Abstract
In this study the treatment efficiency of different ultraviolet (UV)-enhanced ozonation processes for degradation of two surfactants, sodium dodecylbenzene sulfonate [200 mg/L or 0.3 critical micelle concentration (CMC)] and a nonylphenol ethoxylate with 40 oxyethylene units (200 mg/L ~0.5 CMC), were investigated in laboratory-scale experiments at ambient temperature. The absorbance band of the aromatic ring of the surfactants was monitored during the oxidation process. The reduction in chemical oxygen demand (COD) and total organic carbon (TOC) of the surfactant solution was evaluated. The results showed that a combination of UV irradiation and ozonation was considerably more efficient than the individual processes (at least two times more efficient in terms of COD and TOC reductions). The synergistic effect of ozonation and UV irradiation was particularly pronounced when medium-pressure UV irradiation was used. By adding alkali to the solution, the efficiency of the UV-enhanced ozonation increased with respect to COD reduction but decreased with respect to TOC reduction. This indicates partial oxidation with lower degree of mineralization of the surfactants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are used in a wide range of household and industrial applications. Their total consumption in 2003 was 8,600,000 tons, with the majority being anionic and nonionic surfactants [1]. Such extensive use leads to considerable discharge of these compounds into the environment. It is therefore necessary to minimize their environmental impact, and the biodegradability and aquatic toxicity of surfactants are matters of considerable concern today [2].
The harmful effects of anionic surfactants on the environment have been reported and critically discussed elsewhere. They can cause serious environmental pollution with toxic effects on living organisms. Because of their extensive use, a considerable amount of anionic surfactants is released into the environment, causing serious pollution of surface waters [3].
Discharge of alkyl phenol surfactants into sewers is often restricted. These restrictions were established because alkyl phenol polyethoxylate surfactants are biodegraded to alkyl phenols with zero, one or two oxyethylene groups, which tend to adsorb onto sewage sludge and accumulate to concentration up to 1,000 ppm. These hydrophobic residues are much more toxic than the original surfactant, with maximum accepted concentration in the low ppb range. The discharge limit for other surfactants (in natural waters) is typically set at 2 mg/L [4, 5].
Many methods have been developed for extraction and removal of anionic and nonionic surfactants from water by both physical–chemical approaches (adsorption on activated carbon, coagulation/precipitation, filtration, etc.) and biological techniques. Various destructive techniques (oxidation, gamma irradiation, etc.) have also been applied for removal of surfactants from waters [3]. However, few research papers have been devoted to the subject of degradation of surfactants by ozonation (Table 1) [6–12]. Chemical oxygen demand (COD) removal up to 95% and total organic carbon (TOC) removal up to 76% can be obtained by degradation of anionic and nonionic surfactants using oxidation based on ozonation. The oxidant dosage, pH, duration, and chemical structure of the surfactants are the most important parameters affecting the degree of degradation. While the surfactants themselves show little toxicity, their breakdown products, such as alkyl phenols, adsorb readily to suspended solids and are known to exhibit toxic and carcinogenic effects.
The main goal of this research is to study treatment of wastewater containing high concentrations of two surfactants with low biodegradability using advanced oxidation methods based on ozonation. Ozonation alone and in combination with low- and medium-pressure UV irradiation have been investigated.
Materials and Methods
Materials
Sodium dodecylbenzene sulfonate (SDBS) and nonylphenol ethoxylate with 40 oxyethylene units, NPEO40 (Igepal CO-890), were obtained from Sigma–Aldrich. The chemical structures and the most important physical–chemical properties of these surfactants are listed in Table 2. Other chemicals, purchased from Merck, were of analytical grade.
Oxidation of Surfactants
Ozone was produced from dry, purified oxygen (99.99%) in a commercial ozone generator. The ozone generator was from Green Technology Co., Iran and had a capacity of 10 g/h. The oxygen flow rate was controlled at 0.08 L/min for all experiments using a flow controller (Besta model LZB-3WB, Taiwan). The ozone–oxygen mixture was introduced from the reactor bottom via a porous gas diffuser. The concentration of ozone gas in the reactor inlet and outlet was measured by an ozone analyzer (Orbisphere model 3600, Switzerland). The inlet ozone concentration was 55 g/m3 under this condition.
Batch experiments on surfactant degradation were conducted in a 2-L Pyrex glass reactor (inside diameter 12 cm, height 15 cm). A UV-C low-pressure lamp (TUV PL-S 9 W; Philips) equipped with a quartz tube was located vertically at the center of the reactor and immersed in the surfactant solution. A schematic diagram of the experimental setup is provided elsewhere [17]. The incident UV photonic flux inside the reactor was measured by hydrogen peroxide actinometry and was 2.4 × 10−4 Einstein/s [18]. A medium-pressure UV-C lamp (UVOX 150 W, Arda) was also tested as a source of UV irradiation. The O3 + UV reactions were performed at ambient temperature, and no lamp cooling was provided. The laboratory temperature was 27 ± 1 °C.
Wastewater Analysis
The oxidation of surfactants by O3 and O3 + UV was performed using a 1-L solution containing specified concentration of surfactant. Solutions were prepared with distilled water to minimize interferences. The initial concentration of both surfactants was 200 mg/L. Based on CMC values of surfactants (Table 2), the initial concentration of 200 mg/L is almost 0.5 CMC of NPEO40 and 0.3 CMC of SDBS. A magnetic stirrer was used to mix the solution at maximum speed of 1,000 rpm (104.72 rad/s). Samples were withdrawn from the solution at various time intervals and analyzed immediately for assessment of degradation by means of UV spectra and COD and TOC determinations. UV spectra of surfactant solutions at different time intervals were scanned over the range 200–400 nm using a PerkinElmer Lambda 25 spectrophotometer. The calibration curves for SDBS and NPEO40 have been obtained using standard quartz cuvettes with a path length of 1 cm. The absorbance of both surfactant solutions was almost zero in the range 500–700 nm. The absorbance values at λ max = 223 nm over a wide range of SDBS and NPEO40 concentrations showed linear correlation (r 2 > 0.99) even for absorption values greater than 1.
The pH and conductivity of the solutions were checked using a dual pH/conductivity meter (model S47; Mettler Toledo).
The COD, which is a measure of the concentration of all compounds that can be oxidized by the Cr2O 2−7 anion in acidic media, gives the degree of decay of pollutants and intermediates produced during the surfactant degradation. Complete removal of the surfactants is expected to represent the most effective reduction in COD. The COD determination was carried out using oxidation with potassium dichromate in sulfuric acid and heating for 2 h at 150 °C according to Hach method no. 8000, and the analysis was conducted by the procedures described in standard methods [19, 20]. Exact mass balance and mineralization rate calculations based on TOC measurements are helpful to assess the efficiency of the oxidation. The TOC tests were carried out by a colorimetric method using a DR/2500 spectrophotometer according to Hach method no. 10128. Both COD and TOC can be used to evaluate the extent of the oxidation reaction. COD measures the change in the parent structure, and TOC determines the fraction converted to CO2 and H2O (mineralization). In addition, the COD/TOC ratio can be used as a measure of the mean oxidation number of carbon (MOC), see Eq. 1 [21, 22]. A decrease in the COD/TOC ratio of an organic compound indicates partial oxidation with oxygen being incorporated into degradation products. ∆(COD/TOC) can be calculated from Eq. 2 [23, 24].
Results and Discussion
Effect of UV Irradiation Alone
In the present study, two control experiments were separately carried out to test the effect of UV irradiation on the degradation of the anionic surfactant SDBS and the nonionic surfactant NPEO40. The changes in the UV region of the spectra and the change in COD at different time intervals up to 60 min for medium-pressure UV irradiation of these two surfactants were monitored (see Supplementary Material). The trends and results for both surfactants were almost the same. Both the anionic and the nonionic surfactants have one aromatic ring in the chemical structure. The wavelength of maximum absorption of both surfactants appear at λ max = 220–225 nm. The UV spectral region of SDBS shows only small changes after 60 min, and there is no decline in COD. There is a more pronounced change of absorbance at λ max = 223 nm for NPEO40, but the COD removal is only 7–8% after 60 min of UV irradiation (see Supplementary Material). This shows that both surfactants are photolytically stable and that UV radiation alone cannot degrade them significantly.
Ozonation Alone
Ozone is one of the strongest oxidants, with high oxidation potential (2.1 V). It can react with organic pollutants in water and decompose them [25]. Here, we investigated the effect of ozonation alone for degradation of the two surfactants. The changes in the UV spectral region and in COD for SDBS and NPEO40 show that greater degradation and decomposition can be obtained by ozonation in comparison with UV irradiation (see Supplementary Material). The COD reduction reached 42% and 17% after 60 min for ozonation of SDBS and NPEO40, respectively. This shows that SDBS is more susceptible to ozone degradation than NPEO40, indicating that the chemical structure of the surfactant and probably its initial concentration play an important role. Moreover, the UV absorption peak (λ max = 223 nm) decreases with increasing ozonation time.
UV-Enhanced Ozonation
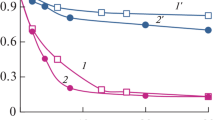
Ozonation in combination with low- and medium-pressure UV irradiation was studied for degradation of SDBS and NPEO40. Ozonation of 200 mg/L NPEO40 solution under UV irradiation with 9 W low-pressure UV-C lamp (Fig. 1) or 150 W medium-pressure UV-C lamp (Fig. 2) was investigated. Both figures show a stronger decrease in the absorption peak at λ max = 223 nm and a more significant decrease of COD than obtained in separate ozonation or UV irradiation experiments. The synergistic effect of ozonation and UV irradiation is particularly pronounced when medium-pressure UV irradiation is applied. A COD reduction of 77% is obtained after 60 min with this procedure. Similar results were obtained with SDBS. The reduction in COD for UV-enhanced ozonation of 200 mg/L SDBS solution under 9 W low-pressure UV-C lamp and 150 W medium-pressure UV-C lamp were 43% and 71%, respectively.
A mechanism of light-induced decomposition of ozone in aqueous solution has been proposed (Eqs. 3, 4) involving homolysis of the ozone molecule and subsequent generation of hydroxyl radicals by reaction of atomic oxygen with water. The hydroxyl radicals may combine to form hydrogen peroxide, which again can be converted to hydroxyl radicals by UV irradiation (<330 nm) (Eqs. 5, 6). However, the intermediate hydroxyl radicals have a higher oxidation potential (2.8 V) than ozone and UV [25–27]. We here propose that they react with the surfactant to produce organic radicals and that this reaction is a critical event in the degradation of the compounds (Eq. 7).
Effect of Alkali on Surfactant Degradation
It is known that ozone will generate hydroxyl radicals in the presence of sodium hydroxide (Eq. 8) [27]. The effect of sodium hydroxide on the UV, ozone, and UV + ozone degradation of SDBS and NPEO40 was studied, and the results in terms of reduction in COD and TOC are collected in Tables 3 and 4. Changes in pH and conductivity occurring during the degradation are also given in the tables.
The decrease in pH and the increase in conductivity obtained are consistent with degradation of the large surfactant molecules into smaller fragments. However, the effect of NaOH on the surfactant degradation is not straightforward. With respect to COD there is a trend towards an increase in COD reduction. The powerful ozone + medium-pressure UV treatment becomes even more effective in the presence of alkali. The situation is different when it comes to TOC reduction. For both SDBS and NPEO40 the ozone + medium-pressure UV treatment is less efficient in the presence of alkali. The high ∆(COD/TOC) values obtained by addition of alkali indicate partial oxidation of the surfactants rather than complete mineralization. The results are consistent with incorporation of oxygen into the degradation products [24].
The differences found in TOC and COD reduction for O3 + UVmp treatment in the absence and presence of NaOH are noteworthy and reflect that the TOC and the COD values provide different information. COD measures the change in the parent structure, whereas TOC gives the fraction converted to CO2 and H2O (mineralization). In contrast to TOC, which often barely decreases with time, COD supplies information on the magnitude of the oxidation steps and can be used for kinetic studies [25, 28].
One may note that addition of alkali to the medium-pressure UV + ozone treatment results in approximately the same slight increase in efficiency in terms of COD and the same strong decrease in efficiency in terms of TOC for the two surfactants. This implies that the degradation mechanisms are similar despite the fact that one surfactant is negatively charged and the other is uncharged.
We believe that the reason why addition of alkali to the reaction medium results in lower TOC reduction compared with the situation without alkali is that the high pH triggers a less useful decomposition of the hydrogen peroxide generated by the UV + ozone treatment, see Eqs. 3–5. It is known that, at high pH, hydrogen peroxide is not decomposed into the reactive hydroxyl radicals but is instead transformed into the perhydroxyl anion, HOO−. The perhydroxyl anion is a strong nucleophile, useful as a reactive species in nucleophilic substitution reactions, but it does not have the oxidizing power of the hydroxyl radical. Thus, the complete oxidation of the surfactants, mineralization, occurs less readily at high pH.
Conclusions
The combination of medium-pressure UV irradiation and ozone is found to be very efficient for degradation of both the anionic surfactant SDBS and the nonionic surfactant NPEO40, as seen by very large decreases in COD and TOC values in a relatively short time. There is clearly a synergistic effect behind the degradation, as neither the UV treatment nor ozonation alone is very efficient. It is likely that the hydroxyl radical, formed in situ during the process, is the main oxidizing species. At high pH the efficiency in terms of TOC reduction goes down, most probably because the hydrogen peroxide generated in the UV + ozone treatment then converts into the perhydroxyl anion, which does not decompose into the hydroxyl radical.
References
Hargreaves T (2003) Surfactants: the ubiquitous amphiphiles, chemistry world, RSC
Huber L, Nitschke L (eds) (2002) Environmental aspects of surfactants. Wiley, Chichester
Cserháti T, Forgács E, Oros G, Cserháti T (2002) Biological activity and environmental impact of anionic surfactants. Environ Int 28:337–348
Vandevivere PC, Bianchi R, Verstraete W (1998) Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies. J Chem Technol Biotechnol 72:289–302
Scott MJ, Jones MN (2000) The biodegradation of surfactants in the environment. Biochim Biophys Acta Biomembr 1508:235–251
Kitis M, Adams CD, Kuzhikannil J, Daigger GT (2000) Effects of ozone/hydrogen peroxide pretreatment on aerobic biodegradability of nonionic surfactants and polypropylene glycol. Environ Sci Technol 34:2305–2310
Beltran FJ, Garcia-Araya JF, Alvarez PM (2000) Sodium dodecylbenzenesulfonate removal from water and wastewater. 2. Kinetics of the integrated ozone-activated sludge system. Ind Eng Chem Res 39:2221–2227
Berna JL (2006) Comments on: removal of the surfactant sodium dodecylbenzene sulphonate from water by simultaneous use of ozone and activated carbon. Water Res 40:1717–1725
Brambilla AM, Calvosa L, Monteverdi A, Polesello S, Rindone B (1993) Ozone oxidation of polyethoxylated alcohols. Water Res 27:1313–1322
Calvosa L, Monteverdi A, Rindone B, Riva G (1991) Ozone oxidation of compounds resistant to biological degradation. Water Res 25:985–993
Vilve M, Hirvonen A, Sillanpää M (2007) Ozone-based advanced oxidation processes in nuclear laundry water treatment. Env Technol 28:961–968
Narkis N, Schneider-Rote M (1980) Ozone-induced biodegradability of a non-ionic surfactant. Water Res 14:1225–1232
Banerjee P, Chatterjee S, Pramanik S, Bhattacharya SC (2007) Interaction of Pyrene-1-Carboxaldehyde with micelles and mixed micelles of polyoxyethylene nonyl phenol (Igepal): a spectroscopic study. Colloids Surf A 302:44–50
Paria S, Yuet PK (2006) Solubilization of naphthalene by pure and mixed surfactants. Ind Eng Chem Res 45:3552–3558
Yang K, Zhu L, Xing B (2006) Enhanced soil washing of phenanthrene by mixed solutions of TX100 and SDBS. Environ Sci Technol 40:4274–4280
Om H, Baker GA, Behera K, Kuma V, Verma KK, Pandey S (2010) Self-probing of micellization within phenyl-containing surfactant solutions. Chem Phys Chem 11:2510–2513
Peternel I, Koprivanac N, Kusic H (2006) UV-based processes for reactive azo dye mineralization. Water Res 40:525–532
Nicole I, Laat JD, Dore M, Duguet JP, Bonnel C (1990) Use of UV radiation in water treatment: measurement of photonic flux by hydrogen peroxide actinometry. Water Res 24:157–168
Kuo WG (1992) Decolorization dye wastewater with Fenton’s reagent. Water Res 26:881–886
American Public Health Association APHA (1992) Standard methods for the examination of water and wastewater, 17th edn. APHA, Washington
Bowers AR, Cho SH, Singh A (1992) Chemical oxidation of aromatic compounds: comparison of H2O2, KMnO4, and O3 for toxicity reduction and improvements in biodegradability. In: Wesley-Eckenfelder W, Bowers AR, Roth JA (eds) Chemical oxidation: technologies for nineties. Technomic, Lanchester
Vogel F, Harf J, Hug A, Rudolf-von-Rohr P (2000) The mean oxidation number of carbon (MOC)—a useful concept for describing oxidation processes. Water Res 34:2689–2702
Narkis N, Schneider-Rotel M (1980) Ozone-induced biodegradability of a non-ionic surfactant. Water Res 14:1225–1232
Alvares ABC, Diaper C, Parsons SA (2001) Partial oxidation by ozone to remove recalcitrance from wastewaters—a review. Environ Technol 22:409–427
Beltran FJ (2004) Ozone reaction kinetics for water and wastewater systems. Levis, CRC imprint, Florida
Ledakowicz S, Miller JS, Olejnik D (2001) Oxidation of PAHs in water solution by ozone combined with ultraviolet radiation. Int J Photoenergy 3:95–101
Gottschalk C, Libra JA, Saupe A (2002) Ozonation of water and waste water. Wiley-VCH, Weinheim
Lopes-de-Morais J, Peralta-Zamora P (2005) Use of advanced oxidation processes to improve the biodegradability of mature landfill leachates. J Hazard Mater 123:181–186
Acknowledgments
We are grateful to Mr. M. Deihimi and Mr. H. Heidari for helping the research team. This research was supported by the International Foundation for Science, Stockholm, Sweden.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tehrani-Bagha, A.R., Nikkar, H., Menger, F.M. et al. Degradation of Two Persistent Surfactants by UV-Enhanced Ozonation. J Surfact Deterg 15, 59–66 (2012). https://doi.org/10.1007/s11743-011-1271-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-011-1271-6