Abstract
Two families of extended surfactants were prepared with the same head groups (carboxylate, sulfate, disodium phosphate) and different intermediate spacer structures. In one there was an average of 7 propylene oxide groups on the side of the tail and an average of 7 ethylene oxide groups on the side of the head, to produce a sequence of two different polarity segments. In the other case the spacer contained the same average numbers of propylene and ethylene oxide groups but in some homogeneous arrangement. The intermediate spacer structure, without ionic head group and in the cases of the carboxylate and sulfate extended surfactants, had a packing density reduction which is associated to the homogeneously alkoxide arrangement in the spacer. Such an arrangement was found to produce about 20% more surface area at the interface, apparently because it results in some plumpness due to the spacer folding to remain close to the interface. Both the critical micelle concentration and occupied interfacial area of the extended surfactant increased with the ionization of the anionic group associated with the electrostatic repulsion effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
So-called extended surfactants contain an intermediate polarity spacer between the conventional hydrophilic head and lipophilic tail groups. This structure was introduced about 15 years ago [1] to mimic the effect of the surfactant and lipophilic linker [2–6] combination in increasing solubilization in bicontinuous microemulsions [7]. Extended surfactants were found to be able to produce a low interfacial tension and a high solubilization with polar oils such as fatty acid esters, diglycerides and even natural triglycerides [1] to an importance not attained by conventional surfactants [8, 9]. The current state of the art attributes these outstanding properties to a smooth transition from a polar to an apolar environment at the interface over a considerable thickness [10].
Only a few experimental species of the alkyl polypropylene oxide sulfate type have been made available by surfactant manufacturers and have been tested in applications such as detergency or solubilization of polar oils [11–16]. Extended surfactants behave in some aspect as conventional ones and may be mixed with other surfactants with fairly linear mixing rules [17, 18]. Some species with other head groups such as carboxylate, C5 and C6 glucosides have been prepared [19–22] but there is not yet a clearly established relationship between the extended surfactant structure and the properties beyond the fact that the alkyl polypropylene oxide sulfate species behave in some aspects as conventional surfactants, with some additional features that seem to depend on the (polypropylene oxide) spacer size. It was found that such an intermediate spacer is part of the hydrophobe and that its size is of paramount importance to solubilize some oils [8, 9]. Additionally it was recently found that the portion of it which is close to the sulfate group, is probably slightly hydrated by water and confers on them a temperature dependency closer to polyethoxylated nonionics rather than to anionics [23].
The present investigation reports on the influence of two structural characteristics not yet studied, i.e. the effect of the anion head group nature, particularly its ionization level, and the influence of the intermediate polarity spacer ordering, on the interfacial adsorption packing and on the association in micelles. A companion paper will deal with the effect of these structural characteristics on the physicochemical formulation aspects of surfactant-oil-water systems.
Synthesis of Different Species: C12/PO7/EO7/HEAD and C12/PO + EO[H7 + 7]/HEAD
Synthesis of Alkoxylated Dodecanol Intermediates
N-dodecanol, synthesis grade from Merck was alkoxylated by Lipesa (San Tomé, Venezuela) according to a standard synthesis method [24–27] and the products were used as received. The basic method involves dissolving a KOH catalyst in a mixture of water and isopropyl alcohol, which is then evaporated. Ethylene oxide and propylene oxide are introduced as gaseous aliquots, which are each a small fraction of the total amount, i.e. 10% for the ethoxylation and propoxylation, and 5% for the mixed alkoxides addition. Each aliquot introduction slightly increases the reactor pressure, and the conversion is followed by the pressure decay. The next aliquot is introduced when the pressure has come to normal and so forth.
Two kinds of products were prepared with the same total amount of an average of 7 propylene oxide and 7 ethylene oxide groups per dodecanol molecule, but with different addition procedures. The first product type so-called “sequential” intermediate was prepared by first adding to dodecanol an average of 7 propylene oxide molecules, and next an average of 7 ethylene oxide molecules. It was labeled C12/PO7/EO7 to indicate that the polypropylene oxide group is located between the alkyl chain and the polyethylene oxide block (Scheme 1).
The second one referred to as a “homogeneous” intermediate was prepared according to the same method but by introducing very small aliquots (1/20th of the total amount) containing a gas mixture with equimolecular proportions of propylene oxide and ethylene oxide. The polyalkoxide block thus contained ethylene and propylene oxides, somehow homogeneously distributed over the spacer because of the introduction of the gas mixture as 20 consecutive small aliquots. The compound was labeled C12/PO + EO[H7 + 7] to indicate the homogeneous distribution of an average of 7 propylene oxide and 7 ethylene oxide groups in the spacer, with essentially no segregation (Scheme 2).
Addition of Ionic Head Group
The sulfate head extended surfactants were synthesized according to the method described elsewhere [21]. Depending of the alkoxylated structure of the intermediate they were labeled C12/PO7/PO7/S or C12/PO + EO[H7 + 7]/S.
The carboxylate head extended surfactants were prepared in a similar way by diluting the corresponding alkoxylated dodecanol intermediate in a mixture of toluene and dimethylsulfoxide (1:1) containing KOH, which was then reacted with sodium chloroacetate for 24 h at ambient temperature under constant stirring. The filtered product was acidified with sulfuric acid and the resulting alkoxylated dodecanol carboxylic acid was extracted with toluene. The toluene was then evaporated and the acid was neutralized with a solution of NaOH in methanol. The final carboxylate head group extended surfactants was obtained by evaporation of the methanol. They were labeled C12/PO7/EO7/C and C12/PO + EO[H7 + 7]/C depending on the sequential or homogeneous nature of the alkoxide intermediate spacer (Scheme 3).
The disodium phosphate extended surfactants were prepared according to the literature to insure the formation of mono-alkyl phosphates [28] by first diluting the alkoxylated intermediate into toluene, which was then reacted with a 10 vol% polyphosphoric acid solution in toluene (added drop by drop) at ambient temperature under constant stirring during 72 h. The aqueous phase was removed and the organic phase was washed with 20 wt% NaCl brine and 2 N hydrochlorohydric acid. The organic phase was then treated with a 5 wt% sodium hydroxide aqueous solution to extract the disodium phosphate species in the aqueous phase. The aqueous phase was acidified again to restore the acid species which were extracted with ethyl ether. The extract was neutralized with a sodium hydroxide solution in methanol until pH 11 was attained, to warranty the formation of the disodium salt as recently shown [29]. Finally, the solvent was evaporated under a vacuum. The final disodium phosphate head group extended surfactants were labeled C12/PO7/PO7/DSP and C12/PO + EO[H7 + 7]/DSP depending on the intermediate spacer structure (Scheme 4).
Results
Alkoxylated dodecanol intermediates
C12/PO7/EO7 and C12/PO + EO[H(7 + 7)] are slightly hydrophilic nonionic surfactants, and hence have a cloud point, which happens to be close to ambient temperature as seen in Table 1.
The cloud point
was essentially the same for both substances, i.e. it is independent of the arrangement of the EO and PO groups in the polyalkoxide chain. However, a slight cloud point decrease was observed as expected [30] as salinity was increased. The sequential arrangement intermediate was found to be very slightly more hydrophilic at zero salinity with a cloud point at 27 °C versus 25.5 °C for the homogeneous arrangement one, which is a difference at the limit of significance, and even less when salt is added. It may be said that the ethylene oxide and propylene oxide contributions to the cloud point, i.e. the tendency to be less water soluble, is basically quite insensitive to the alkoxide species arrangement in the spacer. The cloud point temperature in pure water was similar to the one found for the dodecanol with approximately 4 ethylene oxide groups (C12EO4) [31] which is known to be a nonionic surfactant with balanced hydrophilic-lipophilic tendencies. Hence adding 7 PO groups increases the lipophilicity and needs the addition of about 3–4 EO groups to compensate. This kind of equivalence will be discussed in the next report on phase behavior and optimum formulation.
Tension Variations
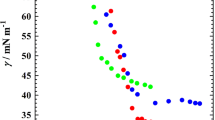
The plots of surface tension versus (log) concentration (Fig. 1) allowed us to calculate the critical micelle concentration CMC (from the breaking point) and the maximum adsorption density Γm or molecular packing (from the slope just before the CMC) indicated as the interfacial area occupied per adsorbed molecule aS calculated in Å2 according to the following relationships [31].
Γm = −(1/2.3 RT) (∂γ/∂logC) at the CMC and when Γm is in mol/1,000 m2, as in Å2 equals aS = 1023/NAΓm.
At 25 °C (298.16°K) the interfacial area in Å2 per molecule may be calculated as
aS = 946.32 n/slope (∂γ/∂logC in mN/m)
where n is 1 for the nonionic species, 2 for the 1:1 anionic sulfate and carboxylate head products, and 3 for the disodium phosphates [31, 32]. In the case of adding 2.9 wt% (0.5 mol/liter) NaCl in the solution of disodium phosphates, n = 1 because the sodium ion concentration was essentially constant and only the phosphate concentration varied. The CMC and area per adsorbed molecules for the different cases are indicated in Table 2.
Effect of Intermediate Spacer Structure on Adsorption at the Interface
In the cases of the intermediate without an ionic head group, and of the carboxylate and sulfate extended surfactants, a packing density reduction is associated to the homogeneously alkoxide arrangement in the spacer. Such arrangement was found to produce about 20% more surface area at interface, presumably because it resulted in some plumpness at the surfactant interfacial “waist” as illustrated in the Fig. 2, and thus induced more steric repulsion between neighboring molecules. Consequently the adsorbed molecule with the homogeneous arrangement in the spacer was less stretched than the one with a sequential arrangement spacer.
It should be remembered that a recent study has reported that the alkyl polypropylene oxide sulfate type extended surfactant (see Fig. 2 center) is not completely stretched [23] as the dodecyl sulfate or ethoxy sulfate (Fig. 2 left), because of the evidence that the first 2–3 propylene oxide groups in the spacer which are close to the head group are likely to be slightly hydrated. As a consequence the polypropylene oxide part of the spacer is probably slightly bent to accommodate some proximity of the interface for the first propylene oxide groups, as shown in Fig. 2 (center) sequential spacer molecule.
This interpretation explains why the C12/PO7/EO7/S occupies 230 Å2 per molecule, compared with 53 Å2 for dodecyl sulfate (C12/S) or 60 Å2 dodecyl ether sulfate (C12/EO2/S) [31].
Following this trend, the intermediate spacer made by homogeneous arrangement mixture of ethylene and propylene oxides would be even more hydrated and thus more bent to stay closer to interface (Fig. 2 right), as corroborated by the 270 Å2 occupied by each molecule. This sequence is illustrated by the three left drawings in Fig. 3 in which the adsorption characteristics are illustrated.
In the case of the disodium phosphate specie, the packing appeared to be extremely loose, with an area per molecule (720 Å2) which was more than two times larger than in other cases (see Fig. 3 right drawing). Moreover there was essentially no difference in packing between the molecules containing sequential and homogeneous arrangements of the alkoxide groups in the spacer. This may be easily interpreted as the dominating influence of the double ionic charge in the head group which produced an electrostatic repulsion, that determined the packing and offset any steric repulsion effect produced by the spacer folding.
Effect of the Ionic Head Group on Adsorption at Surface
The effect of the ionic group was first analyzed by comparing the CMC and area per molecule with the case of the dodecyl alkoxide intermediate C12/PO7/EO7 with no ionic head, which has a low CMC (0.0010 wt% or 0.000011 mol/liter) and occupies 65 Å2 per molecule. The introduction of the carboxylate group raised the area to 170 Å2. The sulfate group generally exhibited slightly more charge because it comes from a stronger acid, and thus the CMC and area per molecules increased slightly more.
This is in agreement with the literature for the non-extended species, e.g., 53 Å2 for the C12SO4 −Na+ that is slightly more than 40–48 Å2 for C11H23COO−Na+ and the C11–O–CH2COO−Na+ depending on conditions.
The disodium phosphate exhibited 2 charges and thus the increase in repulsion was considerably higher and resulted in a large increase in the CMC and in the surface area per molecule, which reached 700 Å2. It may be concluded that the effect of the head group is essentially related to the ionization, the carboxylate being slightly less dissociated than the sulfate, and the disodium phosphate having essentially two times more charges.
The same ranking and progression was found in the homogeneous alkoxide spacer species, with a slightly higher surface area per molecule with none or with a single ionic charge. The explanation is that the alkoxide (sequential or homogenous) spacer was not fully stretched to be positioned perpendicular to interface, and that there was some additional repulsive interaction of steric nature, e.g. some “tail whipping” or “playing elbow” effect, with the carboxylate and sulfate ionic heads. This effect was, however, completely offset by the electrostatic repulsion in the case of the divalent phosphate species whose properties are the same whatever the spacer.
Influence of the Alkoxide Spacer Arrangement on the CMC
It is sometimes difficult to determine the CMC very accurately at the breaking point of the tension—log concentration plot, but it is in general easy to find out which of two plots exhibits a higher or lower CMC, by just overlapping them. Hence, the values shown in Table 2 may be somehow inaccurate, but the ranking of the CMCs according to the surfactant species is unquestionable.
For the nonionic intermediates, the two times lower CMC for C12/PO7/EO7 indicates that the sequentially arranged spacer is more likely to gather in micelles. This may be interpreted as a better segregation between head and tail, and probably a lower interaction with water of the 7 EO and 2–3 PO close to water when compared with the homogeneous arrangement EO + PO/H7 + 7. The bulkier “waist” group results in some steric repulsion that opposes micellization in particular because it weakens the interaction between the tails.
For the two monovalent anionic head group species, again the sequentially arranged spacer systematically exhibited a two-three times lower CMC than the homogeneously arranged spacer counterpart, thus corroborating that the presence of the homogeneous alkoxide spacer tends to increase the interaction with water, independently of the anionic head group.
Of course the occurrence of a double charge in the disodium phosphate species considerably increase the CMC, and the electrostatic effect seems to be much more important that the spacer structure, which has, however, still some minor effect on the CMC.
Influence of the Head Group on the CMC
When the CMCs were compared according to the nature of the head group, it was seen that for both sequential and homogeneous spacers, it was slightly raised by the introduction of the carboxylate, even more with the sulfate, and considerably more with the divalent phosphate. This ranking exactly corroborates the increased hydrophilicity associated with the head group ionization, just in the same way as it was found to influence the repulsion between neighboring adsorbed molecules at the interface.
A Summing up of the General Trends
The comparison of the area per adsorbed molecule at the air-water interface and of the CMC in aqueous solution, indicated that the extended surfactant species may be ranked according to their head group ionization, which increases from the nonionic group to carboxylate, sulfate and disodium phosphate. The more ionized the head group, the higher the CMC and the higher the surface area per adsorbed molecule. On the other hand, the replacement of the polypropylene oxide—polyethylene oxide sequence by an homogeneous mixing of the alkoxides in the spacer tends to increase the surface area per adsorbed molecule and the CMC, with an increased hydrophilicity probably due to a better interaction with water of the uniformly mixed alkoxide spacer, because its closer position with respect to the interface.
References
Miñana-Pérez M (1993) Contribution à la microémulsification d’huiles polaires de synthèse ou naturelles [in French], Ph.D. Dissertation, University of Pau PA, France
Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL (1993) Improving solubilization in microemulsion with additives-part 1: the lipophilic linker role. Langmuir 9:669–672
Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL (1993) Improving solubilization in microemulsion with additives-part 2: long chain alcohols as lipophilic linkers. Langmuir 9:3371–3374
Salager JL, Graciaa A, Lachaise J (1998) Improving Solubilization in microemulsion with additives-part III: lipophilic linker optimization. J Surf Deterg 1:403–406
Sabatini DA, Acosta E, Harwell JH (2003) Linker molecules in surfactant mixtures. Current Opin Colloid Interface Sci 8:316–326
Acosta E, Mai PD, Harwell JH, Sabatini DA (2003) Linker-modified microemulsions for a variety of oils and surfactants. J Surf Deterg 6:353–363
Salager JL, Antón RE, Sabatini DA, Harwell JH, Acosta EJ, Tolosa LI (2005) Enhancing solubilization in microemulsions—State of the art and current trends. J Surf Deterg 8:3–21
Miñana-Pérez M, Graciaa A, Lachaise J, Salager JL (1995) Solubilization of polar oils with extended surfactants. Colloids Surf A 100:217–224
Miñana-Pérez M, Graciaa A, Lachaise J, Salager JL (1995) Solubilization of polar oils in microemulsion systems. Prog Colloid Polym Sci 98:177–179
Salager JL, Scorzza, Forgiarini A, Arandia MA, Pietrángeli G, Manchego L, Véjar F (2008) Amphiphilic mixtures versus surfactant structures with smooth polarity transition across interface to improve solubilization performance. Paper A-17. 7th World Surfactant Congress CESIO, Paris, June 23–25
Smith GA, Hand KR (2006) Enhanced solubilization using extended chain surfactants. US Patent 2006/0211593 A1. 21 Sept 2006
Tongcumpou C, Acosta EJ, Quencer LB, Joseph AF, Scamehorn JF, Sabatini DA, Yanumet N, Chavadej S (2005) Microemulsion formation and detergency with oily soils. III performance and mechanisms. J Surf Deterg 8:147–156
Witthayapanyanon A, Acosta E, Harwell JH, Sabatini DA (2006) Formulation of ultralow interfacial tension systems using extended surfactants. J Surf Deterg 9:331–339
Do L, Witthayapanyanon A, Harwell JH, Sabatini DA (2009) Environmentally friendly vegetable oil microemulsions using extended surfactants and linkers. J Surf Deterg 12:91–99
Witthayapanyanon A, Phan TT, Heitmann TC, Harwell JH, Sabatini DA (2010) Interfacial properties of extended surfactant based microemulsions and related macroemulsions. J Surf Deterg 13:127–134
Phan TT, Harwell JH, Sabatini DA (2010) Effects of triglyceride molecular structure on optimum formulation of surfactant-oil-water. J Surf Deterg 13:189–194
Miñana-Pérez M, Graciaa A, Lachaise J, Salager JL (1996) Systems containing mixtures of extended surfactants and conventional nonionics. Phase behavior and solubilization in microemulsion. 4th World Surfactants Congress (Barcelona, Spain, June 3–7, 1996) Proceedings vol. 2, 226–234. Edited by R. de Lluria for AEPSAT, Barcelona, Spain
Witthayapanyanon A, Harwell JH, Sabatini DA (2008) Hydrophilic-lipophilic deviation (HLD) for characterizing conventional and extended surfactants. J Colloid Interface Sci 325:259–266
Scorzza C, Godé P, Martin P, Miñana-Pérez M, Salager JL, Villa P, Goethals G (2002) Synthesis and surfactant properties of a new “extended” glucidoamphiphile made from D-Glucose. J Surf Deterg 5:331–335
Scorzza C, Godé P, Goethals G, Martin P, Miñana-Pérez M, Salager JL, Usubillaga A, Villa P (2002) Another new family of “extended” glucidoamphiphiles. Synthesis and surfactant properties for different sugar head groups and spacer arm lengths. J Surf Deterg 5:337–343
Fernandez A, Scorzza C, Usubillaga A, Salager JL (2005) Synthesis of new extended surfactants containing a carboxylate or sulfate polar group. J Surf Deterg 8:187–191
Fernandez A, Scorzza C, Usubillaga A, Salager JL (2005) Synthesis of new extended surfactants containing a xylitol polar group. J Surf Deterg 8:193–198
Velázquez J, Scorzza C, Véjar F, Forgiarini A, Anton RE, Salager JL (2010) Effect of the temperature and other variables on the optimum formulation of anionic extended surfactants-alkane-brine systems. J Surf Deterg 13:69–73
Cox MF, Weerasooriya U (1999) Enhanced propoxylation of alcohols and alcohols ethoxylates. J Surf Deterg 2:59–68
Di Serio M, Vairo G, Iengo P, Felippone F, Santacesaria E (1996) Kinetics of ethoxylation and propoxylation of 1- and 2-octanol catalyzed by KOH. Ind Eng Chem Res 35:3848–3853
Santacesaria E, Di Serio M, Garaffa R, Addino G (1992) Kinetics and mechanisms of fatty alcohol polyethoxylation. 1. The reaction catalyzed by potassium hydroxide. Ind Eng Chem Res 31:2413–2418
Santacesaria E, Di Serio M, Garaffa R, Addino G (1992) Kinetics and mechanisms of fatty alcohol polyethoxylation. 2. Narrow range ethoxylation obtained with barium catalysts. Ind Eng Chem Res 31:2419–2421
Tracy DJ, Reieson RL (2002) Commercial synthesis of monoalkyl phosphates. J Surf Deterg 5:169–172
Osanai S, Yanada G, Hidano R, Beppu K, Namiwa K (2010) Preparation and properties of phosphate surfactants containing ether and hydroxy groups. J Surf Deterg 13:41–49
Mahajan RK, Vohra KK, Kaur N, Aswal VK (2008) Organic additives and electrolytes as cloud point modifiers in octylphenol ethoxylate solutions. J Surf Deterg 11:243–250
Rosen M (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley-Interscience, Hoboken
Adamson A, Gast AP (1997) Physical chemistry of surfaces, 6th edn. Wiley, New York
Acknowledgments
The authors thank their industrial associate LIPESA (www.Lipesa.com) for taking care of the propoxylation and ethoxylation reactions to produce intermediate substances.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Forgiarini, A.M., Scorzza, C., Velásquez, J. et al. Influence of the Mixed Propoxy/Ethoxy Spacer Arrangement Order and of the Ionic Head Group Nature on the Adsorption and Aggregation of Extended Surfactants. J Surfact Deterg 13, 451–458 (2010). https://doi.org/10.1007/s11743-010-1216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-010-1216-5