Abstract
The ultimate aerobic biodegradability and toxicity of three ether carboxylic derivative surfactants having different alkyl chains and degrees of ethoxylation were investigated. Ultimate aerobic biodegradability was screened by means of dissolved organic carbon determinations at different initial surfactant concentrations. For comparison, the characteristic parameters of the biodegradation process, such as half-life, mean biodegradation rate, and residual surfactant concentration, were determined. Increased surfactant concentrations decreased mineralization and lengthened the estimated half-life. The results demonstrate that the ultimate aerobic biodegradability is higher for the surfactants with the shortest alkyl chain and highest degree of ethoxylation. Toxicity values of the surfactants, and their binary mixtures, were determined using three test organisms, the freshwater crustacea Daphnia magna, the luminescent bacterium Vibrio fischeri and the microalgae Selenastrum capricornutum. The toxicity is lower for the surfactants with the shortest alkyl chain and highest degree of ethoxylation. The toxicity of binary mixtures of the three ether carboxylate surfactants at a 1:1 weight ratio was also measured. The least toxic mixture is formed by the surfactants having lower individual toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are the most important components in laundry and household cleaning products, comprising 15–40% of the total detergent formulation [1]. The class of anionic surfactants is particularly important, accounting for 60% of the world production [2]. Several of these compounds are biologically not degradable and present a threat to the environment [3]. The massive use of surfactants in detergents and cosmetic formulations and their subsequent disposal in aquatic systems require surfactants to be as environmentally friendly as possible. This implies the need for low-toxicity and biodegradable surfactants. The environmental impact of chemicals is often determined by the ecotoxicity, which is relatively high in the case of surfactants as a result of surface activity and the action against biological membranes [4]. Surfactants have different behaviour and fate in the environment. Non-ionic and cationic surfactants have much higher sorption on soil and sediment than anionic surfactants such as lineal alkyl benzene sulphonate (LAS) [5, 6]. Most surfactants can be degraded by microbes in the environment although some surfactants, such as LAS and dihydrogenated tallow dimethyl ammonium chloride (DTDMAC), and alkylphenols may be persistent under anaerobic conditions [7–9]. LAS was found to degrade in sludge-amended soils with half-lives of 7–33 days [10]. Most surfactants are not acutely toxic to organisms at environmental concentrations and aquatic chronic toxicity of surfactants occurred at concentrations usually greater than 0.1 mg/L [11]. Many studies have been performed on the biodegradability and toxicity of surfactants, the majority of which concern the toxicity of surfactants to small crustaceans such as Daphnia magna [12]. Numerous surfactants are not easily biodegradable; consequently many physicochemical methods of pretreatment such ozonation and other advanced chemical oxidation techniques were developed to eliminate surfactants [13]. There has been an emphasis over the past few years on the development of non-polluting surfactants and builders with improved biodegradability [14]. This growing concern has led to the development of new surfactants, such as the ether carboxylic derivative surfactants.
The ether carboxylic derivative surfactants tested in the present work are anionic surfactants, with the general formula R–O(CH2–CH2O)E–CH2–COO−X, where R is the alkyl chain and X = H+ or Na+. These surfactants improve the foaming quality of the detergent, reducing the irritation level, and therefore they are used as co-surfactants in detergents which have to be in contact with the skin. These surfactants are marketed in concentrated acid form. The ultimate aerobic biodegradability of three ether carboxylic derivative surfactants with different alkyl chains and degrees of ethoxylation has been investigated.
For continued advancement in the search for relationships between toxicity and structural parameters in the field of surfactants, in the present work the ecotoxicity assay with luminescent bacteria, D. magna, and Microalgae was applied to different ether carboxylic derivative surfactants.
The purpose of this paper was to find the relationship between the ultimate biodegradation and the structure of different ether carboxylic derivative surfactants, and the influence of the initial surfactant concentration. Another objective was to determine the toxicity of the ether carboxylic derivative surfactants, and their binary mixtures (1:1 weight), to investigate the toxicological interactions between the surfactants, which take place in natural environments, and how they can affect the toxicity of the mixture, especially when acting in synergism.
Experimental Procedures
Surfactants
The surfactants used in this study were the commercial ether carboxylic derivative surfactants supplied by Kao Corporation (Tokyo, Japan) under the commercial name AKYPO®, and hereafter labelled EC-R8E8, EC-R12–14E3 and EC-R12–14E10. Table 1 shows the degree of ethoxylation (E), the alkyl chain length (R), the % of active matter, and the critical micelle concentration (CMC) of these surfactants. LAS was also supplied by Kao Corporation. The rest of the reagents used were of chemical quality and supplied by Panreac.
Surface Tension Measurements
The CMC values were established by measuring the surface tension of surfactant solutions with different concentrations at 25 °C, using a tensiometer model K11 (KRÜSS GmbH) equipped with a 2-cm platinum plate.
Biodegradation Tests
The biodegradation tests were carried out according to the Organisation for Economic Co-operation and Development (OECD) 301 E test, which is based on the removal of organic compounds measured as dissolved organic carbon (DOC) [15]. A solution of the surfactant, representing the sole carbon source for the microorganisms, is tested in a mineral medium, inoculated and incubated under aerobic conditions in the dark for 21 days. The surfactant solution (for which the biodegradability is to be determined) is inoculated with 0.5 mL of water from a secondary treatment of a sewage-treatment plant (STP) that operates with active sludges. The biodegradation process is monitored by means of the residual surfactant concentration over time by DOC measurements, determined in samples filtered through a 0.45-μm Millipore membrane. Reference assays were performed with an easily biodegradable surfactant (LAS) in order to determine the activity of the microbial population present in the test medium. One flask was used for the blank, one for the reference surfactant, one for abiotic assay, and one for each surfactant concentration tested.
Toxicity Tests
Three toxicity tests were undertaken: the LumiStox® 300 test which employs the luminescent bacterium Photobacterium phosphoreum, the 24-h immobilization test with D. magna (freshwater crustacea), and the 72-h algal growth inhibition test with Selenastrum capricornutum. In the first one, measurements were taken with the measuring system LumiStox® 300, which consists of an instrument for measuring bioluminescence and an incubation unit according to the UNE-EN ISO 11348-2 guideline [16]. The toxicity measurement is based on the luminous intensity of the marine bacteria of the strain Vibrio fischeri NRRL-B-11177 after a certain exposure time to a toxic substance. The luminescent bacteria, dehydrated and frozen at −18 °C, were reactivated with the suspension supplied by Dr. Lange (Dr. Bruno Lange GmbH & Co., Düsseldorf). The assay conditions were pH 7.0, NaCl concentration of 2%, with all the measurements being duplicated for an incubation time of 15 min. When necessary, the sample was filtered prior to the assay. The toxicity values were measured as EC50, which is the surfactant concentration that causes 50% inhibition after 15 min of exposure.
Acute toxicity tests with D. magna were performed in Standard Reference Water (SRW) according to the UNE-EN ISO 6341 guideline [17]. The tests were performed in 100-mL polystyrene vessels, with 50 mL of SRW in each one. Twenty neonates (<24 h) were transferred to vessels containing different concentrations of the test chemical, and the vessels were closed with a polyethylene cap. The neonates were separated from adults every day. There was no feeding and no aeration during the tests which were run at 20 ± 1 °C. Immobility was determined visually after 24 h. For each surfactant, controls and at least five concentrations were used for the determination of the IC50, i.e. the concentration causing 50% inhibition of mobility of Daphnia population. The 72-h algal growth-inhibition test with the microalga S. capricornutum was performed according to the OECD 201 guideline [18]. The procedure consists of filling culture vials with appropriate volumes of nutrient medium and solutions of the surfactant being tested. At the beginning of the test, inoculums of algae were added to the vials to be tested and to the vials of control, and were kept under stable and predetermined incubation conditions.
Inocula were cultivated at 23 ± 1 °C and constant uniform illumination (8,000 lux). After 24, 48 and 72 h the algal density was determined to establish whether growth had been inhibited or stimulated with respect to control. Cell density was estimated by the optical density of the culture at 670 nm.
For all the tests, the surfactant concentration and one control were performed in triplicate for each organism tested. The surfactant concentration in the aquatic bioassays, at the beginning and at the end of the tests, was measured using a total organic carbon (TOC) analyzer for ether carboxylic derivative surfactants and a simplified spectrophotometric method using methylene blue for LAS [19].
Results and Discussion
Biodegradability of Ether Carboxylic Derivative Surfactants
The ultimate biodegradation of the surfactants has been established under aerobic conditions in OECD tests for ready biodegradability [15]. The biodegradation process was monitored by means of the residual surfactant concentration over time by DOC measurements. Duplicate DOC measurements were performed for each sample. It is known that sorption may significantly influence the resulting environmental effects of surfactants and this fact has been studied by some authors [20, 21]. In the biodegradation assays presented here, the sorption could be considered negligible, given the scant biomass formation. Abiotic assays were performed in the presence of HgCl2 to confirm this, and it was found that the values of the residual surfactant remained around 100% over the biodegradation period. These results indicate that the contribution of abiotic processes to the degradation of the surfactants in the biodegradation tests can be dismissed.
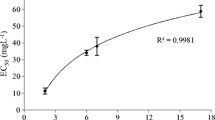
Figure 1 shows the surface tension data versus surfactant concentration for the surfactant EC-R12–14E10. Surface tension data plotted on a semi-log plot for a surfactant will have an approximately linear drop in surface tension followed by a plateau. The concentration at which this discontinuous change in slope occurs is the CMC. CMC data for the ether carboxylic derivative surfactants are shown in Table 1.
Figure 2 shows the time course of the ultimate biodegradation of the surfactants over the degradation period. The initial concentrations in the assays were 25, and 50 mg/L.
For the comparison and quantification of the different biodegradation assays, the following characteristic parameters of the biodegradation profiles were evaluated [22]: half-life (t 1/2), mean biodegradation rate (V M) and the residual surfactant concentration at the end of the assay (S R), which is calculated from the final DOC measurements average. t 1/2 is the time at which the substrate concentration diminishes to half that at the beginning of the biodegradation process. The half-life is calculated by applying graphic methods to the biodegradation profile. V M is defined as the mean velocity of biodegradation reached until achieving 50% biodegradation of the surfactant, and it has been calculated as the quotient between the percentage of biodegradation reached and the time needed to reach this biodegradation value. This parameter provides the speed of the biodegradation process.
Table 1 shows the characteristic parameters of the biodegradation profiles for the ether carboxylic derivative surfactants for all the concentrations assayed. S 0 is the initial concentration of the biodegradation assay in milligrams per litre and Min is the final percentage of mineralization reached at the end of the assay calculated with the following expression:
where the subscripts i and f mean initial and final, respectively.
An analysis of the influence of the initial concentration is presented in Table 1, reflecting that the biodegradation process is slower when the initial concentration increases, i.e. the half-life increases and the mean biodegradation rate decreases. This may be due to the long adaptation time needed by the microorganisms for these surfactants, which are generally not included in conventional detergent formulas.
For EC-R12–14E3 and EC-R12–14E10, the residual surfactant concentration at the end of the assay, S R, is notably augmented with the increasing surfactant concentration. However, for EC-R8E8, the surfactant with the shortest alkyl chain and the highest CMC, the residual surfactant concentration was independent of the initial concentration, and the mineralization percentage rises with the initial concentration.
Current legislation requires a minimum level of 60% of ultimate biodegradation to be reached when applying one of the methods listed in Annex III of Regulation (EC) No. 648/2004 [23]. If this condition is met the surfactant can be considered biodegradable. The surfactant EC-R8E8 fulfils this requirement, yielding 91.9% DOC removal. The surfactants with greater alkyl chain lengths (EC-R12–14E3 and EC-R12–14E10) satisfy this requirement only with an initial surfactant concentration of 25 mg/L (62.13 and 81.42% DOC removal, respectively).
To analyse the influence of the degree of ethoxylation and the size of the alkyl chain on the final biodegradation process, the results for different surfactants at the initial concentrations of 25 and 50 mg/L are compared (Fig. 3).
The surfactant that achieved the greatest biodegradation was EC-R8E8, i.e. the one with the shortest alkyl chain. In comparisons of the surfactants with the same alkyl length, EC-R12–14E3 and EC-R12–14E10, (C12–C14) and different degrees of ethoxylation (3 and 10, respectively), it was found that there were no significant differences.
Toxicity of Ether Carboxylic Derivates Surfactants
The toxicity of the ether carboxylic derivative surfactants, and their binary mixtures, was measured. Toxicity values of the surfactants were determined by applying the 24-h immobilization test with D. magna, the LumiStox® 300 test which employs the luminescent bacteria P. phosphoreum and the 72-h algal growth-inhibition test. These results show that V. fischeri, D. magna and Microalgae do not use the surfactants as sources of carbon. Therefore, the surfactant concentrations remained stable over the time period used in the bioassays. Table 2 shows the toxicity values for the tests with V. fischeri, D. magna and Microalgae, for the different surfactants assayed.
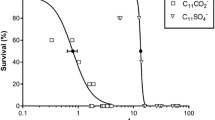
The acute toxicity values of the surfactants ranged from 3.58 to 7.08 mg/L for the surfactant EC-R12–14E3, from 14.18 to 26.01 mg/L for EC-R12–14E10 and from 76.26 to 134.59 mg/L for EC-R8E8. According to the European Union Directive No. 67/548/EEC [24] with the respective amendment No. 7, the above results assign the surfactant EC-R12–14E3 as having class II toxicity (R51), which is regarded as toxic against aquatic organisms. Meanwhile, the surfactants EC-R12–14E10 and EC-R8E8 are classified as harmful (class III R52) and safe, respectively. According to the literature, anionic and non-ionic surfactants are toxic to various aquatic organisms at concentrations of 0.0025–300 mg/L and 0.3–200 mg/L, respectively [25].
For ecological safety, it is further assumed that the theoretically calculated concentration of surfactant in the natural environment should be 100-fold lower than the values of IC50 and EC50 determined experimentally. In this case, no negative environmental impact of the surfactant would be expected. The results of the toxicity tests are typically much higher compared to values that might be found in the environment [26].
The results presented in Table 2 show that V. fischeri was more sensitive to toxic effects from ether carboxylic derivative surfactants than D. magna and Microalgae were. The toxicity is lower for the surfactant with the shortest alkyl chain. The degree of ethoxylation (E) has the reverse effect: the higher degree of ethoxylation the lower toxicity.
Surfactants are often used as co-surfactants in detergent formulas, so the toxicological interactions of the binary mixtures of ether carboxylic derivative surfactants were investigated. The results presented in Table 2 show that D. magna was more sensitive to toxic effects from binary mixtures of ether carboxylic derivative surfactants than V. fischeri and Microalgae were. Microalgae were less sensitive to toxic effects from binary mixtures of ether carboxylic derivative surfactants than the individual surfactants. The least toxic mixture is formed by the surfactants having lower individual toxicity, i.e. surfactants EC-R8E8 and EC-R12–14E10. This result highlights the synergism in the co-occurrence of this class of surfactants.
Comparisons of the toxicity of these surfactants with the typical anionic surfactant LAS show that when V. fischeri and D. magna tests are used, LAS toxicity values are intermediate between the ether carboxylic derivative surfactants assayed. The Microalgae test indicates that LAS is the least toxic surfactant, although the synergic binary mixtures improve these surfactants’ results, and consequently the mixture between EC-R8N8 and EC-R12–14N10 proves less toxic than LAS.
In conclusion, ether carboxylic derivative surfactants can be considered biodegradable. The one with the shortest alkyl chain length and the highest CMC (EC-R8E8) yielded the highest percentage of mineralization. The influence of the initial concentration reflected that the biodegradation process was slower when the initial concentration increased, i.e., the half-life increased, the mean biodegradation rate decreased, and the residual surfactant concentration was notably augmented, except for EC-R8E8, for which the S R was independent of the initial concentration. The toxicity measurements of these ether carboxylic surfactants indicate that the least toxic was the most biodegradable (EC-R8E8). Binary mixture measurements indicate that the least toxic mixture was formed by the surfactant having lower individual toxicity. Moreover, the Microalgae test results indicate that there was synergism in the co-occurrence of these surfactants. The results imply that at low concentrations these surfactants may be considered less damaging to the environment.
References
Scheibel J (2004) The evolution of anionic surfactant technology to meet the requirements of the laundry detergent industry. J Surfact Deterg 7:319–328
Aloui F, Kchaou S, Sayadi S (2009) Physicochemical treatments of anionic surfactants wastewater: effect on aerobic biodegradability. J Hazard Mater 164:353–359
Cain RB (1994) Biodegradation of detergents. Curr Opin Biotechnol 5:266–274
Steber J, Guhl W, Steker N, Schröder F (1995) Alkyl polyglycosides – ecological evaluation of a new generation of nonionic surfactants. Tenside Surf Deterg 32:515–521
Brownawell BJ, Chen H, Zhang W, Westall JC (1997) Sorption of non-ionic surfactants on sediment materials. Environ Sci Technol 31:1735–1741
Fytianos K, Voudrias E, Papamichali A (1998) Bahaviour and fate of linear alkylbenzene sulfonate in different soils. Chemosphere 36:2741–2746
Scott MJ, Jones MN (2000) The biodegradation of surfactants in the environment. Biochim Biophys Acta 1508:235–251
García MT, Campos E, Sánchez-Leal J, Ribosa I (1999) Effect of the alkyl chain length on the anaerobic biodegradability and toxicity of quaternary ammonium based surfactants. Chemosphere 38:3473–3483
Charles W, Ho G, Cord-Ruwisch R (1996) Anaerobic biofloculation of wool scouring effluent: the influence of non-ionic surfactants on efficiency. Water Sci Technol 34:1–8
Ying GG (2005) Fate, behavior and effects of surfactants and their degradation products in the environment. Environ Int 32:417–431
Lewis M, Wee V (1991) Chronic and sublethal toxicities of surfactants to aquatic animals: a review and risk assessment. Water Res 25:101–113
Liwarska-Bizukocj E, Miksch K, Malachowska-Jutsz A, Kalka J (2005) Acute toxicity and genotoxcity of five selected anionic and non-ionic surfactants. Chemosphere 58:1249–1253
White GF, Russel NJ (1993) Biodegradation of anionic surfactants and related molecules. In: Ratledge C (ed) Biochemistry of microbial degradation. Kluwer, Dordrecht, pp 143–177
Yu Y, Zhao J, Bayly A (2008) Development of surfactants and builders in detergent formulations. Chin J Chem Eng 16:517–527
Organisation for Economic Cooperation and Development (OECD) (1993) OECD guidelines for the testing of chemicals volume 1 Section 3: degradation and accumulation, OECD, Paris, France
UNE EN ISO 11348-2 (NORMA UNE EN ISO 11348-2). Determinación del efecto inhibidor de muestras de agua sobre la luminiscencia de Vibrio fischeri (Ensayo de bacterias luminiscentes)
UNE EN ISO 6341 (NORMA UNE EN ISO 6341). Determinación de la inhibición de la movilidad de Daphnia magna Straus (Cladocera, Crustacea) (Ensayo de toxicidad aguda)
Organisation for Economic Cooperation and Development (OECD) (1984) OECD guidelines for the testing of chemicals. Alga growth inhibition test, OECD, Paris, France
Jurado E, Fernández-Serrano M, Núñez-Olea J, Luzón G, Lechuga M (2006) Simplified spectrophotometric method using methylene blue for determining anionic surfactants: Applications to the study of primary biodegradation in aerobic screening tests. Chemosphere 65:278–285
Belanger S, Dorn P, Toy R, Boeije G, Marshall S, Wind T, Van Compernolle R, Zeller D (2006) Aquatic risk assessment of alcohol ethoxylates in North America and Europe. Ecotoxicol Environ Saf 64:85–99
Van Compernolle R, McAvoy D, Sherren A, Wind T, Cano M, Belanger S, Dorn P, Kerr K (2006) Predicting the sorption of fatty alcohols and alcohol ethoxylates to effluent and receiving water solids. Ecotoxicol Environ Saf 64:61–74
Jurado E, Fernández-Serrano M, Núñez-Olea J, Lechuga M (2007) Primary biodegradation of commercial fatty-alcohol ethoxylate surfactants: characteristic parameters. J Surfact Deterg 10:145–153
EC Regulation (2004) Regulation (EC) No 648/2004 of the European Parliament and the Council of 31 March 2004 on detergents. (DO L 104, 04/08/2004)
European Economic Community (EEC). Council Directive of 27 June 1967 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (67/548/EEC)
Pettersson A, Adamsson M, Dave G (2000) Toxcity and detoxification of Swedish detergents and softener products. Chemosphere 41:1611–1620
Szwach I, Hreczvch W and Fochtman P (2002) Comparative evaluation of environmental impact of fatty alcohol ethoxylates and fatty acid methyl ester ethoxylates as nonionic surfactants. In: A Chain (ed) Proceedings of the 5th world conference on detergents: reinventing the industry—opportunities and challenges. Montreux, Switzerland, pp 163–165
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jurado, E., Fernández-Serrano, M., Lechuga, M. et al. Environmental Impact of Ether Carboxylic Derivative Surfactants. J Surfact Deterg 15, 1–7 (2012). https://doi.org/10.1007/s11743-011-1278-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-011-1278-z