Abstract
In order to develop models that can predict the environmental behavior and effects of chemicals, reliable experimental data are needed. However, for anionic surfactants the number of ecotoxicity studies is still limited. The present study therefore aimed to determine the aquatic ecotoxicity of three classes of anionic surfactants. To this purpose we subjected daphnids (Daphnia magna) for 48 h to alkyl carboxylates (CxCO2−), alkyl sulfonates (CxSO3−), and alkyl sulfates (CxSO4−) with different carbon chain lengths (x). However, all surfactants with x > 11 showed less than 50% immobility at water solubility. Hence, EC50 values for only few surfactants could be gathered: C9CO2− (16 mg L−1), C11CO2− (0.8 mg L−1) and C11SO4− (13.5 mg L−1). Data from these compounds showed an increase in ecotoxicity with a factor 4.5 per addition of a hydrocarbon unit to the alkyl chain, and a factor 20 when replacing the sulfate head group by a carboxylate head group. Unfortunately, we could not test carboxylates with a broader variety of chain lengths because solubility limited the range of chain length that can be tested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Numerous new organic chemicals are produced yearly for application in industry and consumer products (CEFIC 2014). For environmental risk assessment of new and existing chemicals, an understanding of their environmental behavior and effects is required, but for anionic surfactants the number of ecotoxicity studies is still limited. For the development of predictive models such as quantitative structure–activity relationships (QSARs) for surfactants, more experimental data for these group of compounds are therefore needed. Although some toxicity tests on surfactants have been performed thus far (Schüürmann 1990; Roberts 1991; Versteeg et al. 1997; Wong et al. 1997; Dyer et al. 2000; Roberts and Costello 2003; Boeije et al. 2006; Hodges et al. 2006; Qi et al. 2011), the data is still too limited to compare the effect between different surfactant groups (i.e., surfactants with different head group structures), certainly for anionic surfactants. In this study, we therefore focused on generating aquatic ecotoxicity data for anionic surfactants from three different surfactant groups.
Anionic surfactants are high production volume chemicals which are present in many consumer products and consequently also in the environment (Sanderson et al. 2006; CEFIC 2014). Their amphiphilic and electrostatic properties make them very efficient compounds for the detergent industry. At the same time, these properties result in a very different environmental behavior compared to e.g. neutral organic compounds (Jones et al. 2003; Guo and Gaiki 2005). Unlike for common neutral organic pollutants, their accumulation and potential effects can therefore not always be correlated with predicted octanol–water partition constants (log Kow) (Tolls and Sijm 1995).
The ecotoxicity of organic compounds (quantified by the concentration causing a 50% effect; EC50 value) is usually determined in standardized Daphnia magna acute ecotoxicity tests according to OECD guideline 202 (OECD 2004). For some surfactants within a specific surfactant group (i.e., homologues sharing the same head group), toxicity is observed to increase with increasing alkyl chain length due to increased hydrophobicity (Roberts 2000; Roberts et al. 2013; Barmentlo et al. 2015). At the same time, hydrophobicity affects the bioavailability of surfactants by decreasing the solubility, but also by increasing sorption to other phases (Pittinger et al. 1989). Bioavailability of anionic surfactants is also influenced by the electrostatic characteristics of the head group, which can result in ion-pairing with divalent inorganic cations (e.g., Ca2+ or Mg2+) (Rodriguez et al. 2001; Yan et al. 2010). The standard medium in the D. magna toxicity test (OECD 2004) contains a relatively high total ionic strength that includes divalent cations and solubility problems can therefore be expected for some surfactants. The determination of EC50 values for (ionic) compounds with a low solubility using OECD guideline 202 can therefore be challenging. However, since experimental data for anionic surfactants is still much needed, the aim of the present study was to employ the standardized D. magna ecotoxicity test to determine the aquatic ecotoxicity of three classes of anionic surfactants: alkyl carboxylates, alkyl sulfonates, and alkyl sulfates.
Materials and Methods
All test compounds had a typical surfactant structure containing a hydrophobic alkyl chain and a hydrophilic ionized head group. Sodium salts of linear alkyl sulfates (CxSO4−; with alkyl chain lengths C11, C13, C15 and C16) and linear alkyl sulfonates (CxSO3−; C11, C13, C14 and C15) were obtained from Research Plus (South Plainfield, NJ). Sodium salts of linear alkyl carboxylates (CxCO2−; C9, C11, C13, C14, and C15) were obtained from Sigma-Aldrich, (Zwijndrecht, The Netherlands). All organic compounds had purities higher than 98%. Ammonium acetate was purchased from Sigma-Aldrich. Methanol was obtained from Biosolve (Valkenswaard, The Netherlands). Ultrapure water was obtained from a Millipore water purification system (resistivity > 18 MΩ/cm, Merck Chemicals, Amsterdam, The Netherlands).
The daphnid D. magna Straus was selected as test organism to determine the aquatic ecotoxicity of surfactants. Juvenile daphnids (clone 4) aged < 24 h were obtained from adults between 2 and 3 weeks old. Continuous cultures were maintained in Elendt M4 medium and fed with the alga Chlorella vulgaris. At regular intervals (about every 3 months), acute toxicity tests were performed with the reference toxicant K2Cr2O7 to check whether the sensitivity of the daphnids culture was within the limits (EC50, 24 h = 0.6–2.1 mg L−1) as set by the guideline (OECD 2004). The medium used in the toxicity experiments consisted of the standard OECD medium that was prepared according to OECD guideline 202, containing 266 mg L−1 CaCL2·2H2O, and 112 mg L−1 MgSO4·7H2O. Concentrations of KCl and NaHCO3 were 5 and 65 mg L−1 respectively (OECD 2004). The test media was buffered to pH 7 ± 0.3 with NaOH (66 mg L−1) and 3-(N-morpholino)propanesulfonic acid (MOPS; 1.046 g L−1).
The D. magna were exposed to the selected compounds in 48 h immobility tests (OECD 2004). Per experiment five test concentrations, a solvent control (0.25% methanol without the test compound) and a control were tested with four replicates per treatment. Each replicate consisted of a glass tube filled with 20 mL of test solution, spiked with 50 µL (0.25% of total volume) methanol containing the test compound. The tubes were randomly distributed in a climate controlled fume hood (20 ± 1°C), with a light–dark regime of 16:8 h. The experiment was started by introducing five neonates (younger than 24 h) into each tube. After 48 h, the number of animals not responding to stimulation was scored. Hardness, oxygen concentration, temperature and pH were measured at the start and the end of the experiments and were within the range prescribed by OECD guideline 202 (OECD 2004). The concentration of the test compounds was analyzed by extracting a 200 µL water sample from each replicate at the start and the end of the experiment, an injection standard was added and the sample was subsequently diluted with 750 µL of methanol and stored in a freezer (− 18°C) until chemical analysis.
All anionic surfactants were detected with a triple quadrupole mass spectrometer (MDS SCIEX API 3000 MS/MS System from Applied Biosystems, Bleiswijk, The Netherlands) with a Turbo Ion spray source operated at 400°C. A solvent delay switch (Da Vinci, Rotterdam, The Netherlands) was used to prevent introduction of inorganic constituents from water samples into the MS. Chromatograms were integrated with Analyst 1.4.2 (Applied Biosystems). Concentration–response relationships and the corresponding 48 h EC50 values were calculated according to Haanstra et al. (1985) by fitting a logistic curve (Eq. 1) to the percentage of mobility (100% − immobilization) versus the surfactant concentration in the water phase.
where y(x) is the mobility at concentration x (in %), a is the EC50 value (in mg L−1), b is the slope of the curve, c is y(0) which equals the average mobility of the control and x is the surfactant concentration in water (in mg L−1). Data analyses were performed with SPSS software (IBM Corp 2013) and Graphpad Prism Version 7.0 (GraphPad Software 2017).
Results and Discussion
A total of 14 surfactants with varying alkyl chain lengths from three surfactant groups (alkyl sulfates, alkyl sulfonates, and alkyl carboxylates) were tested. Due to their hydrophobicity and electrostatic charge, anionic surfactants with long alkyl chains often poorly dissolve in water containing inorganic cations. We therefore decided to first test the effect of saturated water solutions at maximum aqueous solubility (Sw) on the daphnids. To this end we stirred an excess of compound for 48 h in standard OECD medium under the standard conditions of the D. magna toxicity tests (OECD 2004). For the compounds that caused more than 50% immobility of the daphnids at Sw, a concentration range was tested in order to obtain concentration–response relationships and to derive EC50 values.
We were unable to dissolve alkyl sulfonates (CxSO3−) in the OECD medium at sufficiently high concentrations to cause any effect. This may have been a result of the presence of (divalent) cations in the aqueous phase. Cations are known to affect the hydration of anionic surfactants and often lowers their critical micelles concentration (CMC) (Yan et al. 2010). Divalent cations such as Ca2+ and Mg2+ can furthermore form ion pairs containing two surfactant monomers and one divalent cation, or form bridges between monomers and charged sites on sorbents (Haftka et al. 2015). For the alkyl carboxylates (CxCO2−) and the alkyl sulfates (CxSO4−), compounds with an alkyl chain longer than C11 were badly soluble in the OECD medium and showed less than 50% immobility at Sw. Hence, no further ecotoxicity tests were performed for these compounds.
Because of the solubility problems of the tested compounds in the OECD medium, EC50 values for only few anionic surfactants could be generated: C9CO2−, C11CO2− and C11SO4−. Because one pair of these surfactants contains equal alkyl chain lengths and different surfactant head groups (C11CO2− and C11SO4−), and another pair (C9CO2− and C11CO2−) differs in chain length with equal head group, we had two single opportunities to evaluate the effect of head group structure and alkyl chain length on the toxicity of the anionic surfactants. However, note that these interpretations are based on only a single pair of surfactants. For C9CO2−, C11SO4− and C11CO2− analyzed concentrations were respectively ± 10%, ± 10% and ± 30% lower compared to nominal concentrations. During the 48 h D. magna toxicity experiments 100% control survival was recorded. From the dose–response curve of C11SO4− an EC50 value of 13.5 mg L−1 was derived (95% CI 13.2–13.8 mg L−1) (Fig. 1). We were unable to find any EC50 values of C11SO4− in literature as most studies focused on C12SO4−. Persoone et al. (1989) reported an EC50 value of 9.6 mg L−1 for C12SO4− in a D. magna 24 h toxicity test and Dyer et al. (1996) found an EC50 value of 5.5 mg L−1 in a 48 h Ceriodaphnia dubia toxicity test (comparable sensitivity to D. magna (Versteeg et al. 1997)). Both values are in line with our data for C11SO4−, as toxicity generally increases from 24 to 48 h exposure and an EC50 value of 5.5 mg L−1 is close to the expected EC50 concentration increase when a hydrocarbon (–CH2–) unit is added to the alkyl chain of C11SO4− (see next paragraph). The dose–response curve of C11CO2− provided an EC50 concentration of 0.80 mg L−1 (95% CI 0.7–0.9 mg L−1) (Fig. 1). Toxicity data for D. magna are scarce for C11CO2−, a 36x higher EC50 value (EC50 = 29 mg L−1) was reported by Lundahl and Cabridenc (1978) in a 24 h ecotoxicity test, and an EC50 value of 1.3 mg L−1 was reported by the European Chemical Agency (2014). While, we were unable to acquire the exact experimental details of the toxicity test of Lundahl and Cabridenc (1978), their analysis was performed using the Methylene Blue Active Substance (MBAS) essay which is meanwhile retracted as a standard method by ASTM.
Effect of head group on ecotoxicity of C11SO4− and C11CO2− to Daphnia magna after 48 h of exposure. Both dose–response curves were calculated according to Haanstra et al. (1985). The EC50 concentrations are plotted with their 95% confidence intervals as solid black symbols (the 95% confidence interval of C11SO4− is too small to be seen)
Comparing the dose–response curves and EC50 values for C11SO4− and C11CO2− shows that the head group has a significant effect on ecotoxicity (Fig. 1). The alkyl chains of both compounds are of the same length and the effect of hydrophobicity is subsequently similar (Hammer et al. 2017). Therefore, the difference in EC50 values is likely a result from the different molecular properties of the surfactant head groups (SO4− vs. CO2−). Besides the head group structure, the most notable distinction between the properties of these two surfactant groups is the difference in pKa [4.8 for CxCO2− (Haynes 2015), and − 3.6 for CxSO4− (COSMOlogic 2015)]. The pKa value is partly a result of the charge distribution over a molecule and shows what fraction of the compound is in the ionic form at certain pH. While these compounds are in the OECD medium both for > 99% present in their ionic (de-protonated) form, the difference charge distribution between both molecules still affects their behavior in the aqueous phase and their interaction with other phases. For example, alkyl carboxylates are much better hydrated than alkyl sulfates (Vlachy et al. 2009), which also affects their electrostatic interaction with sorbents (Rabin and Stillian 1994). Furthermore, the difference in charge distribution may affect the uptake of the anionic surfactants in cell membranes due to their zwitterionic properties (Scherer and Seelig 1989). Badly hydrated compounds are usually more affected by local charges and have more difficulty to partition into membranes than well hydrated compounds (Jing et al. 2009; Roberts et al. 2013). The C11CO2− surfactant may therefore partition more effectively into cell membranes of the daphnids compared to C11SO4− which explains why alkyl carboxylates were approximately 20 times more toxic compared to their sulfated counterparts.
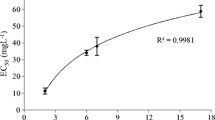
The effect of the alkyl chain length on surfactant toxicity was studied by comparing the EC50 values of C9CO2− and C11CO2−. The dose–response curve for C9CO2 showed an EC50 concentration of 16.0 mg L−1 (95% CI 14.8–17.3 mg L−1), see Fig. 2. Just like for the previously discussed surfactants, literature data on the toxicity of C9CO2− to D. magna is inconsistent and details about the experimental setup were difficult to obtain. We were able to find two EC50 concentrations from literature: first, again a very high EC50 concentration of 135 mg L−1 from a 24 h D. magna toxicity test by Lundahl and Cabridenc (1978). Second, a reported EC50 value of 16 mg L−1, which is equal to our experimentally derived EC50 value and originates from a report of the European Chemical Agency (2013). The results from Lundahl and Cabridenc are questionable (see previous paragraph) and both studies lack experimental details about medium composition and only mention the duration of the tests. Toxicity between C11 and C9 carboxylate differed with a factor of ∼ 23 compared (Fig. 2), which is a factor of ∼ 4.5 per hydrocarbon unit added to the alkyl chain. This is somewhat higher than the increments found for other surfactant groups in previous studies [between 2.4 and 3.4 (Lundahl and Cabridenc 1978; Maki and Bishop 1979; Hodges et al. 2006)]. An increase in the alkyl chain length increases the hydrophobicity of the compound and thus increases the sorption to the membrane lipid (Könnecker et al. 2011). At longer alkyl chain lengths (> C11) the toxicity is expected to further increase, but this effect is not detectible using the D. magna toxicity test due the low solubility of the compounds in the OECD medium. The factor ~ 4.5 increase in toxicity with addition of a carbon atom to the alkyl chain is based on only two chemicals. This data set is limited and could be regarded as a shortcoming of the study. Unfortunately, we could not test more compounds because of the solubility problems (limits) of the longer chain carboxylates in the calcium rich test medium of the Daphnia test. Another test organism that requires another medium composition (less calcium) could avoid this shortcoming.
Effect of alkyl chain length on ecotoxicity of C11CO2− and C9CO2− on Daphnia magna after 48 h of exposure. Both dose–response curves were calculated according to Haanstra et al. (1985). The EC50 concentrations are plotted with their 95% confidence intervals as solid black symbols
The main reason why ecotoxicity could not be detected for most of the test compounds probably lies in the presence of cations in the aqueous solution of the D. magna tests, which can affect the solubility and bioavailability of anionic surfactants. In an attempt to generate more ecotoxicity data, we decided to change the composition of the original OECD medium and study the effect of divalent cation concentration on the ecotoxicity of C9CO2− and C11CO2−. To this end, four different media were prepared with different concentrations of Ca2+ and Mg2+, while maintaining original Ca2+:Mg2+ ratio (Naddy et al. 2002). A concentration of Ca2+ of 10 mg L−1 was selected as the lowest concentration, because lower concentrations will affect with D. magna survival (Hessen et al. 2000). The highest concentration of Ca2+ tested was 80 mg L−1, conform with the original OECD guideline 202. The resulting EC50 concentrations varied slightly, but did not differ significantly between medium compositions. Hence, the medium with the lowest ionic strength may already contain enough cations to cause precipitation of anionic surfactants.
The D. magna toxicity test is a well-accepted and standardized toxicity test which has generated ecologically relevant toxicity data for many organic compounds. However, the medium proposed in the OECD guideline for D. magna is of high ionic strength and this can result in solubility problems for compounds that are already barely soluble in water and for compounds that maintain an electrostatic charge (Waaijers et al. 2013). The D. magna toxicity test appeared unable to produce reliable results for most of the surfactants tested in this study. For hazard assessment purposes of anionic surfactants, alternative approaches should therefore be investigated that either exclude the influence of divalent cations present in the test medium or endpoints should be selected that are affected at concentrations below the aqueous solubility of the surfactants. Furthermore, because anionic surfactants are known to have an affinity for soil surfaces and organic matter (Rico–Rico 2009) toxicity tests that include sediment living organisms (e.g. Lumbriculus variegatus or Chironomus riparius) may be more suitable for the production of toxicological endpoint data. Despite the obstacles that occurred with anionic surfactants during the D. magna tests, we were able to determine the effect of surfactant alkyl chain length and head group composition on the aquatic ecotoxicity of a select group of anionic surfactants. However, these interpretations were based on only a single pair of surfactants.
References
Barmentlo SH, Stel JM, van Doorn M et al (2015) Acute and chronic toxicity of short chained perfluoroalkyl substances to Daphnia magna. Environ Pollut 198:47–53. https://doi.org/10.1016/j.envpol.2014.12.025
Boeije GMG, Cano ML, Marshall SJ et al (2006) Ecotoxicity quantitative structure-activity relationships for alcohol ethoxylate mixtures based on substance-specific toxicity predictions. Ecotoxicol Environ Saf 64:75–84. https://doi.org/10.1016/j.ecoenv.2005.08.009
CEFIC (2014) The European Chemical Industry Council. http://www.cefic.org/Facts-and-Figures/Chemicals-Industry-Profile/. Accessed 1 Jan 2018
COSMOlogic GmbH (2015) COSMOtherm. pp 1–77
Dyer SD, Lauth JR, Morrall SW et al (1996) Development of a chronic toxicity structure–activity relationship for alkyl sulfates. Environ Toxic Water 295–303
Dyer SD, Stanton DT, Lauth JR, Cherry DS (2000) Structure-activity relationships for acute and chronic toxicity of alcohol ether sulfates. Environ Toxicol Chem 19:608–616. https://doi.org/10.1002/etc.5620190312
European Chemical Agency (2013) Regulation (EU) No. 528/2012 concerning decanoic acid
European Chemical Agency (2014) Regulation (EU) No. 528/2012 concerning lauric acid
GraphPad S (2017) GraphPad Prism version 7.00 for Windows
Guo Y, Gaiki S (2005) Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J Chromatogr A 1074:71–80. https://doi.org/10.1016/j.chroma.2005.03.058
Haanstra L, Doelman P, Voshaar JHO (1985) The use of sigmoidal dose response curves in soil ecotoxicological research. Plant Soil 84:293–297. https://doi.org/10.1007/BF02143194
Haftka JJH, Hammer J, Hermens JLM (2015) Mechanisms of neutral and anionic surfactant sorption to solid-phase microextraction fibers. Environ Sci Technol 49:11053–11061. https://doi.org/10.1021/acs.est.5b02901
Hammer J, Haftka JJ-H, Scherpenisse P et al (2017) Fragment-based approach to calculate hydrophobicity of anionic and nonionic surfactants derived from chromatographic retention on a C18 stationary phase. Environ Toxicol Chem 36:329–336. https://doi.org/10.1002/etc.3564
Haynes WM (2015) CRC handbook of chemistry and physics, 96th edn. CRC Press, Florida
Hessen DO, Alstad NEW, Skardal L (2000) Calcium limitation in Daphnia magna. J Plankton Res 22:553–568. https://doi.org/10.1093/plankt/22.3.553
Hodges G, Roberts DW, Marshall SJ, Dearden JC (2006) The aquatic toxicity of anionic surfactants to Daphnia magna—a comparative QSAR study of linear alkylbenzene sulphonates and ester sulphonates. Chemosphere 63:1443–1450. https://doi.org/10.1016/j.chemosphere.2005.10.001
IBM Corp (2013) IBM SPSS statistics for Windows version 22.0.0. IBM Corp, Armonk
Jing P, Rodgers PJ, Amemiya S (2009) High lipophilicity of perfluoroalkyl carboxylate and sulfonate: Implications for their membrane permeability. J Am Chem Soc 131:2290–2296. https://doi.org/10.1021/ja807961s
Jones PD, Hu W, De Coen W et al (2003) Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem 22:2639–2649. https://doi.org/10.1897/02-553
Könnecker G, Regelmann J, Belanger S et al (2011) Environmental properties and aquatic hazard assessment of anionic surfactants: physico-chemical, environmental fate and ecotoxicity properties. Ecotoxicol Environ Saf 74:1445–1460. https://doi.org/10.1016/J.ECOENV.2011.04.015
Lundahl P, Cabridenc R (1978) Molecular structure-biological properties relationships in anionic surface-active agents. Water Res 12:25–30. https://doi.org/10.1016/0043-1354(78)90191-4
Maki AW, Bishop WE (1979) Acute toxicity studies of surfactants to Daphnia magna and Daphnia pulex. Arch Environ Contam Toxicol 8:599–612. https://doi.org/10.1007/BF01055040
Naddy RB, Stubblefield WA, May JR et al (2002) The effect of calcium and magnesium ratios on the toxicity of copper to five aquatic species in freshwater. Environ Toxicol Chem 21:347–352
OECD (2004) Test No. 202: Daphnia sp. acute immobilisation test. OECD Publishing, Paris
Persoone G, Van de Vel A, Van Steertegem M, De Nayer B (1989) Predictive value of laboratory tests with aquatic invertebrates: influence of experimental conditions. Aquat Toxicol 14:149–167. https://doi.org/10.1016/0166-445X(89)90025-8
Pittinger CA, Woltering DM, Masters JA (1989) Bioavailability of sediment-sorbed and aqueous surfactants to Chironomus riparius (midge). Environ Toxicol Chem 8:1023–1033. https://doi.org/10.1002/etc.5620081108
Qi P, Wang Y, Mu J, Wang J (2011) Aquatic predicted no-effect-concentration derivation for perfluorooctane sulfonic acid. Environ Toxicol Chem 30:836–842. https://doi.org/10.1002/etc.460
Rabin S, Stillian J (1994) Practical aspects on the use of organic solvents in ion chromatography. J Chromatogr A 671:63–71. https://doi.org/10.1016/0021-9673(94)80222-X
Rico-Rico Á (2009) Linear alkylbenzene sulfonates in the aquatic environment: study of the analysis, sorption processes and sediment toxicity. Utrecht University, Utrecht
Roberts DW (1991) QSAR issues in aquatic toxicity of surfactants. Sci Total Environ 109–110:557–568. https://doi.org/10.1016/0048-9697(91)90209-W
Roberts DW (2000) Aquatic toxicity—are surfactant properties relevant? J Surfactants Deterg 3:309–315. https://doi.org/10.1007/s11743-000-0134-z
Roberts DW, Costello J (2003) QSAR and mechanism of action for aquatic toxicity of cationic surfactants. QSAR Comb Sci 22:220–225. https://doi.org/10.1002/qsar.200390015
Roberts DW, Roberts JF, Hodges G et al (2013) Aquatic toxicity of cationic surfactants to Daphnia magna. SAR QSAR Environ Res 24:417–427. https://doi.org/10.1080/1062936X.2013.781538
Rodriguez CH, Lowery LH, Scamehorn JF, Harwell JH (2001) Kinetics of precipitation of surfactants. I. Anionic surfactants with calcium and with cationic surfactants. J Surfactants Deterg 4:1–14. https://doi.org/10.1007/s11743-001-0155-7
Sanderson H, Dyer SD, Price BB et al (2006) Occurrence and weight-of-evidence risk assessment of alkyl sulfates, alkyl ethoxysulfates, and linear alkylbenzene sulfonates (LAS) in river water and sediments. Sci Total Environ 368:695–712. https://doi.org/10.1016/j.scitotenv.2006.04.030
Scherer PG, Seelig J (1989) Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry 28:7720–7728. https://doi.org/10.1021/bi00445a030
Schüürmann G (1990) QSAR analysis of the acute toxicity of oxyethylated surfactants. Chemosphere 21:467–478. https://doi.org/10.1016/0045-6535(90)90017-N
Tolls J, Sijm DTHM (1995) A preliminary evaluation of the relationship between bioconcentration and hydrophobicity for surfactants. Environ Toxicol Chem 14:1675–1685. https://doi.org/10.1002/etc.5620141007
Versteeg DJ, Stanton DT, Pence MA, Cowan C (1997) Effects of surfactants on the rotifer Brachionus calyciflorus in a chronic toxicity test and in the development of QSARs. Environ Toxicol Chem 16:1051–1058. https://doi.org/10.1002/etc.5620160527
Vlachy N, Jagoda-Cwiklik B, Vácha R et al (2009) Hofmeister series and specific interactions of charged headgroups with aqueous ions. Adv Colloid Interface Sci 146:42–47. https://doi.org/10.1016/j.cis.2008.09.010
Waaijers SL, Hartmann J, Soeter AM et al (2013) Toxicity of new generation flame retardants to Daphnia magna. Sci Total Environ 463–464:1042–1048. https://doi.org/10.1016/j.scitotenv.2013.06.110
Wong DCL, Dorn PB, Chai EY (1997) Acute toxicity and structure-activity relationships of nine alcohol ethoxylate surfactants to fathead minnow and Daphnia magna. Environ Toxicol Chem 16:1970–1976. https://doi.org/10.1002/etc.5620160929
Yan H, Yuan S-L, Xu G-Y, Liu C-B (2010) Effect of Ca2+ and Mg2+ ions on surfactant solutions investigated by molecular dynamics simulation. Langmuir 26:10448–10459. https://doi.org/10.1021/la100310w
Acknowledgements
The present study was supported by the Dutch Technology Foundation STW, which is part of The Netherlands Organization for Scientific Research and is partly funded by the Ministry of Economic Affairs (Stichting voor de Technische Wetenschappen). Additional funding was received from Deltares (Utrecht, The Netherlands) and Environmental Risk Assessment and Management (ERASM), which is a partnership of European detergent and surfactant products. We would like to thank BSc students Tessa de Bruin, Remy Mulders, and Linde de Herder from the University of Amsterdam for their support with the experimental work and Rineke Keijzers from Ecofide for supplying the daphnids.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hammer, J., Tukker, A.M., Postma, J.F. et al. Solubility Constraints on Aquatic Ecotoxicity Testing of Anionic Surfactants. Bull Environ Contam Toxicol 101, 99–104 (2018). https://doi.org/10.1007/s00128-018-2361-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2361-1