Abstract
Alkylphenolpolyethoxylates whose bioresistant metabolites (alkylphenols, short-chain alkylphenolpolyethoxylates and alkylphenoxycarboxylic acids) are compounds with hormonal activity that pose a serious threat to living organisms are among the main types of nonionic surfactants widely used in industry and everyday life. Advanced oxidation processes (AOPs)—in particular, photocatalytic processes—are capable of effectively removing such impurities from an aqueous medium. However, photocatalytic oxidation of some synthetic surfactants with oxygen until their complete mineralization proceeds slowly. The combined use of a photocatalyst and oxidizing agents that are stronger than oxygen contributes to an increase in the degree of mineralization of organic impurities and a decrease in the reaction time compared to those achieved with separate treatment by both methods, which gives rise to the lower cost of water purification and improves the environmental friendliness of the combined process. The photocatalytic processes of degradation of nonionic surfactant octylphenol ethoxylate (Triton X-100) in an aqueous medium by ozone (O3), by ozone and UV radiation jointly (O3/UV), and by ozone and atmospheric oxygen in a TiO2 Degussa P-25 suspension (O3/TiO2/UV and O2/TiO2/UV) under UV irradiation with a DB-15 low-pressure mercury quartz lamp (λ = 254 nm) have been studied. The parameters of photocatalytic ozonation have been determined to ensure the nearly complete destruction of Triton X-100 (97% according to total organic carbon). The advantages of photocatalytic ozonation of a Triton X-100 solution in comparison with ozonation, O3/UV treatment, and photocatalytic oxidation with air oxygen are demonstrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Alkylphenol polyethoxylates (APEOs) belong to the main types of nonionic surfactants widely used in various industries and everyday life. As a result of large-scale consumption and insufficiently effective treatment of domestic and wastewaters at existing facilities, APEOs and their metabolites (nonylphenol and octylphenol, APEOs and alkylphenoxycarboxylic acids with number of oxyethyl chains (nEO) of 1–2) are widespread everywhere in the environment (freshwater, coastal and seawater ecosystems, bottom sediments, soils, sewage sludge, etc.) [1, 2].
Nonylphenol and octylphenol, and their short-chain ethoxylated derivatives are highly toxic to aquatic organisms and are considered compounds that destroy the endocrine system [1, 3]. The maximum permissible concentrations (MPCs) of 4-nonylphenol and 4-tert-octylphenol for surface water sources within the European Union are currently 0.3 and 0.1 μg/dm3, respectively [1]. The US Environmental Protection Agency (US EPA) recommends that a maximum concentration of nonylphenol in fresh water should be 28 μg/dm3. At the same time, the concentration of nonylphenol according to chronic criteria should not exceed 6.6 μg/dm3 in fresh water and 1.7 μg/dm3 in salt water [1]. In this regard, the development of more effective, economically acceptable, and environmentally friendly technologies for removing these substances from water remains an extremely relevant task.
Ozonation and some Advanced Oxidation Processes (AOPs) involving ozone have been used for long time in practical applications in natural and wastewater treatment processes [1, 4–6]. The partial destruction of nonionic surfactants, including alkylphenol ethoxylates, by ozone to improve bioavailability and the destruction of alkylphenols themselves by ozone and OH radicals have been studied for several decades [1–3, 7–13].
Nonionic surfactants of this type and alkylphenols themselves react quite intensively with molecular ozone [1, 7, 14]. The rate constant for the reaction of nonylphenols with ozone was 3.8 × 104–2.01 × 106 mol–1 s–1, but the rate of their interaction with OH radicals was two to five orders of magnitude higher, i.e., 7.6 × 108–6.8 × 109 mol–1 s–1 [1, 2, 15]. Therefore, simultaneous UV irradiation (λ = 254 nm) accelerated the destruction of the aromatic ring of the nonylphenol ethoxylate molecule by ozone in the pH range of 6–9 [7, 8, 10].
The shallow destruction of pollutants is a substantial disadvantage of ozonation. As was noted in many studies [1, 3, 8, 11, 12], the rapid primary transformation of alkylphenol ethoxylates and other nonionic surfactants in the process of ozonation or O3/UV treatment was accompanied by a low degree of mineralization or its almost complete absence, which was from 0 to 25% according to the total organic carbon (TOC) and up to 40% according to the chemical oxygen demand (COD). A high degree of mineralization of nonionic surfactants by ozone (about 95% according to TOC) is possible with the ozonation time eight to ten times longer than that required for a similar degree of their primary destruction and, consequently, with economically unacceptable doses of ozone [9].
A decrease in the concentration of nonionic surfactants from the initial value by about 40–50% with the degree of mineralization from 0 to 40% according to TOC was acceptable when combining the chemical and biochemical oxidation processes, since the task of preliminary chemical oxidation was only to reduce the bioresistance of organic substances to accelerate biodegradation processes during further purification [1, 8, 11, 13].
Ozonation and some AOPs are also considered as tertiary treatment for municipal wastewater treatment plants or industrial wastewater before discharge into municipal sewers [16–18]. The removal of nonylphenol microimpurity (C = 1 μg/dm3) from domestic wastewater by 70%, i.e., up to the MPC value for surface waters in the EU (0.3 μg/dm3), was achieved with an ozone dose of 12–20 mg/dm3 for 15–42 min at estimated operating costs from 2 to 4 Eurocents/m3 [18]. The use of combined processes (O3/H2O2, O3/UV) increased the degree of mineralization of organic impurities in biologically treated domestic wastewater up to 70% in 2 h, while the maximum degree of mineralization during ozonation was 1.7–2 times lower [16]. Tertiary ozonation also reduced the estrogenic activity of water [17].
The maximum rate and the maximum degree of destruction of resistant organic compounds, including various surfactants, were achieved under the simultaneous treatment with ozone, UV radiation, and a photocatalyst [5, 19–23]. Thus, the integration of ozonation and photocatalysis enables using the advantages of both processes and their synergy, and makes it possible to overcome their individual limitations [19–21]. Photocatalytic ozonation really shows high synergy indices in the destruction of organic impurities in wastewater [19–21]. However, the range of organic compounds, the destruction of which has been investigated by this method at least on a laboratory scale, is currently limited. Highly dispersed titanium dioxide was used as a photocatalyst in most of these studies [5, 23], and metal ions were used less often [5, 6].
The aim of this study was to investigate the photocatalytic destruction of a nonionic surfactant (Triton X-100) by ozone in an aqueous suspension of titanium dioxide (O3/TiO2/UV) and to evaluate the advantages of this oxidation method compared to others (O3, O3/UV, and O2/TiO2/UV).
The rate and degree of photocatalytic oxidation of organic compounds are influenced by the chemical nature and concentration of the substrate and oxidant, the physicochemical properties and concentration of the catalyst, the spectrum and intensity of UV radiation, the pH of the solution, and other process parameters [19–21, 24]. In addition, the degree of destruction of the substrate during ozonation and related AOPs substantially depends on the rate of ozone supply (νoz) [5, 7, 23, 25]:
where (νv OAM is the supply rate of the ozone–air mixture, dm3/min; Coz is the concentration of ozone in the OAM, mg/dm3; and Vs is the volume of the solution, dm3.
The values of this parameter in published studies are not always suitable for practical use in water purification processes [23]. Excessively high or low rates of O3 supply and an insufficient intensity of UV irradiation increase the dose of ozone or the duration of treatment required for a given degree of destruction of the substrate [7].
EXPERIMENTAL
The object of study was a solution of octylphenol ethoxylate (Triton X-100 from Merck) with average composition C8H17C6H4O(C2H4O)nH with number nEO of oxyethyl units of 2–12, average value n = 10 [26], and MW = 646 Da in distilled water (C0 50 mg/dm3, A224(0) 0.82, COD0 114 ± 2 mg O/dm3, TOC0 31.6 mg/dm3, and pH0 = 5.8 ± 0.2). Commercial TiO2 Degussa P-25 (81% anatase, 19% rutile, SBET 56 m2/g, and crystallite size about 30 nm) [27] at a concentration of 0.5 g/dm3 was used as a photocatalyst.
Ozonation (O3), UV irradiation (photolysis), O3/UV treatment, photocatalytic ozonation (O3/TiO2/UV), and photocatalytic oxidation with air oxygen (O2/TiO2/UV) of Triton X-100 solutions were carried out at a temperature of 20 ± 2°C on a laboratory setup equipped with a computer system for recording technological parameters of the ozonation process [23], in a cylindrical quartz reactor (d = 3.6 cm, V = 0.44 dm3) equipped with a disperser in the lower part for supplying OAM or air, a spherical defoamer from above (V ~ 1 dm3) and a peristaltic pump that circulates the suspension from the bottom up (v = 0.15 dm3/min) to intensify the mixing and washing off the foam into the solution.
Ozone was obtained in a laboratory ozonator with a capacity of 1 g O3/h from air oxygen that was supplied from a cylinder with compressed air and was dried on silica gel. Taking into account the previous experience [7, 23], the rate of ozone feed to the reactor (νoz) during the oxidation of the Triton X-100 solution was 2.0 ± 0.1 mg/(dm3 min) at a constant rate of OAM supply (about 0.07 dm3/min), and the ozone concentration in the OAM was 13.5 ± 1.5 mg/dm3. The solution/suspension was irradiated with UV by a DB-15 low-pressure mercury quartz lamp (λmax = 254 nm) located on the side parallel to the axis of the reactor at a distance of 5 cm from its wall.
The duration of treatment of Triton X-100 solutions (V = 0.44 dm3) by the above-mentioned methods varied in the range from 2 to 120 min. After photocatalytic oxidation for 20–120 min, the catalyst was separated from the Triton X-100 solution by centrifugation (8000 rpm) or filtration through a microfilter (0.45 μm).
The process of destruction of Triton X-100 was controlled by three parameters (P). The change in the concentration of Triton X-100 was monitored by the spectrophotometric method (according to the change of absorbance A224). The total concentration of organic compounds in the reaction mixture was estimated by the amount of chemical oxygen consumption (COD) and the concentration of total organic carbon (TOC). The absorption spectra of Triton X-100 solutions were recorded using a Shimadzu UV-2450 spectrophotometer, and the TOC concentration was determined using a Shimadzu TOC-VCSN analyzer. Test solutions from HACH (Germany), an ECO 8 thermoreactor (VELP Scientifica), and an Orion AQ4000 photometer were used to determine the COD value.
RESULTS AND DISCUSSION
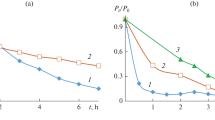
The UV absorption spectra of Triton X-100 solutions treated with ozone and O3/UV differed markedly only in the initial period of oxidation (t ≤ 15 min). Thus, some acceleration of the decomposition of the aromatic ring of the Triton X-100 molecule was noted at the initial stage of O3/UV treatment compared to ozonation. In particular, the concentrations of Triton X-100 and its primary aromatic intermediates in a solution during ozonation and O3/UV treatment decreased by about 80% in 13 and 8 min, respectively. However, the degrees of further Triton X-100 conversion according to A224 by both methods barely differed (87% in 30 min) (Fig. 1).
At the same time, O3/UV treatment substantially increased the degree of destruction of Triton X-100 by COD compared to ozonation (30 and 18% in 30 min, respectively) (Fig. 1). With an increase in the treatment duration the advantages of photoozonation, with respect to the degree of destruction of the primary decomposition products of Triton X-100, substantially increased in comparison with ozonation and UV irradiation (Figs. 2b and 2c).
The rapid primary destruction of the structure of the Triton X-100 molecule by ozone (80–88% according to A224 in 20–60 min) was accompanied by a decrease in the COD by 16–21% and a very small degree of mineralization (up to 5% according to TOC in 60 min) (Fig. 2). At the same time, the O3/UV treatment that is close to ozonation in the kinetics of the primary destruction of Triton X-100 (Fig. 2a) dramatically accelerated the process of mineralization of intermediates and increased the degrees of destruction according to COD and TOC (see Figs. 2b and 2c, respectively). Thus, there was a change in the qualitative composition of the components of Triton X-100 solutions during ozonation without a significant change in the total amount of organic substances, according to generalized indicators. Similar dependences were noted earlier for other compounds of this type [1, 8].
It should be noted that UV irradiation itself caused considerable decomposition of Triton X-100 (79% in 1.5 h according to A224), though it was slower than conversion with other oxidation methods (Fig. 2a). Moreover, the degrees of photolytic destruction of Triton X-100 according to COD and TOC were higher than those achieved with ozonation (see Figs. 2b and 2c, respectively). The possibility of photolytic destruction of alkylphenol ethoxylates under UV-C irradiation was also shown in [28]. According to [28], the degrees of primary destruction and mineralization of NPEO10 after UV irradiation of its solution (C0 = 100 mg/dm3, and pH0 = 7) for 1.5 h were 75 and 9%, respectively.
In our study, the degree of adsorption of Triton X-100 on TiO2 Degussa P-25 was 8–9% in 1 h for Ccat = 0.5–1 g/dm3 (4.5 mg of substance per 1 g of TiO2), which is approximately three times less than that for a similar concentration of sodium alkylbenzene sulfonate (anionic surfactant) [27]. Nevertheless, photocatalytic oxidation of the Triton X-100 solution with air oxygen substantially accelerated its decomposition in all three controlled parameters when compared to those for photolysis (Figs. 2a–2c). However, it is clearly seen from Fig. 2 that the degree of nonionic surfactant mineralization was limited by this method, though the maximum degree of preliminary destruction of Triton X-100 was close to those achieved in oxidation processes involving ozone. Photocatalytic ozonation ensured the maximum rate of destruction and the maximum degree of mineralization of Triton X-100 (Figs. 2b and 2c).
The relative efficiency of the studied methods of Triton X-100 destruction for the same treatment time (t = 1 h) is clearly seen in Fig. 3. Among the studied oxidation processes, UV irradiation gives the minimum degree of primary destruction of Triton X-100 (61% according to A224), but it was accompanied by a substantial degree of mineralization (36% and 32% according to COD and TOC, respectively). Three processes (O3, O3/UV, and O2/TiO2/UV) provided almost the same high degree of primary destruction of Triton X-100 (88 ± 1%), but the degrees of destruction according to generalized indicators during ozonation (21 and about 5% according to COD and TOC, respectively) were substantially inferior to similar indicators for other studied AOPs (47 ± 1% and 39 ± 1% according to COD and TOC, respectively). The highest degrees of destruction of Triton X-100 according to all three indicators were achieved in 1 h with photocatalytic ozonation (95%, 60%, and 59% according to A224, COD, and TOC, respectively).
The relative efficiencies of the studied methods of destruction of Triton X-100 were evaluated by comparing the values of the pseudo-first-order destruction rate constants determined from the slopes of straight lines in the ln(P0/Pt) – f(t) coordinates, where P denotes A224, COD, or TOC (Table 1). Taking into account the extremely fast primary transformation of the Triton X-100 molecule in processes that involve ozone, the effective initial rate constants of Triton X-100 destruction according to A224 were calculated for t ≤ 20 min (Table 1). The effective rate constants of Triton X-100 destruction according to generalized indicators (COD and TOC) were calculated for all processing methods and compared for those time ranges in which their reliability is undoubted (R2 ≥ 0.95) (Table 1).
Compared to photolysis, the initial rate constants of Triton X-100 destruction according to A224 (ΔA ≤ 80%, t = 10 min) in the cases of ozonation and O3/UV treatment were higher by factors 9 and 13, respectively. Moreover, the rate constant of primary photochemical (O3/UV) destruction of Triton X-100 at this stage was about 1.5 times higher than that for ozonation. The rate constants of Triton X-100 decomposition according to COD and TOC (ΔCOD ≤ 50%, and ΔTOC ≤ 40%, respectively) during O3/UV treatment for 1 h were higher than those achieved during ozonation by factors 4.5 and 9, and those achieved during photolysis by factors 1.8 and 1.5, respectively (Table 1).
Compared to photolysis, photocatalytic oxidation by oxygen air for 1 h (ΔCOD ≤ 50%, and ΔTOC ≤ 40%) gave 2.9, 2.6, and 1.8 times higher rate constants Triton X-100 decomposition according to A224, COD, and TOC, respectively. It should be noted that the rate constants of Triton X-100 destruction in the case of UV irradiation in this time interval (20–60 min) were 1.5–2 times higher than in the case of ozonation.
At the initial stage (20 min), the rate constant of Triton X-100 destruction according to A224 during photocatalytic ozonation was 5.8, 1.2, and 1.3 times higher than the rate constants achieved in the processes of photocatalytic oxidation with air oxygen, O3/UV treatment, and ozonation, respectively. The mineralization rate constants of Triton X-100 during photocatalytic ozonation for 1 h (ΔCOD and ΔTOC ≤ 60%) were higher by factors of 1.2–1.3, 1.6–1.7, and 7.5–14 than those for photocatalytic oxidation of air oxygen, O3/UV treatment, and simple ozonation, respectively (Table 1).
As can also be seen from Table 1, the rate constants of Triton X-100 destruction at the initial stage (t ≤ 20 min) according to A224 in the O3/TiO2/UV, O2/TiO2/UV, O3/UV, and O3 oxidation processes exceed the analogous rate constants of Triton X-100 destruction according to COD and TOC by factors of 7–8, 4–10, 6–16, and 7–11, respectively; however, only threefold acceleration was achieved in the photolysis process.
It should also be noted that the mineralization rate constants of Triton X-100 substantially increased when using two AOPs with ozone (O3/TiO2/UV and O3/UV) with oxidation durations of over 1.5 h (by factors of 4.9–7.2 and 2.5–3, respectively), while the opposite trend was observed in the process of photocatalytic oxidation with air oxygen, in which the mineralization rate constant was lower by a factor of 4.3–5.5 (see Table 1).
Photocatalytic ozonation of a Triton X-100 solution for 2 h with νoz = 2.0 ± 0.1 mg/(dm3 min) ensured its almost complete destruction (by 100% according to A224 and COD, and by 97% according to TOC) (see Fig. 4). High degrees of Triton X-100 destruction (95% according to A224 and 91% according to COD) were also achieved during the indicated time period in the process of O3/UV treatment of the Triton X-100 solution, but the degree of mineralization was substantially lower in this case (84%). Photocatalytic oxidation of the Triton X-100 solution with air oxygen in a comparable period of time (2 h) provided only a high degree of its preliminary destruction (92%), but the degrees of destruction according to COD and TOC were substantially lower (71 and 55%, respectively) (see Fig. 4). Moreover, a slight increase in the degree of destruction according to TOC with an increase in the duration of oxidation by this method from 1.5 to 2 h (see Fig. 2c) indicates an extremely low rate of mineralization of intermediate decomposition products of the substance under study. A substantial increase (by 20%) in the degree of Triton X-100 destruction according to TOC in the O3/TiO2/UV system with an increase in the duration of oxidation from 1.5 to 2 h (Table 2) indicates the possibility of achieving its complete mineralization with a small additional increase in the duration of treatment.
With the studied parameters of O3/TiO2/UV and O3/UV treatment of the Triton X-100 solution, the degree of Triton X-100 destruction was determined by the dose of absorbed ozone (Doz). With equal Doz values, a higher degree of Triton X-100 destruction according to TOC is achieved in the process of photocatalytic ozonation compared to O3/UV treatment. Moreover, the specific consumption of the oxidant in the former process is lower (see Table 2).
Thus, almost complete mineralization of Triton X-100 is achieved only by photocatalytic ozonation of its solution for 2 h with νoz = 2.0 ± 0.1 mg/(dm3 min) (see Fig. 4 and Table 2). The specific values of ozone consumption in this process were 2.8 mg/mg Triton X-100, 1.0 mg/mg COD, and 4.4 mg/mg TOC (Table 2). In the case of O3/UV treatment, the maximum degree of Triton X-100 destruction was lower, and the specific consumption of the oxidant for its mineralization was 16% higher (Table 2).
Heterogeneous photocatalytic ozonation is a very complex reaction system that involves chemical, catalytic, and photocatalytic reactions with a huge number of variables to investigate [19]. The advantages of photocatalytic ozonation of Triton X-100 in comparison with O3/UV treatment are a higher theoretical yield of OH radicals (1.0 vs. 0.5 mol per 1 mol of decomposed O3, respectively), the possibility of using a wider range of UV radiation (λ < 380 nm vs. λ < 310 nm, respectively) [25, 29], and the availability of photogenerated holes on the TiO2 surface [19]. The advantages of photocatalytic ozonation compared to photocatalytic oxygen oxidation are a higher yield of OH radicals from ozone compared to oxygen (one electron is needed to generate one hydroxyl radical from ozone, and three electrons are needed to generate one hydroxyl radical from oxygen) [20].
The primary oxidation process during the reactions of ozone and OH radicals with alkylphenol ethoxylates can be, depending on the oxidation method and process parameters, both the hydroxylation and breakage of the aromatic ring and the cleavage and/or depolymerization of the ethoxyl chain with the formation of oligomers with a smaller number of ethylene glycol units, low molecular weight glycols and alcohols, 4-octylphenol or 4-nonylphenol, aldehydes, ketones, aliphatic mono- and dicarboxylic acids, and keto-, oxy-, and polyoxycarboxylic acids, as well as H2O2 [1, 7, 25, 28].
The results of this study and the previously published study [3, 7] confirm that the initial stage of APEO decomposition in an environment close to neutral in the processes involving ozone is aromatic ring breakage that leads to the formation of aldehydes, saturated and unsaturated aliphatic acids, and oxyacids with the gradually decreasing (from C6 to C1) number of carbon atoms in molecules, which ends with the formation of CO2 and H2O [30]. During the photocatalytic oxidation of alkylphenol ethoxylates with oxygen, the cleavage reactions of the aromatic ring and ethoxylate chains are considered to be competitive processes, and the conversion of longer ethoxylate chains with the formation of short-chain APEOs is considered to be a primary process [1, 25]. After some initial induction period, the cleavage of the aromatic ring becomes an increasingly important degradation pathway. During the photocatalytic destruction of an alkyl radical, OH radicals can nonselectively attack all carbon atoms with the formation of a mixture of oxy- and carbonyl intermediates, carboxylic acids, and, ultimately, CO2.
In the structure of the Triton X-100 molecule, only about 18% of the TOC concentration is associated with carbon of the aromatic ring, which in our conditions is primarily attacked by molecular ozone and OH radicals. About 59% of the TOC concentration is due to carbon of the oxyethyl chain, which is also prone to destruction by OH radicals, and 23.5% of the TOC concentration is composed by carbon of the aliphatic radical (C8H17), which is mineralized with the slowest rate, probably, in the photo-Kolbe reaction involving the oxidation of the terminal CH3 groups of the aliphatic chain sequentially to carbonyl and carboxyl groups with subsequent decarboxylation (i.e., elimination of to CO2) [23].
CONCLUSIONS
Among other studied oxidation methods (O3, O3/UV, O2/TiO2/UV, and photolysis), photocatalytic ozonation (O3/TiO2/UV) is the most effective way of destroying nonionic surfactant Triton X-100 in an aqueous medium. Only photocatalytic ozonation with an ozone feed rate of 2.0 ± 0.1 mg/(dm3·min) ensures almost complete mineralization of Triton X-100 (97% according to TOC) in 2 h with a specific ozone consumption of 4.4 mg/mg TOC. The maximum degree of photocatalytic mineralization of Triton X-100 with air oxygen for the indicated time period is 55% according to TOC, and the process of O3/UV treatment under similar conditions gives the degree of mineralization equal to 84% according to TOC with a higher specific ozone consumption (5.1 mg/mg TOC). At the same time, the advantage of photocatalytic ozonation compared to photocatalytic oxidation with oxygen and O3/UV treatment increases during the second hour of treatment, apparently at the stage of mineralization of aliphatic resistant products of Triton X-100 cleavage. The UV irradiation process itself (λ = 254 nm) is also accompanied by partial mineralization of Triton X-100 (35% according to TOC in 1.5 h). Ozonation provides only rapid primary destruction of the structure of Triton X-100 (88% according to A224 in 1 h) with a very small degree of mineralization (about 5% according to TOC).
REFERENCES
Crini, G., Cosentino, C., Bradu, C., Fourmentin, M., Torri, G., Ruzimuradov, O., Arslan-Alaton, I., Tomei, M.C., Derco, J., Barhoumi, M., Prosen, H., Malinović, B.N., Vrabel’, M., Huq, M.M., Soltan, J., Lichtfouse, E., and Morin-Crini, N., Advanced treatments for the removal of alkylphenols and alkylphenol polyethoxylates from wastewater, in Emerging Contaminants, vol. 2: Remediation, Environmental Chemistry for a Sustainable World, vol. 66, Morin-Crini, N., Lichtfouse, E., and Crini, G., New York: Springer, 2021, pp. 305–398.
Ike, M., Asano, M., Belkada, F.D., Tsunoi, S., Tanaka, M., and Fujita, M., Degradation of biotransformation products of nonylphenol ethoxylates by ozonation and UV/TiO2 treatment, Water Sci. Technol., 2002, vol. 46, pp. 127–132. https://doi.org/10.2166/wst.2002.0727
Ledakowicz, S., Perkowski, J., Bulska, A., Jamroz, T., and Sencio, B., Ozonation impact on degradation and toxicity of non-ionic surfactants, Ozone: Sci. Eng., 2005, vol. 27, no. 6, pp. 437–445.
Miklos, D.B., Remy, C., Jekel, M., Linden, K.G., Drewes, J.E., and Hübner, U., Evaluation of advanced oxidation processes for water and wastewater treatment, A critical review, Water Res., 2018, vol. 139, pp. 118–131. https://doi.org/10.1016/j.watres.2018.03.042
Rekhate, C.V. and Srivastava, J.K., Recent advances in ozone-based advanced oxidation processes for treatment of wastewater, A Review, Chem. Eng. J. Adv., 2020, vol. 3, p. 100031. https://doi.org/10.1016/j.ceja.2020.100031
Joseph, C.G., Farm, Y.Y., Taufiq-Yap, Y.H., Pang, C.K., Nga, J.L.H., and Puma, G.L., Ozonation treatment processes for the remediation of detergent wastewater: A comprehensive review, J. Environ. Chem. Eng., 2021, vol. 9, no. 5, p. 106099.
Goncharuk, V.V., Vakulenko, V.F., Shvadchina, Yu.O., Sova, A.N., Nevinnaya, L.V., and Sidorenko, Yu.V., The effect of UV radiation on kinetics of nonylphenol ethoxylate destruction by ozone in water, Khim. Tekhnol. Vody, 2004, vol. 26, no. 4, pp. 1–9.
Goncharuk, V.V., Vakulenko, V.F., Klimenko, N.A., Shvadchina, Yu.O., Samoylenko, L.S., Sidorenko, Yu.V., and Topkin, Yu.V., Selection of modes in oxidative treatment of solutions of nonionic surfactants prior to biosorption on activated carbon, Khim. Tekhnol. Vody, 2005, vol. 27, no. 1, pp. 1–12.
Uchiyama, T., Kobayashi, H., Znad, H.T., Tokumura, M., and Kawase, Y., Dynamic performance of ozonation treatment for nonionic surfactants (polyoxyethylene alkyl ether) in a bubble column reactor, Ozone: Sci. Eng., 2007, vol. 29, no. 1, pp. 65–72. https://doi.org/10.1080/01919510601113414
Tehrani-Bagha, A.R., Nikkar, H., Menger, F.M., and Holmberg, K., Degradation of two persistent surfactants by UV-enhanced ozonation, J. Surfactants Deterg., 2012, vol. 15, pp. 59–66.
Lechuga, M., Fernández-Arteaga, A., Fernández-Serrano, M., Jurado, E., Burgos, A., and Ríos, F., Combined use of ozonation and biodegradation of anionic and non-ionic surfactants, J. Surfactants Deterg., 2014, vol. 17, pp. 363–370. https://doi.org/10.1007/s11743-013-1480-2
Gieldowska-Bulska, A., Perkowski, J., and Kos, L., The application of ozone in the decomposition of aqueous solutions of non-ionic surfactants, Ozone: Sci. Eng., 2004, vol. 26, pp. 217–225.
Tam, L.T.M., Phuong, L.D., Ninh, N.T., Nhat, N.M., Dan, N.P., Ha, P.T.S., Chi, D.H.L., and Phong, N.T., Ozonation for nonylphenol ethoxylates removal from raw water for drinking water supply, J. Sci. Technol., 2015, vol. 53, no. 3A, pp. 1–6.
Derco, J., Gotvajn, A.Ž., Čižmárová, O., Dudáš, J., Sumegová, L., and Šimovičová, K., Removal of micropollutants by ozone-based processes, Processes, 2021, vol. 9, p. 1013.
Felis, E. and Miksch, K., Nonylphenols degradation in the UV, UV/H2O2, O3 and UV/O3 processes—Comparison of the methods and kinetic study, Water Sci. Technol., 2015, vol. 71, no. 3, pp. 446–453.
Mecha, A.C., Onyango, M.S., Ochieng, A., and Momba, M.N., Impact of ozonation in removing organic micro-pollutants in primary and secondary municipal wastewater: Effect of process parameters, Water Sci. Technol., 2016, vol. 74, no. 3, pp. 756–765. https://doi.org/10.2166/wst.2016.276
Derco, J., Dudas, J., Valickova, M., Sumegova, L., and Murinova, S., Removal of alkylphenols from industrial and municipal wastewater, Chem. Biochem. Eng. Q., 2017, vol. 31, pp. 173–178. https://doi.org/10.15255/CABEQ.2016.1021
Bertanza, G., Pedrazzani, R., Papa, M., Mazzoleni, G., Steimberg, N., Caimi, L., Montani, C., and Dilorenzo, D., Removal of BPA and NPnEOs from secondary effluents of municipal WWTPs by means of ozonation, Ozone: Sci. Eng., 2010, vol. 32, no. 3, pp. 204–208. https://doi.org/10.1080/01919511003795303
Xiao, J., Xie, Y., and Cao, H., Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation, Chemosphere, 2015, vol. 121, pp. 1–17.
Mehrjouei, M., Müller, S., and Möller, D., A review on photocatalytic ozonation used for the treatment of water and wastewater, Chem. Eng. J., 2015, vol. 263, pp. 209–219. https://doi.org/10.1016/j.cej.2014.10.112
Mecha, A.C. and Chollom, M.N., Photocatalytic ozonation of wastewater: A review, Environ. Chem. Lett., 2020, vol. 18, pp. 1491–1507.
Zsilák, Z., Fónagy, O., Szabó-Bárdos, E., Horváth, O., Horváth, K., and Hajós, P., Degradation of industrial surfactants by photocatalysis combined with ozonation, Environ. Sci. Pollut. Res., 2014, vol. 21, pp. 11126–11134.
Shvadchina, Yu.O., Vakulenko, V.F., Sova, A.N., and Goncharuk, V.V., Photocatalytic destruction of anionic SAS by ozone and oxygen, J. Water Chem. Technol., 2013, vol. 35, no. 5, pp. 195–202. https://doi.org/10.3103/S1063455X13050019
Shokri, A. and Sanavi Fard, M., A critical review in the features and application of photocatalysts in wastewater treatment, Chem. Pap., 2022, vol. 76, pp. 5309–5339. https://doi.org/10.1007/s11696-022-02256-3
Hegedüs, P., Szabó-Bárdos, E., Horváth, O., Horváth, K., and Hajós, P., TiO2-mediated photocatalytic mineralization of a non-ionic detergent: Comparison and combination with other advanced oxidation procedures, Materials, 2015, vol. 8, p. 231–250.
Nagarnaik, P.M. and Boulanger, B., Advanced oxidation of alkylphenol ethoxylates in aqueous systems, Chemosphere, 2011, vol. 85, no. 5, pp. 854–860.
Shvadchina, Yu.O., Vakulenko, V.F., Levitskaya, E.E., and Goncharuk, V.V., Photocatalytic destruction of anionic SAS with oxygen and hydrogen peroxide in the TiO2 suspension, J. Water Chem. Technol., 2012, vol. 34, no. 5, p. 218–226.
Karci, A., Arslan-Alaton, I., Olmez-Hanci, T., and Bekbolet, M., Degradation and detoxification of industrially important phenol derivatives in water by direct UV-C photolysis and H2O2/UV-C process: A comparative study, Chem. Eng. J., 2013, vol. 224, pp. 4–9. https://doi.org/10.1016/j.cej.2012.11.049
Tichonovas, M., Krugly, E., Jankunaite, D., Racys, V., and Martuzevicius, D., Ozone-UV-catalysis based advanced oxidation process for wastewater treatment, Environ. Sci. Pollut. Res., 2017, vol. 24, no. 21, pp. 17584–17597. https://doi.org/10.1007/s11356-017-9381-y
Suzuki, H., Araki, S., and Yamamoto, H., Evaluation of advanced oxidation processes (AOP) using O3, UV, and TiO2 for the degradation of phenol in water, J. Water Process Eng., 2015, vol. 7, pp. 54–60. https://doi.org/10.1016/j.jwpe.2015.04.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Kadkin
About this article
Cite this article
Shvadchina, Y.O., Vakulenko, V.F. & Sova, A.M. Deep Destruction of the Triton X-100 Nonionic Surfactant in Water by Advanced Oxidation Processes Involving Ozone. J. Water Chem. Technol. 45, 99–108 (2023). https://doi.org/10.3103/S1063455X23020108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X23020108