Abstract
A novel series of cationic surfactants containing Schiff base groups were synthesized by condensation of fatty amines namely: dodecyl, tetradecyl, hexadecyl and octadecyl amine and 4-diethyl aminobenzaldehyde. The chemical structures of these surfactants were confirmed using elemental analysis, FTIR spectra, 1H-NMR and atomic absorption spectroscopy. Surface properties of the synthesized compounds were determined using surface tension techniques. The results of the surface tension measurements showed good surface behaviors of these compounds in their aqueous solutions. The surface activities were found to be greatly influenced by the chemical structures of the synthesized compounds. The synthesized cationic Schiff bases were evaluated as corrosion inhibitors for carbon steel in different acidic media (HCl and H2SO4) at different doses (400, 200, 100, 50, 25 ppm). The corrosion inhibition efficiencies of these inhibitors were calculated using the corrosion rates of the carbon steel in the studied media and found in the acceptable range of the commercially used inhibitors. Also the chemical structure of these inhibitors was found of great influence on their inhibiting efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Schiff bases are one of the important classes of organic compounds which have many interesting properties and extensive applications in biology and materials science [1–3]. The Langmuir–Blodgett films of amphiphilic Schiff base compounds have been investigated and their interesting features revealed [4, 5]. The main properties studied focused on the coordination with transition metal ions, and some organized molecular films of these Schiff base derivatives have been reported. Using the Langmuir–Blodgett technique, it is convenient to form ordered layer structures by the transferring monolayers from water surface-to-solid substrate. This ordered deposition offers potentially greater control over molecular orientation and inter-molecular packing for these materials, which could lead to more and more creations of new types of thin-film devices especially in the field of electronics and optics. Research work on the behavior of Langmuir and LB films has been attracting increasing attention in recent years. The formation of bilayer membranes from single-chain amphiphiles in dilute aqueous solution is mainly determined by the balance between the interactions among the hydrophobic tails and that among the hydrophilic head groups and the molecular geometry as well. Through any of the following ways to enhance the assembly between amphiphiles, namely the introduction of a rigid segment [6], H-bonding [7], protonation of the head groups and coordination [8], stable bilayers are obtainable. Synthesis of novel bilayer-forming amphiphiles are of great interest as the introduction of rigid segments promotes the formation of not only a hydrophobic assembly but also a microenvironment containing functional units [9]. The inhibition of steel corrosion by acids has been previously studied by various researchers using different organic compounds [10–12]. These compounds in general are adsorbed onto the metal surface blocking the active corrosion sites. Several Schiff bases have been investigated as corrosion inhibitors for various metals and alloys in acidic media. In this study, novel cationic amphiphiles containing Schiff base groups were synthesized from condensation reactions of 4-diethyl aminobenzaldehyde and different fatty amines. Their chemical structures were confirmed using different analytical tools. The surface activity–chemical structure relationship was discussed. The synthesized cationic Schiff bases were evaluated as corrosion inhibitors for carbon steel in different acidic media (HCl and H2SO4) at different doses (400, 200, 100, 50, 25 ppm). The corrosion inhibition efficiencies of these inhibitors were calculated using the corrosion rates of the carbon steel in the studied media.
Experimental
Synthesis of Schiff Bases
A 0.5 mol sample of 4-diethylaminobenzaldehyde was condensed with 0.5 mol samples of fatty amines namely; dodecyl, tetradecyl, hexadecyl or octadecyl amines in the presence of 250 mL of ethyl alcohol as a solvent. The reaction mixture was refluxed for 6 h and then left overnight until the product was precipitated. The product was filtered then washed with petroleum ether and recrystallized twice from ethanol. The final product was dried under vacuum at 40 °C [13]. The chemical structures of the Schiff bases produced are presented in Scheme 1.
Synthesis of Cationic Surfactants of Schiff Base Derivatives
Cationic form of the synthesized Schiff bases were obtained throughout direct interaction between equimolar amounts of the synthesized Schiff bases and methyl iodide in ethanol. The reaction mixture was refluxed for 8 h, and then left overnight for complete precipitation of the cationic quaternary ammonium compounds. The product was filtered and recrystallized for three times from ethanol to produce the pure cationic Schiff base surfactants [14] (Scheme 2).
Surface Tension Measurements
Surface tension measurements were obtained using a De-Nouy Tensiometer Kruss K-6 with a platinum ring. Freshly prepared aqueous solutions of Schiff base cationics and their transition metal complexes were used over a concentration range of 10–0.01 mM/L at 25 °C. Apparent surface tensions were measured three times for the sample with a 2-min interval between each reading [15].
Emulsification Efficiency and Interfacial Tension Measurements
Emulsification efficiencies were determined by measuring the time required for separation of 9 mL of pure water from a strongly shaken emulsion of 10 mL (0.1%) surfactant solution and 10 mL of paraffin oil at 25 °C. Interfacial tension measurements were made for the same concentration (0.1%) using paraffin oil as the nonpolar phase at 25 °C [15].
Corrosion Measurement
A weight-loss technique [16] was used to measure the inhibiting efficiency to corrosion of the prepared inhibitors for carbon steel in 2 N HCl and 2 N H2SO4 solutions at 25 °C for different periods (24 and 48 h). The experiments were performed with carbon steel specimens having a composition (wt.%): 0.21 C, 0.035 Si, 0.51 Mn, 0.82 P, and the remainder Fe. Each specimen was machined into regular shapes of 18 cm2 cross-sectional area. The specimens were sequentially abraded with different emery papers, degreased with acetone, washed with distilled water and dried. Corrosive solutions of 2 N HCl and 2 N H2SO4 in the absence and presence of the synthesized inhibitors at concentrations of 400, 200, 100, 50 and 25 ppm were prepared with doubly distilled water.
Analyses
Microelemental analyses were done using a Vario Elementar instrument, FTIR spectroscopic analyses were done using a Fourier-transform infrared spectrophotometer, 1H-NMR spectroscopic analyses were done using a Bruker model DRX-300 NMR spectrometer with TMS as an internal standard and atomic absorption spectroscopy (AAS) was carried out using a Hitachi 180/80 atomic absorption spectrometer.
Results and Discussion
Chemical Structure
Elemental Analyses
The elemental analyses of the synthesized cationic Schiff base amphiphiles showed that the values expected and found of the different elements are very close to each other, indicating the purity of the synthesized compounds (Table 1).
FTIR Spectroscopy
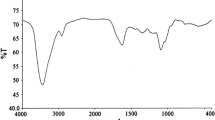
The synthesized Schiff bases showed a complete disappearance of the absorption band at 1,734 cm−1 corresponding to the carbonyl group and the appearance of a new band at 1,658 cm−1 corresponding to a –C=N– group. The IR spectra of the synthesized cationic Schiff base amphiphiles showed new absorption bands at 3,130 and 1,400 cm−1 corresponding to a [N+] group (vibration and elongation, respectively). Bands at 2,980 and 2,870 cm−1 corresponding to CH2 and CH3, respectively, and 940–980 cm−1 corresponding to a benzene nucleus appeared in all of the synthesized compounds indicating the presence of all function groups expected in the synthesized compounds.
1H-NMR Spectroscopy
The 1H-NMR spectra of the synthesized compounds showed a characteristic triplet signal at 0.792 ppm corresponded to methyl groups, a multiple signal at 1.028 ppm (CH2) with an integrated number of hydrogen protons for the alkyl chain, a triplet signal at 2.423 ppm, 4H for methylene groups in the tertiary amine group and multiple signals at 7.585 ppm of the phenyl protons.
Surface Properties of Quaternary Ammonium Cationic Schiff Bases
The relation between the surface tension and the concentration of the synthesized cationic Schiff bases surfactants is represented in Fig. 1. The relation is typically found for cationic surfactants in their solutions [17]. The critical micelle concentration (CMC) values of the synthesized amphiphiles are gradually decreased by increasing the length of the alkyl chains attached to the synthesized molecules (Fig. 2). The lowest CMC value was observed at 0.73 mM/L for the surfactant containing a hexadecyl chain. While, the highest value was observed at 2.63 mM/L for the dodecyl chain derivative at 25 °C. The octadecyl derivative showed an exceptional higher value at 1.26 mM/L at 25 °C. That could be due to the geometrical conformation occurred in the alkyl chain. This conformation (coiling) causes a compactness in SB-18 molecules which decreases their actual volume. Hence, the molecules act as they if they were shorter than the hexadecyl derivative (SB-16) and longer than the tetradecyl derivative (SB-14; Table 2). The effectiveness values (πcmc) also increased gradually according to the hydrophobic chain lengths. The highest depression in the surface tension values at CMC was recorded for the hexadecyl derivative (SB-16) at 44 mN/m. The octadecyl derivative (SB-18) showed an πcmc value at 35 mN/m. This could be explained by the conformation factor. The efficiency values (Pc20) of the targeted surfactants (Table 2) showed their good tendency to modify the surface activity of their solutions at considerably low concentrations. The maximum surface excess (Γmax) values describe the accumulation extent of surfactant molecules at the solution interface. Increases in the Γmax values indicates that the water molecules exert a high repulsion force on the surfactant molecules, hence the later escape to the interface therefore Γmax values increased. Increasing Γmax values indicates that the molecules wouldn’t undergo to the bulk of their solutions to form micelles at lower concentrations, which coincides with the high CMC values of SB-12 derivative. Longer chain lengths showed lower Γmax values (Table 2). The minimum surface area occupied by each molecule at the interface (A min) is greatly influenced by the number of repeated methylene groups in the hydrophobic chains. Increasing the hydrophobic chain length increases the area occupied by each molecule at the interface. The maximum area occupied by individual molecule at the interface was found for SB-16 derivative which equals to 27.05 Å2. SB-18 derivative showed smaller A min than the SB-16 derivative due to the coiling which had occurred in the hydrophobic chain [18].
Thermodynamic Aspects
The thermodynamic characteristics of the synthesized cationic Schiff base amphiphiles were studied using the calculated values of adsorption and micellization free energies ΔG ads, ΔG mic. These data were calculated using the thermodynamic Eqs. 1, 2, [19] (Table 2).
The micellization free energies of the targeted surfactants in their aqueous media showed negative sign, that indicates the spontaneity of the micellization process. The driving force of micelles formation is the repulsion occurring between the hydrophobic chains and the polar medium. In addition, the stabilization occurring for the micelles formed through the attraction forces between the positively charged head groups and the water molecules. The association of the counter ions (I−) in the attracted system increases the stability of the formed micelles. Obviously, increasing the hydrophobic chain length increases the negativity of ΔG mic values. This reinforces the idea of increasing the micellization tendency by increasing the number of methylene groups along the hydrophobic chains. The gradual increase in the negativity of ΔG mic agrees with the change sequence of the critical micelle concentration values (Table 2). On the other hand, the adsorption free energy change of the studied surfactants showed an exact response as ΔG mic by increasing the hydrophobic chain lengths.
The negativity of both ΔG ads and ΔG mic indicates that the two processes occurred spontaneously. The closeness between the two values (ΔG mic and ΔG ads) reveals the equilibrium between the two phases of the surfactant molecules (adsorbed and micellized phase). Also, the slight increase of ΔG ads values showed that these molecules tend to adsorb at the air–water interface till complete surface coverage is reached, then, the micelles formed. Hence, the micellization and adsorption processes are governed by the thermodynamic aspects. The chemical structure of these molecules is the main factor influencing their thermodynamic aspects. The equivalence between adsorption and micellization tendencies qualifies these surfactants as being applicable in the interfacial applications including emulsification, corrosion inhibition, and biocidal application [20]. In addition, in micellar applications including solubilization, drug delivery and drug carrier purposes.
Interfacial Tension and Emulsification Efficiency
Interfacial tension (IT) values of the synthesized cationic Schiff bases were measured between their aqueous solutions and paraffin oil at 25 °C (Table 2). Table 2 shows higher IT values for the dodecyl derivatives (SB-12; 12 mN/m). Increasing the hydrophobic chain length decreases the interfacial tension considerably. On the other hand, the emulsification efficiency of the surfactants in oil–water or nonpolar–polar systems is a reflection of the ability of surfactant molecules to locate at the boundary lines between the two phases. Hence, the most adsorbed surfactant molecules at the interface are the most powerful emulsifying agents for the emulsions. The emulsification efficiency in this study was measured as the time required for separation of 9 mL of pure water from the emulsion formed from 10 mL of surfactant solution and 10 mL of paraffin oil. Increasing the time required for separation of the desired amount of water from the emulsified system indicates the stability of that emulsion, and vice versa. Table 2 shows that derivatives containing shorter hydrophobic chains exhibit low emulsification efficiencies.
Corrosion Inhibition Efficiency
The calculations of the corrosion rate in millimeter per year (mpy) were made using the following formula:
where, K is a constant (3.45 × 106), T is time of exposure in hours, A is the area in cm2, W is the mass loss (g), D is the density of the steel used.
While, the corrosion inhibition efficiency of the synthesized corrosion inhibitors was calculated from the corrosion rate data as follows:
where, η is the corrosion inhibition efficiency, Cr is the corrosion rate in the absence of inhibitors, Cr′ is the corrosion rate in the presence of inhibitors.
Figure 3 represents the variation of the corrosion inhibition efficiencies of the synthesized cationic inhibitors by increasing the hydrophobic chain length. It is clear that increasing the alkyl chain length from 12 to 14, 16 and 18 carbon atoms decreases the inhibition efficiency of the inhibitors. This effect is inverted in several studies [21, 22], but agreed with our previous work on some Schiff base derivatives [13]. The inhibition of the corrosion processes by the cationic inhibitors in acidic media revealed the adsorption of these inhibitors at the metal surface. The inhibition efficiency was increased by increasing the surface concentration of the inhibitors at the metal surface. This decrease in the inhibition efficiency was attributed to two reasons. The first is the gradual decrease in the critical micelle concentration values (CMC) by increasing the hydrophobic chain lengths. The decrease in CMC values indicates the high tendency of these amphiphiles towards micellization. Hence, the adsorbed molecules at the interface will decrease. The second reason is the surface concentration, i.e., the concentration of the inhibitor molecules at the interface was decreased by increasing the hydrophobic chain length (Table 2). Hence, the uncovered area of the metal surface will increase. The two reasons are responsible for increasing the corrosion rate and consequently the corrosion inhibition efficiency will decrease.
In spite of the corrosion inhibition efficiency being decreased by increasing the hydrophobic chain length, their values were still high and ranged between 99.92 and 97.25% at 400 ppm and 99.88 and 95.69% at 200 ppm. In addition, the corrosion rates ranged between 0.01 and 0.09 mpy at 400 and 200 ppm for 24 h. While, at 48 h, the efficiency was ranged between 98.33 and 94.77% at 400 ppm and 98.11–94.4% at 200 ppm with a corrosion rates ranged between 0.26 and 0.85 mpy at 400 and 200 ppm. These values are considered to be in the acceptable range in the relatively high acidic concentrations (2 N HCl and H2SO4). The high values of the corrosion inhibition efficiencies of the synthesized inhibitors can be seenin Table 3. The high corrosion inhibition efficiencies of the synthesized inhibitors are revealed by their chemical structure. The presence of the positively charged (N+) group and unsaturated imine group in addition to the π-electron cloud on the benzene ring facilitates the adsorption of these inhibitors at the metal surface. That adsorption occurred on the positive and negative centers on the metal surface which decreases the electrochemical dissolution of the metal to ions in the solution (Eqs. 3 and 4).
Figure 4 represents the effect of the different inhibitor doses on the corrosion inhibition efficiency of the synthesized inhibitors. At higher inhibitor doses (400 ppm) the corrosion inhibition efficiency was found to have its maximum values. In contrast, decreasing the inhibitor dose decreases the inhibition efficiency considerably. The ratio of surface coverage (θ) of the metal by inhibitor molecules was calculated at different doses using the formula:
where, θ is the ratio of the area of metal surface covered by the inhibitor molecules, CR′ is the corrosion rate in the presence of inhibitors and CR is the corrosion rate in the absence of inhibitors. The decrease in the inhibitor efficiency at lower doses can be attributed to the decrease in the surface coverage values (θ) of the metal surface by inhibitor molecules at a lower dose (200 ppm). While, at the higher dose (400 ppm) the surface coverage of the metal by inhibitor molecules increased, hence, the corrosion inhibition efficiencies increased (Tables 3, 4).
The Schiff base derivatives are characterized by their strong tendency towards complexation with the transition metal ions. Also, the complexation tendency is increased by introducing several function groups to their chemical structures. In the case of the targeted inhibitors, the quaternary ammonium group [(CH3CH2)2N+CH3] increases their complexation tendency extremely. The complexation between the metal ions and the Schiff bases is by the donation of the lone pairs of electrons of the nitrogen atoms in the azomethine groups (–N=C–) and the vacant d-orbital of the metal ions [1, 9, 20].
Complexation reaction between the synthesized compounds and the Fe2+ ions 
This facilitates the complexation process between the dissociated Fe2+ ions from the slow corrosion reaction and the adsorbed Schiff base inhibitors. Hence, the adsorbed inhibitors on the metal surface decreased with time and the corrosion rate increased. Consequently, the corrosion inhibition efficiency decreases by increasing the contact time between the inhibitor molecules and the metal ions as shown in Fig. 5.
HCl acid produces only one proton while H2SO4 acid produces two protons upon their dissociation in the solutions. Hence, the attacking power of H2SO4 is double of that in HCl as shown in Eqs. 5, 6, and 7.
It is clear from Fig. 6 that the corrosion inhibition efficiencies of the synthesized inhibitors in the H2SO4 acidic medium are lower than those in the HCl medium. This effect can be attributed to the protonating power of the two corrosive acids. Increasing the protonating power of the acid increases its aggressive action on the metal, which lowers the influence of the inhibitors on the protection process. As a result, the inhibition efficiency decreases.
In conclusion, the synthesized cationic Schiff base amphiphiles are characterized by high corrosion inhibition efficiency for carbon steel in HCl and H2SO4 media. Also, their inhibition efficiencies as corrosion inhibitors were in the acceptable range referring to the corrosion rate which ranged between 0.26 and 0.85 mpy. Hence, these inhibitors can be considered as efficient additives for metal processing in acidic media and also for short circle petroleum industries (short circle processes are the processes which take place in relatively short time as acid cleaning of the pipeline from scale which take about 4 h of washing the pipelines with acids to remove the precipitated scale).
References
Cerchiaro G, Aquilano K, Filomeni G, Rotilio G, Ciriolo MR, Ferreira AMDC (2005) Isatin-Schiff base copper(II) complexes and their influence on cellular viability. J Inorg Biochem 99:1433–1440
Vancoa J, Svajlenova O, Racanskac E, Muselıka J, Valentova J (2004) Antiradical activity of different copper(II) Schiff base complexes and their effect on alloxan-induced diabetes. J Trace Elem Med Biol 18:155–161
Patroniak V (2005) New dinuclear nonaazadentate Schiff base podates of rare earth(III) ions. Polish J Chem 79:57–64
Dhathathreyan A (2004) Interaction of rigid Schiff base amphiphiles with water structure modifiers at air/water interface. J Colloids Surf A Physicochem Eng Asp 236:45–50
Jiao T, Liu M (2006) Substitution controlled molecular orientation and nanostructure in the Langmuir–Blodgett films of a series of amphiphilic naphthylidene-containing Schiff base derivatives. J Colloid Interface Sci 299:815–822
Ebara Y, Itakura K, Okahata Y (1996) Kinetic studies of molecular recognition based on hydrogen bonding at the air−water interface by using a highly sensitive quartz-crystal microbalance. Langmuir 12:5165–5170
Negm NA, Mahmoud SA (2003) Effect of structure on the physicochemical properties of nonionic phosphate amphiphiles. Egypt J Petrol 12:11–20
Negm NA, Morsy SMI, Badawi AM (2005) Biological activities of some novel cationic metallo micelles. Egypt J Chem 48(5):79–88
Kim JY, Ji YJ, Ha HJ, Chae HK (2003) Synthesis of unusual rhenium complexes with Schiff bases. Bull Korean Chem Soc 24(4):504
Emregul KC, Akay AA, Atakol O (2005) The corrosion inhibition of steel with Schiff base compounds in 2 M HCl. Mater Chem Phys 93:325–329
Emregul KC, Duzgun E, Atakol O (2006) The application of some polydentate Schiff base compounds containing aminic nitrogen as corrosion inhibitors for mild steel in acidic media. Corros Sci 48:3243–3260
Sorkhabia HA, Shaabanib B, Seifzadeha D (2005) Corrosion inhibition of mild steel by some Schiff base compounds in hydrochloric acid. Appl Surf Sci 239:154–164
Negm NA, Zaki MF (2008) Corrosion inhibition efficiency of nonionic Schiff base amphiphiles of p-aminobenzoic acid for aluminum in 4 N-HCl. J Colloids Surf A Physicochem Eng Asp 322:97–102
Negm NA, El-Farargy A, Zaki MF, Mahmoud SA, Abdel Rahman N (2008) Cationic Schiff base amphiphiles: 1. Synthesis, characterization and surface activities of cationic surfactants bearing Schiff base groups and their Mn(II), Cu(II) and Co(II) complexes. Egypt J Petrol 17(2):15–25
Negm NA, Aiad IA (2007) Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surfact Deterg 10:87–92
ASTM (1987) Hand book of corrosion, 13:376
Negm NA, Mohamed AA, El-Awady MY (2004) Influence of structure on the cationic polytriethanol ammonium bromide derivatives. I. Synthesis, surface and thermodynamic properties. Egypt J Chem 47(4):369–381
Zheng O, Zhao JX (2006) Solubilization of pyrene in aqueous micellar solutions of gemini surfactants C12-s-C12·2Br. J Colloid Interface Sci 300:749–754
Azzam EMS, Negm NA, Gad EAM (2004) Surface and solubilization activities of 1-amino-2-alkyloxy–naphthalene-4-sodium sulfonates. Ads Sci Technol 22(8):663
Nair R, Shah A, Baluja S, Chanda S (2006) Synthesis and antibacterial activity of some Schiff base complexes. J Serb Chem Soc 71(7):733
Shokry H, Yuasa M, Sekine I, Issac RM, El-Baradie HY, Gomma GK (1998) Corrosion inhibition of mild steel by Schiff base compounds in various aqueous solutions. Corros Sci 39(1):2173
Walker ML (2004) Corrosion inhibitor. Canadian Patent CA 2,482,513
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Negm, N.A., Zaki, M.F. & Salem, M.A.I. Synthesis and Evaluation of 4-Diethyl Amino Benzaldehyde Schiff Base Cationic Amphiphiles as Corrosion Inhibitors for Carbon Steel in Different Acidic Media. J Surfact Deterg 12, 321–329 (2009). https://doi.org/10.1007/s11743-009-1156-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1156-0