Abstract
Perfluorinated and hydrocarbon surfactants are important contaminants in wastewater. In this study, degradation of surfactants including PFOS, LAS, and CTAB under electron beam irradiation is investigated under different irradiation conditions (0–10 kGy absorbed dose, pH 3–11). Fulvic acid and H2O2 are used as active species inhibitors to study the effect of OH and eaq− on surfactants degradation. Irradiation degradation products of surfactants are analyzed by liquid chromatography-mass spectrometry. The degradation mechanisms of surfactants are summarized according to the experimental results. It shows that surfactants degradation is promoted at a high absorbed dose and pH value. The degradation rate of PFOS, LAS, and CTAB is 93.8%, 89.1%, and 80.6% maximum, respectively. The eaq− plays a dominant role in the breakage of C–C bonds in surfactants. The bonds of C–F, C–S, and C–N are mainly destroyed by OH. The degradation of surfactants follows pseudo-first-order kinetics. Degradation products of surfactants include various long and short-chain molecules. Furthermore, concentrations of short-chain products increase with absorbed dose because of further degradation of long-chain products. This study shows the potential application for electron beam irradiation technology to remove perfluorinated and hydrocarbon surfactants from wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants, which are the common organic compounds composed of carbon chains, have been widely used in the fields of chemical production, water treatment, agriculture, medicine, and biotechnology. Some kinds of surfactants generated by industrial production are harmful contaminants for the human and natural environment due to their bioaccumulation, toxicity, and persistence. Perfluorinated surfactants and hydrocarbon surfactants are two typical types of surfactants. A representative perfluorinated surfactant, perfluorooctane sulfonate (PFOS, C8F17SO3H), has been detected in the environment and wildlife [1, 2]. PFOS has excellent molecular stability because of the high strength of C–F bonds (116 kcal/mol) [3]. Therefore, it has attracted the attention of researchers for the removal of PFOS from water and soil [4, 5]. Linear alkylbenzene sulphonate (LAS) and cetyl trimethyl ammonium bromide (CTAB) are typical anionic and cationic hydrocarbon surfactants respectively. They are also important organic contaminants in domestic and industrial sewage. It has raised wide concern for these surfactants abuse in recent decades.
Various treatment methods including adsorption [6,7,8], photolytic method [9,10,11], oxidation/reduction method [12], sonochemical decomposition [5, 13], thermolysis [14, 15], and electron beam irradiation [16,17,18] are used to remove surfactants from solution. In these methods, electron beam irradiation is a new treatment technique for surfactants decomposition with potential applications. Electron beam has relatively strong penetration. Organic molecules can be degraded by the active species, e.g.,·OH and eaq−, which are produced by the splitting of water under electron beam irradiation (Eq. 1) [19]:
where the numbers in brackets are the yields (mmol/J) of those species per unit of radiation at pH 7. Electron beam irradiation shows an efficient removal ability for TWEEN20, a common nonionic surfactant from laundry effluent [20]. The concentration of LAS decreases observably after irradiation of electron beam [21]. According to the researches of Kim et al. [16, 22], it shows good degradation rates for PFOS (> 60%) under electron beam irradiation absorbed doses of 2000 kGy with the PFOS initial concentration of 10–100 mg/L. Furthermore, PFOS degradation rates are 32–57% at 500 kGy absorbed dose with initial concentrations of 10–40 mg/L [23]. It is reported by Kowald et al. [17] that 41% PFOS is degraded at a relatively low absorbed dose (50 kGy) under initial concentrations between 0.00025 and 0.1 mg/L. Although some studies of surfactants degradation under electron beam irradiation have been reported, systematic researches and comparisons about the degradation of perfluorinated and hydrocarbon surfactants under different irradiation conditions are still insufficient to our knowledge. With the help of effective contrast of different surfactants degradation under variable electron beam irradiation conditions, treatment results can be achieved according to different processing demands, processing conditions, and technical foundation. Therefore, systematic study can provide the theoretical basis for the treatment of wastewater containing multiple surfactants.
In this study, the degradations of three kinds of surfactants, PFOS, LAS, and CTAB, under 0–10 kGy absorbed doses are investigated. The irradiation systems with different pH values and irradiation absorbed doses are carried out to investigate the degradation rates of surfactants under different treatment conditions. Fulvic acid (FA) and H2O2 could react with OH and eaq− respectively. Therefore, they are used as active species inhibitors to investigate the crucial roles of OH and eaq− in the degradation process of surfactants. The irradiation experiments are carried out to study the degradation kinetics of surfactants under different conditions. Furthermore, the mineralization mechanisms of surfactants under irradiation are summarized. The result shows that the degradation rate of PFOS, LAS, and CTAB under 10 kGy absorbed dose at pH 11 is 93.8%, 89.1%, and 80.6% maximum, respectively. eaq− plays a dominant role in the break of C–C bonds of surfactants. The degradation of surfactants follows pseudo-first-order kinetics at different pH. Various long and short-chain species are generated in the process of irradiation degradation. This study confirms that electron beam irradiation has potential application for the removal of perfluorinated and hydrocarbon surfactants from solutions.
Materials and methods
Materials and reagents
The reagents of PFOS (99%), LAS (99%), and CTAB (99%) used in the experiment study were purchased from Wengjiang Chemical Reagent Company (Wengjiang, China). Fluoride standard samples were purchased from Anpu Biotechnology (Shanghai,China). Methanol (99%) and ammonium acetate (99%) were purchased from Sinopharm Chemical Reagent Company (Beijing,China). All the other chemicals which were used in this study were analytical grade and used without purification. All of the solution samples in the experiment were prepared with deionized water.
Irradiation experiments at different conditions
Surfactant solution (PFOS, LAS, or CTAB) was prepared with the initial concentration of 400 mg/L. 5 mL surfactant solution was added into the quartz tube. The irradiation samples were carried out at different pH values and doses. The pH values of the samples were adjusted to 3–11 with 1 M NaOH and HCl solution. Absorbed doses of systems were set as 0–10 kGy with a DD1.2 dynamitron electron accelerator (Xianfeng Electric Machinery Factory, Shanghai, China; Electron energy range of 0.8–1.2 MeV, a maximum beam current of 1 mA). The sample was placed at 30 cm below the radiation source. The irradiation time was determined by the equation (Eq. 2):
where T was the irradiation time (h), f was the utilization factor of radiant energy (≈0.7), D was the absorbed dose (kGy), V was acceleration voltage (V) and I was the current of the electron beam (A). The absorbed dose was measured with dichromate solution method [24]. The irradiated samples after irradiation were sealed in plastic bags at 4 °C before being analyzed. The time interval between irradiation and measurement was one hour for all samples. The concentrations of surfactants and species of surfactants degradation product in solution were measured by Liquid Chromatogram-Time of Flight Mass Spectrometer (1260 LC- G6200 TOFMS, Agilent), which was equipped with ZORBAX Eclipse XDB-C18 (4.6 × 150 mm, 5.0 μm) and Agilent Poroshell 120 EC-C18 column (3.0 × 100 mm, 2.7 μm, injection volume 20 μL) in series. The mobile phase was composed of 2 mmol/L ammonium acetate and methanol, with a flow rate of 300 μL/min and a column temperature of 40 °C. The mass spectrometry system was operated with ion spray voltage − 4.5 kV, atomization temperature 160 °C, carrier gas of nitrogen (9 L/min, 325 °C), and capillary voltage + 3.5 kV. The concentrations of F− and SO42− in solution were measured by an anion chromatography (DionexICS-2000, USA) with guard column (IonPac AG19-HC, 4 mm × 50 mm), separation column (IonPac AS19-HC, 4 mm × 250 mm) and autosampler (250 μL injection volume). The mobile phase was 25 mmol/L KOH solution (1.2 mL/min). The suppressor current was set as 75 mA.
Effects of ·OH and eaq − on surfactants degradation
OH and eaq− are the active species which contribute to the degradation of surfactants in alkaline solution under electron beam irradiation. In order to investigate the effect of each kind of active species on surfactants degradation separately, two different active species inhibitors were used to mask the interference from unwanted active species. FA and H2O2 were used in the irradiation systems as active species inhibitors for OH and eaq− respectively. The concentrations of active species inhibitors in the systems were set as 0–150 mg/L at pH 11. The initial concentration of surfactant was 400 mg/L. The absorbed doses were in the range of 2–10 kGy. The degradation rates of surfactants in different irradiated samples are obtained to analyse the degradation mechanisms of surfactants.
Surfactants degradation kinetics
In degradation kinetics studies, the initial concentration of surfactant in solution was set as 400 mg/L. The irradiation experiments were carried out at pH 3, 5, 7, 9, and 11, respectively. The concentrations of surfactants in the systems at different irradiation time (0–350 s) were measured. Furthermore, the results of degradation kinetics of surfactants under irradiation of electron beam were fitted with the pseudo-first-order kinetic model (Eq. 3):
where C was the concentrations (mg/L) of PFOS at time t (s), C0 was the initial concentration of PFOS (mg/L), k was reaction rate constant (s−1).
Results and discussion
Effect of irradiation conditions on surfactants degradation
The degradation rates of surfactants at different pH and absorbed doses are shown in Fig. 1. The degradation rate of PFOS increases with the absorbed dose at the same pH (Fig. 1a). Typically, for the system at pH 3, the degradation rate of PFOS increases from 2.2 to 29.2% with absorbed dose (from 2 to 10 kGy). The other samples present a similar trend. Furthermore, the PFOS degradation rate increases with the system pH value under the same absorbed dose. The minimum degradation rates in the system of pH 3 are 2.2–29.2%. The samples in pH 11 show the highest degradation rates (40.8–93.8%). The variation trend of PFOS degradation rates in different pH systems in this research accords with that of previous reports [25].
The degradation rates of LAS and CTAB (Fig. 1b, c) present similar trends with that of PFOS. The degradation rates increase with pH value at the same absorbed dose. For example, the degradation rates of LAS at pH 11 systems are in the range of 50.9% to 89.1% under absorbed dose 2–10 kGy, which are apparently higher than those in pH 3 systems (10.1–34.3%). Furthermore, it shows higher degradation rates for hydrocarbon surfactants than the degradation rates of perfluorinated surfactants at pH < 11. At absorbed dose 10 kGy, LAS is degraded by 34.3%, 49.8%, 61.0%, and 77.7% at pH 3, 5, 7, and 9, respectively. With the same absorbed dose, CTAB degradation rates are 33.9%, 49.4%, 65.8%, and 69.5% at pH 3, 5, 7, and 9, respectively. As a contrast, the degradation rates of PFOS are 29.2%, 37.1%, 48.6%, and 62.0%, which are relatively lower than those in LAS and CTAB systems. This is possible because of the higher molecular stability of perfluorinated surfactant than that of hydrocarbon surfactants.
However, PFOS is degraded no less than LAS and CTAB at strong alkaline (pH 11). Irradiation degradation of surfactants is promoted in an alkaline environment with following reasons. The hydrated electron eaq− which plays an important role in the decomposition of organic molecules is consumed by large amounts of H+ in a high acidity environment (Eq. 4). Under alkaline conditions, eaq− is generated by reaction of OH− with H· (Eq 0.5). The eaq− has a lower redox potential (− 2.9 eV) than that of H· (− 2.1 eV). This indicates that eaq− has stronger reduction ability than H·. Degradation of surfactants is improved more significantly by eaq−. Therefore, the degradation of surfactants is promoted in high pH levels. The effect of surfactants’ molecular stability on their degradation is reduced to a certain extent.
Effects of ·OH and eaq − on surfactants degradation
Under the same absorbed dose, the addition of FA has little influence on the degradation rate of PFOS (Fig. 2a). Furthermore, the concentration of F− in solution decreases with the increase of FA concentration (Fig. 2c). As the ·OH generated from radiation decomposition of water is consumed by FA, which indicates that ·OH causes breaking of the bond of C–F rather than the bond of C–C. As a contrast, the degradation rate of PFOS decreases with the increase of H2O2 concentration with the same absorbed dose (Fig. 2b). H2O2 consumes the eaq− in solution. So, the result indicates that C–C bond is disrupted by eaq−. In addition, F− concentration in solution decreases with the increase of H2O2 concentration, which indicates that eaq− also destroys the C–F bond of PFOS.
As shown in Fig. 3, the variation trends of degradation rates of LAS with different concentrations of active species inhibitors are similar to those of PFOS. It indicates that ·OH breaks the bond of C–S, and eaq− destroys C–C bond and C–S bond. For the surfactant of CTAB, the degradation rate of CTAB and concentration of NH4+ are both relatively constant with the increasing of FA concentration under the same absorbed dose (Fig. 4a, c). It shows that the presence of ·OH has little influence on C–C bond and C–N bond. eaq− plays a dominant role in the break of the C–C bond and C-N bond of CTAB.
Surfactants degradation kinetics
The degradation kinetics and fitting results of surfactants degradation are shown in Fig. 5 and Table 1. It is shown that the concentration of PFOS decreases with increasing irradiation time (Fig. 5a). Furthermore, degradation of PFOS follows pseudo-first-order kinetics at different pH (R2 > 0.97). Consistent with results above, degradation of PFOS is promoted in alkaline medium. The PFOS degradation rate constant at pH 11 is about 8 times of that at pH 3. The first-order rate constants for initial pH values of 3, 5, 7, 9, and 11 are 0.0010 s−1, 0.0014 s−1, 0.0020 s−1, 0.0028 s−1, and 0.0080 s−1 respectively, showing that the efficiencies depend on the pH of the systems. The variation trends of degradation kinetics of LAS and CTAB are similar to that of PFOS (Fig. 5b, c). Nonetheless, hydrocarbon surfactants have significantly higher degradation velocity than PFOS at the same pH value. The first-order degradation rate constants of LAS and CTAB at pH 11 are 0.0245 s−1 and 0.0181 s−1, respectively. Furthermore, when the pH is below 11, degradation rate constants of PFOS (0.0028 s−1 at pH 9) are significantly lower than those of LAS and CTAB (0.0154 s−1 and 0.0133 s−1 respectively at pH 9). It indicates that perfluorinated surfactant is more difficult to be degraded than hydrocarbon surfactants. This may be caused by the better stability of C–F bonds in perfluorinated surfactants.
Degradation products of surfactants
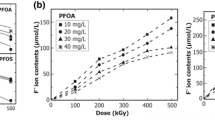
The irradiation degradation products of PFOS under 10 kGy dose are shown in Fig. 6a. The irradiation degradation products of PFOS are C7F15COO− and short-chain perfluorinated carboxylic acids (PFCAs) including C6F13COO−, C5F11COO−, C4F9COO−, C3F7COO−, C2F5COO−, and CF3COO−. The concentrations of degradation products present different trends with increasing doses. The concentrations of CF3COO−, C4F9COO−, C2F5COO−, and C3F7COO− increase with the increasing absorbed dose. Among these products, CF3COO− is generated mostly (maximum 14.3 mg/L) with increasing absorbed dose. The concentrations of relative longer-chain PFCAs, such as C7F15COO− and C6F13COO−, decrease with increasing absorbed dose under the condition of high irradiation (4–10 kGy). This may be due to that longer chain molecules are degraded into short chains, such as CF3COO−, etc.
The irradiation degradation products of LAS include HOOCCH2COOH, HOOCC2H4COOH, C8H9SO3−, C10H13SO3−, C12H17SO3−, C14H21SO3−, and C16H25SO3− (Fig. 7). The concentrations of HOOCCH2COOH, HOOCC2H4COOH, C8H9SO3−, and C10H13SO3− increase with absorbed dose. The concentrations of long-chain molecules, such as C12H17SO3−, C14H21SO3−, and C16H25SO3−, decrease under high absorbed doses. The results show that the C-S bond between the benzene ring and SO30− is difficult to be destroyed by irradiation.
The irradiation degradation products of CTAB are N(CH3)3, C3H7COO−, C5H11COO−, C7H15COO−, C9H19COO−, C11H23COO−, C13H27COO−, and C15H31COO− (Fig. 8). Similar with the cases of PFOS and LAS, the concentrations of short-chain molecules (C3H7COO−, C5H11COO−, and C7H15COO−) increase with absorbed dose. The long chain products, such as C11H23COO−, C13H27COO−, and C15H31COO−, decrease at high-level irradiation. Furthermore, N(CH3)3 and C9H19COO− are relatively constant when absorbed dose is more than 6 kGy. This may be caused by the balance of degradation of N(CH3)3, C9H19COO−, and long-chain products.
Degradation mechanisms of surfactants under irradiation
Degradation mechanisms of three kinds of surfactants are speculated and summarized according to experimental results. PFOS is defluorinated by ·OH which is generated from splitting of water under electron beam irradiation. Desulfurization reaction occurs in this process. Furthermore, the end of the carbon chain is oxidized to carboxylic acid (Eq. 6). Further degradation of C7F15COO− is caused by the breaking of the C–C bond because of eaq− (Eq. 7) [23]. Methanoic acid and F− are the split products of this process. Through this reaction, long-chain products of PFOS are degraded gradually in the unit of CF2 (Eq. 8).
For the irradiation degradation of LAS, eaq− plays a dominated role in the fracture of the carbon chain. The long-chain products are degraded in the unit of C2H4. CH3COO− is generated as end product in this reaction (Eq. 9). ·OH takes part in the reaction of benzene removal and degradation (Eq. 10, 11 and 12). Benzene ring is removed from the carbon chain, and is decomposed into COOHC2H4COOH and COOHCH2COOH with the existence of ·OH.
The C-N bond of CTAB is destroyed by eaq− (Eq. 13). The end of the carbon chain is oxidized to carboxylic acid. N(CH3)3 is further decomposed into HCOO− and NH4+ (Eq. 14). Production and consumption amount of N(CH3)3 might be in relative balance. Therefore, the concentration of N(CH3)3 in the system is constant at a high radiation dose. Similar with the case of LAS degradation, long-chain products are degraded with the effect of eaq− in the unit of C2H4, which is eventually oxidized to CH3COO− (Eq. 15). Large amounts of formic acid and acetic acid are generated in the irradiation degradation of surfactants. But formic acid and acetic acid are not shown in the mass spectra of degradation products due to their volatilization in the mass spectrometry measuring system.
It is a complicated chemical process for the degradation of surfactants under irradiation. Many kinds of unstable intermediate products may be generated and consumed during irradiation degradation [16, 23, 26]. More sophisticated analysis techniques are needed to investigate the intermediate processes and products in the irradiation system. The results in this study illustrate the essential mechanisms of irradiation degradation of surfactants under electron beam irradiation. It shows a good potential application for electron beam irradiation technology in the treatment of surfactants wastewater.
Conclusions
The degradation of PFOS, LAS, and CTAB under electron beam irradiation is promoted at a high absorbed dose and pH value. The maximum degradation rate of PFOS, LAS, and CTAB under 10 kGy absorbed dose at pH 11 is 93.8%, 89.1%, and 80.6%, respectively. eaq− plays a dominant role in the breakage of the C–C bond of surfactants. The bonds between C and other atoms or groups, such as C–F, C–S, and C–N bonds, are destroyed by ·OH. The degradation of surfactants follows pseudo-first-order kinetics at different pH values. Degradation products of the surfactants include various long and short-chain molecules. Concentrations of long-chain products decrease at a high absorbed dose. Whereas short-chain products concentrations increase with absorbed dose. Formic acid, acetic acid, and inorganic ions are the final products of irradiation degradation of surfactants. This work provides a theoretical basis and research reference to remove perfluorinated and hydrocarbon surfactants from solutions by using electron beam irradiation.
References
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40:3463–3473
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35:1339–1342
Liu DD, Xiu ZM, Liu F, Wu G, Adamson D, Newell C, Vikesland P, Tsai AL, Alvarez PJ (2013) Perfluorooctanoic acid degradation in the presence of Fe(III) under natural sunlight. J Hazard Mater 262:456–463
Mahinroosta R, Senevirathna L (2020) A review of the emerging treatment technologies for PFAS contaminated soils. J Environ Manag 255:109896
Moriwaki H, Takagi Y, Tanaka M, Tsuruho K, Okitsu K, Maeda Y (2005) Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ Sci Technol 39:3388–3392
Zhou Q, Deng SB, Yu Q, Zhang QY, Yu G, Huang J, He HP (2010) Sorption of perfluorooctane sulfonate on organo-montmorillonites. Chemosphere 78:688–694
Pan M-M, Li Q, Xu L (2020) Efficient adsorption of perfluoroalkyl acids by the quaternized hierarchically porous polystyrene-divinylbenzene. Chem Eng J 386:123990
Cheng HF, Sabatini DA (2002) Simultaneous uptake of anionic surfactants and micellar-solubilized contaminants using anion-exchange resins. Water Res 36:2062–2076
Jin L, Zhang P, Shao T, Zhao S (2014) Ferric ion mediated photodecomposition of aqueous perfluorooctane sulfonate (PFOS) under UV irradiation and its mechanism. J Hazard Mater 271:9–15
Kishimoto N, Doda K (2018) Effects of pH and coexisting chemicals on photolysis of perfluorooctane sulfonate using an excited xenon dimer lamp. Water Sci Technol 77:108–113
Levichev N, Lagunova Y, Seliverstov AF, Kireev SG, Tumashevich K, Shashkovskiy SG, Ershov BG (2020) Photodecomposition of sodium dodecyl sulfate under high-intensity pulsed UV radiation of continuous spectrum and hydrogen peroxide. Desalin Water Treat 196:131–136
Trojanowicz M, Bojanowska-Czajka A, Bartosiewicz I, Kulisa K (2018) Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) - a review of recent advances. Chem Eng J 336:170–199
Singla R, Grieser F, Ashokkumar M (2009) Kinetics and mechanism for the sonochemical degradation of a nonionic surfactant. J Phys Chem A 113:2865–2872
Hori H, Nagaoka Y, Murayama M, Kutsuna S (2008) Efficient decomposition of perfluorocarboxylic acids and alternative fluorochemical surfactants in hot water. Environ Sci Technol 42:7438–7443
Lee YC, Lo SL, Chiueh PT, Liou YH, Chen ML (2010) Microwave-hydrothermal decomposition of perfluorooctanoic acid in water by iron-activated persulfate oxidation. Water Res 44:886–892
Kim T-H, Lee S-H, Kim HY, Doudrick K, Yu S, Kim SD (2019) Decomposition of perfluorooctane sulfonate (PFOS) using a hybrid process with electron beam and chemical oxidants. Chem Eng J 361:1363–1370
Kowald C, Brorman E, Shankar S, Klemashevich C, Staack D, Pillai SD (2021) PFOA and PFOS breakdown in experimental sand, laboratory-grade water, investigation-derived groundwater and wastewater effluent samples at 50 kGy electron beam dose. Radiat Phys Chem 180:109323
Petrovic M, Gehringer P, Eschweiler H, Barcelo D (2007) Radiolytic decomposition of multi-class surfactants and their biotransformation products in sewage treatment plant effluents. Chemosphere 66:114–122
Getoff N (1996) Radiation-induced degradation of water pollutants—state of the art. Radiat Phys Chem 47:581–593
Selambakkannu S, Othman NAF, Abu Bakar K, Thailan KM, Karim Z (2021) Degradation of surfactants from domestic laundry effluent by electron beam irradiation. Mater Today Proc 46:1807–1812
Borrely SI, Romanelli MF, Pereira MCC, da Silva GP, Mesquita LCA, de Moraes MCF (2009) Radiation processing of detergents and possible environmental benefits. Nukleonika 54:61–64
Kim T-H, Yu S, Choi Y, Jeong T-Y, Kim SD (2018) Profiling the decomposition products of perfluorooctane sulfonate (PFOS) irradiated using an electron beam. Sci Total Environ 631–632:1295–1303
Ma S-H, Wu M-H, Tang L, Sun R, Zang C, Xiang J-J, Yang X-X, Li X, Xu G (2017) EB degradation of perfluorooctanoic acid and perfluorooctane sulfonate in aqueous solution. Nucl Sci Tech 28:1–8
Han B, Ko J, Kim J, Kim Y, Chung W, Makarov IE, Ponomarev AV, Pikaev AK (2002) Combined electron-beam and biological treatment of dyeing complex wastewater. Pilot plant Exp Radiat Phys Chem 64:53–59
Wang Y, Zhang PY, Pan G, Chen H (2008) Ferric ion mediated photochemical decomposition of perfluorooctanoic acid (PFOA) by 254 nm UV light. J Hazard Mater 160:181–186
Deng Y, Liang Z, Lu X, Chen D, Li Z, Wang F (2021) The degradation mechanisms of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) by different chemical methods: a critical review. Chemosphere 283:131168–131168
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (21771045), the project of Young Talents of China National Nuclear Corporation, University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2018013), Heilongjiang Provincial Postdoctoral Science Foundation (No. LBH-Z18232). The authors would like to thank Heilongjiang Institute of Atomic Energy for irradiation experiments and measurement.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiao, C., Men, X., Li, Z. et al. Degradation of representative perfluorinated and hydrocarbon surfactants by electron beam irradiation. J Radioanal Nucl Chem 331, 1691–1699 (2022). https://doi.org/10.1007/s10967-022-08224-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08224-1