumar SL, Gopiraman M, Saravana Kumar M, Sreekanth A (2011) 2- Acetylpyridine-N(4)-morpholine thiosemicarbazone (HAcpMTSc) as a corrosion inhibitor on mild steel in HCl. Ind Eng Chem Res 50:7824–7832
Tan YJ, Bailey S, Kinsella B (1996) An investigation of the formation and destruction of corrosion inhibitor films using electrochemical impedance spectroscopy (EIS). Corros Sci 38:1545–1561
Zaafarany I, Abdallah A (2010) Ethoxylated fatty amide as corrosion inhibitors for carbon steel in hydrochloric acid solution. Int J Electrochem Sci 5:18–28
Quraishi MA, Ahamad I, Singh AK, Shukla S, Lal B, Singh V (2008) N-(Piperidinomethyl)-3-[(pyridylidene)amino]isatin: a new and effective acid corrosion inhibitor for mild steel. Mater Chem Phys 112:1035–1039
Kalaiselvi P, Chellammal S, Palanichamy S, Subramanian G (2010) Artemisia pallens as corrosion inhibitor for mild steel in HCl medium. Mater Chem Phys 120:643–648
Fouda AS, Al-Sarawy AA, El-Katori EE (2006) Pyrazolone derivatives as corrosion inhibitors for C-steel in hydrochloric acid solution. Desalination 201:1–13
Putilova IN, Balezin SA, Arannik VP (1960) Metallic corrosion inhibitors. Pergamon Press, London 55
Gomma MK, Wahdan MH (1995) Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater Chem Phys 39:209–213
Tang L, Li X, Mu G, Li L, Liu G (2006) Synergistic effect between 4-(2-pyridylazo) resorcin and chloride ion on the corrosion of cold rolled steel in 0.5 M sulfuric acid. Appl Surf Sci 252:6394–6401
Ali SA, Al-Muallem HA, Saeed MT, Rahman SU (2008) Hydrophobic-tailed bicycloisoxazolidines: A comparative study of the newly synthesized compounds on the inhibition of mild steel corrosion in hydrochloric and sulfuric acid media. Corros Sci 50:664–675
Oguzie EE (2007) Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros Sci 49:1527–1539
Ostovari A, Hoseinieh SM, Peikari M, Shadizadeh SR, Hashemi SJ (2009) Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (lawsone, gallic acid, α-d-glucose and tannic acid). Corros Sci 51:1935–1949
Hosseini M, Mertens FL, Ghorbani M, Arshadi RM (2003) Asymmetrical Schiff bases as inhibitors of mild steel corrosion in sulphuric acid media. Mater Chem Phys 78:800–808
Quraishi MA, Khan MAW, Ajmal M, Muralidharan S, Iyer SV (1996) The influence of some thiazole derivatives on the corrosion of mild steel in hydrochloric acid. Anti Corros Methods Mater 43:5
Ebenso EE, Ekpe UJ, Ita I, Offiong OE, Ibok UJ (1999) Effect of molecular structure on efficiency of amides and thiosemicarbazones used for corrosion inhibition of mild steel in hydrochloric acid. Mater Chem Phys 60:79–90
Shriver DF, Atkins PW, Langford CH (1994) Inorganic chemistry, 2nd edn. Oxford University Press, Oxford, pp 239–260
Emregul KC, Kurtaran R, Atakol O (2003) An investigation of chloride-substituted Schiff bases as corrosion inhibitors for steel. Corros Sci 45:2803–2817
Hasanov R, Sadıkoglu M, Bilgic S (2007) Electrochemical and quantum chemical studies of some Schiff bases on the corrosion of steel in H2SO4 solution. Appl Surf Sci 253:3913–3921
Emregul KC, Akay AA, Atacol O (2005) The corrosion inhibition of steel with Schiff base compounds in 2 M HCl. Mater Chem Phys 93:325–329
Acknowledgments
We thank the Director of the National Institute of Technology at Tiruchirappalli for his encouragement.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kesavan, D., Tamizh, M.M., Gopiraman, M. et al. Physicochemical Studies of 4-Substituted N-(2-Mercaptophenyl)-Salicylideneimines: Corrosion Inhibition of Mild Steel in an Acid Medium. J Surfact Deterg 15, 567–576 (2012). https://doi.org/10.1007/s11743-012-1338-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1338-z