Abstract
Extended surfactants containing an intermediate-polarity spacer, such as polypropylene oxide, in between the hydrophilic head and the hydrocarbon tail are known to result in superior solubilization and low interfacial tension, though they exhibit slow kinetics. The present work seeks to evaluate both equilibrium and kinetic aspects of extended-surfactant-based micro- and macroemulsions. The interfacial morphology of the extended surfactant membrane, i.e., characteristic length (ξ) and interfacial rigidity (E r) at optimum middle-phase microemulsion conditions, was characterized using the net-average curvature model. The results showed that extended surfactants resulted in a relatively rigid interfacial membrane compared with conventional surfactants having similar hydrocarbon chain length. In addition, both ξ and E r parameters increased with the length of the polypropylene oxide spacer. Increasing E r values correlated to the slow coalescence rates of extended surfactant emulsified systems. Two alternative approaches (the addition of combined linkers and co-surfactant) are shown to overcome the slow kinetics of coalescence while maintaining desirable high solubilization and low interfacial tension.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the Winsor R-concept [1–4], an effective way to produce microemulsions with greater solubilization and lower interfacial tension (IFT) is to equally increase the interaction of surfactant for both oil and water. While this can be achieved by increasing the hydrophilicity of the surfactant head and the length of the hydrocarbon tail, this approach is limited by the loss in solubility associated with long hydrocarbon tails. Extended surfactants have been proposed as an alternative surfactant structure to achieve the Winsor potential without sacrificing water solubility [4–7].
By definition, extended surfactants are surfactants in which intermediate-polarity groups such as short-chain polypropylene oxide (POs) or polypropylene–polyethylene oxide (POs–EOs) groups are inserted between the surfactant hydrophilic head and hydrocarbon tail [4–6]. Due to the presence of intermediate-polarity groups, extended surfactants not only increase the tail length but also offer a smoother interfacial transition from a polar aqueous to nonpolar oil region [3, 4]. As a result, extended surfactants are capable of forming middle-phase microemulsions with high solubilization and ultralow IFT for a wide range of oils, in particular long-chain alkanes, triglycerides, and vegetable oils [5–9]. Detailed discussions and proposed structures of extended surfactant can be found in the literature [3–11].
In spite of having attractive properties desirable for practical uses, extended-surfactant-based microemulsions face the challenge of poor kinetics. Our previous work [7] reported that the equilibration time for optimum middle-phase microemulsions produced by extended surfactants is in the range of weeks to months. Such slow kinetic systems will not be adequate for application processes which operate under short contact times, e.g., detergency, hard-surface cleaning, surfactant-enhanced separation, etc.
It is widely accepted that the surfactant structure at the interface (“membrane”) dictates both equilibrium and dynamic properties of microemulsion and emulsion systems [2, 12–17]. These properties include solubilization capacity, IFT reduction, phase behavior, rate of solubilization, and coalescence kinetics of macroemulsion droplets. Although many different parameters have been proposed as membrane characteristics, the present work focuses on two essential parameters: the characteristic length (ξ) and the interfacial rigidity (E r). They are preferred because they yield vital information linked to both equilibrium and dynamic properties of microemulsion systems, as discussed further below. In addition, the net-average curvature model (NAC) [18, 19] can readily be used to estimate these membrane properties (both ξ and E r) based on simple phase behavior and IFT data.
The characteristic length accounts for the surfactant tail plus associated oil or water molecules in the surfactant membrane, thereby determining the maximum solubilization capacity of the microemulsion system. De Gennes and Taupin [14] and Acosta et al. [18] indicated that the thickness of surfactant layer (ξ) is a function of the length parameter (L), which was later found to be an extended length scaling from the surfactant tail [18]. As for the solubilization parameter (SP), a larger value of the characteristic length suggests a higher solubilization and also results in a lower IFT. Another important property associated with the ξ parameter is the rigidity of the surfactant membrane or the interfacial rigidity (E r). Based on a series of phase studies with linker molecules [17, 20–22], systems with higher ξ values produced a rigid surfactant membrane (high E r values).
While ξ accounts for equilibrium microemulsion properties such as solubilization capacity and IFT, the E r property relates to the bending modulus, K [12, 23], a key parameter controlling dynamic properties (e.g., coalescence kinetic) of microemulsion systems. Helfrich [13] proposed a mathematical equation to evaluate the K modulus in terms of the free energy required to create a new dynamic interfacial area. His work suggested that a high K-value corresponds to a rigid interfacial film, for which more energy is required to deform the membrane as two droplets approach, thereby leading to a slower coalescence rate. With this relationship, the kinetics of microemulsion equilibration may be viewed as an indirect measurement of macroemulsion coalescence rate [17], an approach which will be used in the current study as well.
Because of their unique structure, extended surfactants are likely to produce a thick and rigid interfacial membrane, which will result in a slow coalescence rate and a prolonged equilibration time. This issue is addressed in the present report after corroborating the ability of extended surfactants to form middle-phase microemulsions with desirable high solubilization and low IFT. Then, the membrane properties of the extended-surfactant-based microemulsions will be characterized using the NAC model. This information will allow comparison of interfacial thickness (ξ) and membrane rigidity (E r) between extended surfactants and conventional surfactants having similar tail lengths. Finally, we explore approaches (i.e., the addition of combined linkers and co-surfactants) to overcome the slow coalescence rate of extended-surfactant-based systems, while still maintaining favorable low IFT and high solubilization for practical uses.
Experimental Procedures
Materials
Surfactants evaluated in this work are classified into two main groups: conventional and extended surfactants. In the first group, sodium dodecyl sulfate (SDS) and bis(2-ethylhexyl) sulfosuccinate sodium salt (SDOSS, trade name Aerosol-OT) were utilized as representatives of conventional anionic surfactants. SDS (97% active) and SDOSS (99%+) were purchased from Sigma–Aldrich. The second group of surfactants evaluated is the extended surfactants containing various numbers of propoxylated groups (PO) inserted between the sulfate head and hydrocarbon tail [R–(PO) x –SO4Na]. The hydrocarbon tail of the extended surfactants studied consisted of branched 12–13 carbons (C12,13). These R–(PO) x –SO4Na surfactant samples were provided by Sasol North American Inc. (Lake Charles, LA); see Table 1. The 50 and 100 B indicated after the surfactant formulation represent the degree of branching of hydrocarbon tail: 50% and 100%, respectively. All conventional and extended surfactants were used as received.
Sodium chloride (99%+), sec-butanol (99%+, anhydrous), and straight-chain alkanes, namely octane (99%+), decane (99%+), dodecane (99%+), and hexadecane (99%+), were purchased from Sigma–Aldrich (Saint Louis, MO). Sodium mono- and dimethyl-naphthalenesulfonate (SMDNS, 99%) were supplied by Akzo Nobel (Houston, TX). All chemicals stated above were used as received.
Methods
Microemulsion phase studies were performed in flat-bottom vials with Teflon-lined screw caps using standard methods [6, 7, 20, 21]. Equal volumes of oil and water (5 mL) were added into the vial at different NaCl concentrations (salinity scan). The tubes were then placed in a temperature-controlled water bath at 27 °C. The samples were shaken once a day for the first 3 days and left to equilibrate for at least 2 weeks. When the systems reached equilibrium, the relative phase volumes and interfacial tension (IFT) values were quantified for each sample to determine the optimum condition (in this case, the salinity that produced the lowest IFT and equal volumes of oil and water solubilized in the middle phase, so that SPo and SPw are equal) is known as the optimum salinity (S*) for the system.
Equilibrium interfacial tension (IFT) was measured between the excess water and excess oil phases of pre-equilibrated middle-phase microemulsion samples using a spinning drop tensiometer (University of Texas, model 500). The excess water phase of a middle-phase microemulsion (which was the dense phase) was added into the spinning drop tube. Then 1–3 μL of the excess oil phase was subsequently injected into the same tube. The IFT measurement was recorded after 15 min of spinning.
Characteristic length (ξ*) and interfacial rigidity (E r ) of extended-surfactant-based microemulsions at the optimum condition were evaluated using the NAC approach [18], The interfacial rigidity at the optimum condition (Er) can be calculated by the following equation [18]:
where γ* is the interfacial tension (either between middle phase and excess oil or between middle phase and excess water) at optimum condition, Er is the interfacial rigidity expressed in kBT units at 300 K, and ξ* is the characteristic length at the optimum condition (Å).
Coalescence rate was estimated using the turbidity device developed by Acosta et al. [17] using a standard green light-emitting diode (LED) light source and a cadmium sulfide cell (CDS) detector. The change in the resistance was then registered via a multimeter (METEX M3850D) and converted into turbidity (τ). Coalescence samples were obtained by gently hand-shaking the optimum middle-phase microemulsions (approximate shaking rate of 20 strokes per minute) for 30 s prior to placing in the device with the light source and detector aligned at the center of the test tubes to follow the rate of macroemulsion coalescence as the equilibrium middle-phase microemulsion state is approached.
From the turbidity results, a plot of inverse turbidity (1/τ) versus time (t) yields the coalescence kinetic constant (k c) of the corresponding macroemulsion at the optimum condition.
where τ and τ 0 are the turbidity of samples at time t and that of the blank solution [17]. Note that Eq. 2 was established based on the assumption that a decrease in turbidity as time elapses is proportional to a decrease in the number of drops per unit volume [17]. Therefore, the k c value indicates the rate of change of number of droplets per unit volume in the system. A higher k c value indicates a faster coalescence of macroemulsion droplets and a quicker equilibration time toward the original middle-phase microemulsion system.
Results and Discussion
Interfacial Tension and Solubilization Properties of Extended-Surfactant-Based Microemulsions
In this study, the ability of extended surfactants to form microemulsions is compared with a conventional surfactant having a similar hydrophilic head and hydrocarbon tail. Due to a limited number of extended surfactants available, the extended surfactant C12,13–(PO)8–SO4Na (50% branch) and the conventional surfactant C12–SO4Na (SDS) were the closest pair available and were studied at 0.07 M concentration at 27 °C.
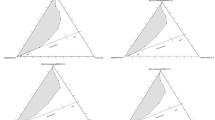
Figure 1 and Table 2 summarize important microemulsion properties [i.e., optimum salinity (S *), solubilization parameter (SP*), and interfacial tension (IFT*)] of the C12,13–(PO)8–SO4Na and the SDS/sec-butanol systems. As expected from Winsor’s R concept [1, 2], both surfactant systems exhibit an increase in optimum salinity (S *) with increasing oil alkane carbon number (ACN), a typical trend observed in the literature [2, 6, 7, 24]. However, it is interesting to mention that, while an increase in oil ACN results in a decrease in the minimum IFT value for the C12,13–(PO)8–SO4Na extended surfactant, the opposite trend is observed with the SDS/sec-butanol system. Furthermore, according to data in Fig. 1, although the IFT* property of C12,13–(PO)8–SO4Na decreases with increasing oil ACN, the SP* value remains relatively constant, an unusual behavior which deviates from the Chun Huh relationship [25, 26] which indicates that SP* and IFT are inversely proportional.
Characteristic Length (ξ *) and Interfacial Rigidity (E r) of Extended Surfactant Membrane at Optimum Condition
As suggested in the introduction, with additional PO groups in the surfactant tails, extended surfactants are likely to evidence higher characteristic length (ξ *) (thicker surfactant membrane) and more rigid surfactant membranes (E r). Table 3 summarizes data on solubilization parameter, characteristic length, and interfacial rigidity of conventional and extended surfactant formulations for hexadecane at the optimum condition. Since the results show a consistent trend between the solubilization parameter and characteristic length, only the ξ * value will be used as an indicator for system solubilization in later discussion. In the case of the conventional surfactant SDS and alcohol mixture, it is interesting to note that the calculated characteristic length is lower than the reported value for a usual tail length of SDS (11.64 Å versus 16 Å) [18]. The low ξ * value indicates a weak surfactant-oil interaction which is likely the result of the high sec-butanol concentration and thus the low surfactant adsorption density at the interface. In respect to Eq. 1, the E r value is strongly influenced by the ξ * property; so the E r value of the SDS/sec-butanol system is found to be very low (0.2 k B T). A value of E r < 1 k B T is quite common for systems containing high amount of short-chain alcohol or hydrophilic linker [17, 18]. Furthermore, the low E r value in the SDS/sec-butanol systems is also consistent with the fact that their emulsion droplets coalesced quickly after shaking ceased (≤10 min).
In contrast to the SDS/sec-butanol system, extended surfactant systems exhibit much higher ξ * values (Table 3), which are a function of the number of PO groups. The results show the ξ * value of extended surfactant notably increases from 211.8 Å to 270.1 Å on increasing the number of PO groups from four to eight. Since a higher ξ * value means a thicker surfactant membrane and better solubilization power of microemulsion systems, this suggests that additional PO groups increase the total length of extended surfactant molecules and thus their solubilization potential.
Consistent with the ξ * parameter, the E r values are found to increase with increasing number of PO groups inserted into extended surfactants. The calculated E r values for the C12,13–(PO)4–SO4Na and C12,13–(PO)8–SO4Na surfactants with 100% branching are 6.9 k B T and 8.3 k B T, respectively. From the literature [12], the E r value for typical middle-phase microemulsion systems corresponds with the bending modulus (K) of 1 k B T, whereas E r ≈ 10 k B T suggests the presence of lipid bilayers. Based on this scale, the relatively high rigidity of the extended surfactant membrane leads to two potential explanations. First, it is possible that the extended surfactants may form a middle-phase microemulsion that coexists with liquid crystals. These liquid crystals could wrap around the emulsion droplets, inhibiting droplets from coalescing, as has been reported by others [2, 27–29]. Another potential explanation of the high-rigidity film is the steric hindrance caused by the bulky PO attachment and branch-tailed nature of extended surfactant. This effect could potentially prevent the approach of dispersed emulsion droplets or a thinning of the plane-parallel film.
Fish Diagram of a Single Extended Surfactant
An alternative way to present the microemulsion phase behavior is by showing a plot between surfactant concentration (M) and salinity (wt %) of the surfactant/brine/oil system. This type of plot is often portrayed in the gamma shape, thus referred to as the gamma or fish diagram [2, 10, 17].
Figure 2 is a fish diagram of the C12,13–(PO)8–SO4Na/brine/hexadecane system. According to the diagram, the C12,13–(PO)8–SO4Na 50B surfactant shows a vertical orientation, similar to what is typically observed for a high-purity single-component conventional anionic surfactant system. Furthermore, the fish diagram also offers two key surfactant concentrations: the critical microemulsion concentration (CμC) and the lowest surfactant concentration where a single phase (or Winsor type IV) microemulsion is formed, sometimes called the X point [2, 3, 21]. This information is highly valuable for developing a cost-effective formulation. The CμC indicates the minimum surfactant concentration required to form the “first drop” of middle-phase microemulsion and attain the lowest IFT value [6, 21], while the lowest surfactant concentration where a single phase (or Winsor type IV) microemulsion is formed guides formulators for the solubilization power of surfactant formulation [2–4]. From Fig. 2, the CμC value and the lowest point forming Winsor type IV of the C12,13–(PO)8–SO4Na surfactant are reported as 0.37 mM (0.03 wt%) and 0.20 M (13 wt%), respectively.
Approaches to Increase the Kinetics of Coalescence with Systems Containing Extended-Surfactant-Based Microemulsions
Upon shaking the optimum system samples, the three equilibrated phases (excess oil, middle phase microemulsion, and excess water) are mixed and emulsified into a single pseudophase (macroemulsion). Since this emulsion is thermodynamically unstable, it starts to coalesce and returns to the equilibrium three-phase system as time elapses. Acosta et al. [17] used this correlation to track the kinetics of middle-phase microemulsion equilibration by measuring the turbidity of the emulsion as a function of time during coalescence (the coalescence rate). In this work, we propose that, to enhance the rate of coalescence, the rigidity of extended surfactant membrane must be reduced. Literature reports indicate that adding certain additives, such as short-chain alcohol, linkers, and branch-tailed co-surfactants, helps reduce the film rigidity (E r) [2, 4, 14, 17, 22, 30–32]. However, whether alcohols, linkers or co-surfactants are used, they must be selected with caution since our objective is to expedite the rate of coalescence without jeopardizing the IFT and solubilization properties of microemulsion systems.
In this section, linkers and co-surfactant molecules were utilized to modify the rigidity of the extended surfactant membrane. The four extended surfactant systems evaluated were the C12,13–(PO)4–SO4Na-alone, the C12,13–(PO)4–SO4Na/SMDNS/dodecanol, the C12,13–(PO)4–SO4Na–/SMDNS/oleyl alcohol, and the C12,13–(PO)4–SO4Na/SDOSS systems, all at 27 °C. The extended surfactant C12,13–(PO)4–SO4Na was used at constant concentration of 0.07 M unless otherwise stated. The amount of hydrophilic (SMDNS) and lipophilic linkers (dodecanol or oleyl alcohol) added was kept constant at 0.03 M. In the case of the C12,13–(PO)4–SO4Na/SDOSS system, the total surfactant concentration was fixed at 0.07 M with a 50/50 C12,13–(PO)4–SO4Na-to-SDOSS ratio.
Table 4 summarizes the ξ * and E r values of the extended surfactant and mixtures at the optimum condition. Compared with the C12,13–(PO)4–SO4Na-alone system, the ξ * and E r values decrease considerably with the addition of combined linkers and the co-surfactant SDOSS, while the IFT values remain relatively constant. This result highlights that the extended surfactant is a key component in producing the low IFT property. As mentioned above, the presence of additives such as linkers or co-surfactants produces two different effects. A reduction in the ξ * value indicates a decrease in solubilization, while a decrease in E r value suggests a less rigid surfactant membrane, which corresponds to a faster coalescence rate (also a faster equilibration time).
Figure 3 plots inverse turbidity as a function of time during the coalescence of the emulsified system. The results show that the turbidity curve of the C12,13–(PO)4–SO4Na surfactant alone remains relatively constant over the entire study period (total of 1 h), while the turbidity of the C12,13–(PO)4–SO4Na mixtures drops quickly (1/τ increases rapidly) and reaches a plateau within 10 min. As noted in Eq. 2, the turbidity curve is correlated to the coalescence kinetic constant (k c). Higher k c values indicate a faster coalescence of macroemulsions and quick equilibration time of microemulsion systems.
The inverse turbidity curves during coalescence at 27 °C of the samples containing the 0.07 M C12,13–(PO)4–SO4Na-alone, the 0.07 M C12,13–(PO)4–SO4Na/0.03 M SMDNS/0.03 M dodecanol, the 0.07 M C12,13–(PO)4–SO4Na/0.03 M SMDNS/0.03 M oleyl alcohol, and the 0.035 M C12,13–(PO)4–SO4Na/0.035 M SDOSS mixtures. Turbidity samples resulted from shaking the optimum middle-phase hexadecane microemulsions
Among formulations, the steepest slope (highest k c value) is observed with the C12,13–(PO)4–SO4Na/SMDNS/dodecanol system, while the smallest slope (smallest k c value) is attained with the C12,13–(PO)4–SO4Na-alone system; recall from before that higher k c values mean greater rate of coalescence and faster approach to the equilibrium middle-phase microemulsion system. The calculated k c values of studied systems are listed in Table 4. The C12,13–(PO)4–SO4Na/SMDNS/dodecanol system has a k c value three orders of magnitude higher than the C12,13–(PO)4–SO4Na-alone system (1.2E+00 versus 4.0E-03 cm/s, respectively), indicating a faster coalescence rate while still providing good solubilization capacity and low IFT properties. Another added benefit of the linker modified formulation is that the magnitude of solubilization power, IFT reduction, and the kinetics of coalescence can be adjusted/optimized by simply changing the type of linker molecules, as seen in the example of the C12,13–(PO)4–SO4Na–/SDMNS/oleyl alcohol formulation.
In the case of the C12,13–(PO)4–SO4Na/SDOSS system, the inverse turbidity plot shows a two-step increase in the 1/τ value (Fig. 3), which is different from prior systems. In previous work, SDOSS was found to be much more hydrophobic than the extended surfactants [7]. By considering the difference in their hydrophilic–lipophilic nature, it is suggested that the system with C12,13–(PO)4–SO4Na and SDOSS may produce two different types of surfactant membranes or may transition over time in the composition at the interface, with the high ratio of SDOSS to C12,13–(PO)4–SO4Na exhibiting different properties than the high fraction of C12,13–(PO)4–SO4Na at the interface. In Table 4, two distinctive k c values are calculated from the individual slopes in the C12,13–(PO)4–SO4Na/SDOSS system. In addition, although the bicontinuous middle phase (MP) might tend to be the continuous phase in macroemulsion systems, given the disproportionate volumes between the MP and excess oil and water phases, this MP phase could also be a dispersed phase in some cases. Therefore, the case of the Winsor III microemulsion possibly consists of the coalescence of multiple emulsion systems (e.g., O/W, W/O, O/MP, and W/MP), and the coalescence rates are slowest for systems containing the MP as a continuous phase [15, 33]. Regarding this fact, it is possible that the C12,13–(PO)4–SO4Na/SDOSS system may produce multiple emulsions. Further study is required to gain improved understanding of this mixture behavior. Nonetheless, from a practical perspective, the equilibration time is ≤10 min as compared with 2 weeks for the extended surfactant alone, thereby again illustrating the ability of this co-surfactant to improve the system’s kinetics.
References
Winsor PA (1948) Solvent properties of amphiphilic compounds. Trans Faradays Soc 44:736
Bourrel M, Schecter R (1988) Microemulsions and related systems. Marcel Dekker, New York
Salager JL (1999) Microemulsions. In: Broze G (ed) Handbook of detergents: part A: properties. Marcel Dekker, New York, pp 253–302
Salager JL, Anton RE, Sabatini DA, Harwell JH, Acosta EJ, Tolosa LI (2005) Enhancing solubilization in microemulsions—state of the art and current trends. J Surfactant Deterg 8(1):3–21
Miñana-Pérez M, Graciaa A, Lachaise J, Salager JL (1995) Solubilization of polar oils with extended surfactants. Colloid Surf A 100:217–224
Witthayapanyanon A, Acosta EJ, Harwell JH, Sabatini DA (2006) Formulation of ultralow interfacial tension systems using extended surfactants. J Surfactant Deterg 9(4):331–339
Witthayapanyanon A, Harwell JH, Sabatini DA (2008) Hydrophilic-lipophilic deviation (HLD) method for characterizing conventional and extended surfactants. J Colloid Interface Sci 325:259–266
Yanatatsaneejit U, Rangsunvigit P, Scamehorn JF, Chavadej S (2005) Removal by froth flotation under low interfacial tension conditions I: foam characteristics, coalescence time, and equilibrium time. Sep Sci Technol 40:1537
Childs J, Acosta EJ, Scamehorn JF, Sabatini DA (2005) Surfactant-enhanced treatment of oil-based drilling cuttings. J Energy Res Technol 127:153–162
Do L, Witthayapanyanon A, Harwell JH, Sabatini DA (2009) Environmentally friendly vegetable oil microemulsions using extended-surfactants and linkers. J Surfactant Deterg 12:91–99
Salager JL, Scorzza C, Forgiarini A, Arandia MA, Pietrangeli G, Manchego L, Vejar F (2008) Amphiphilic mixtures versus surfactant structures with smooth polarity transition across interface to improve solubilization performance. 7th World Surfactant Congress, Paris, France
Microemulsions http://www.chm.bris.ac.uk/eastoe/surf_Chem/3%20Microemulsions.pdf
Helfrich WZ (1973) Elastic properties of lipid bilayers: theory and possible experiments. Naturforsch 28C:693
De Gennes PG, Taupin C (1982) Microemulsions and the flexibility of oil/water interfaces. J Phys Chem 86:2294–2304
Binks BP (1998) Emulsions-recent advances in understanding. In: Binks BP (ed) Modern aspects of emulsion science. Royal Society of Chemistry, Great Britain, pp 1–55
Carroll BJ (1981) The kinetics of solubilization of nonpolar oils by nonionic surfactant solutions. J Colloid Interface Sci 79:126–135
Acosta EJ, Le MA, Harwell JH, Sabatini DA (2003) Coalescence and solubilization kinetics in linker-modified microemulsions and related systems. Langmuir 19:566–574
Acosta EJ, Szekeres E, Harwell JH, Sabatini DA (2003) Net-average curvature model for solubilization and supersolubilization in surfactant microemulsions. Langmuir 19:186–195
Acosta E (2004) Modeling and formulation of microemulsions: the net-average curvature model and the combined linker effect. Dissertation, University of Oklahoma
Acosta E, Mai PD, Harwell JH, Sabatini DA (2003) Linker-modified microemulsions for a variety of oils and surfactants. J Surfactant Deterg 6(4):353–363
Acosta EJ, Harwell JH, Sabatini DA (2004) Self-assembly in linker-modified microemulsions. J Colloid Interface Sci 274:652–664
Sabatini DA, Acosta E, Harwell JH (2003) Linker molecules in surfactant mixtures. Curr Opin Colloid Interface Sci 8:316–326
Langevin D, Meunier J (1994) Interfacial tension: theory and experiment. In: Gelbart WM, Ben-Shaul A, Roux D (eds) Micelles, membranes, microemulsions and monolayers. Springer-Verlag, New York
Miñana-Pérez M, Graciaa A, Lachaise J, Salager JL (1995) Solubilization of polar oils in microemulsion systems. Progr Colloid Polym Sci 98:177–179
Huh C (1979) Interfacial tension and solubilizing ability of a microemulsion phase that coexists with oil and brine. J Colloid Interface Sci 71:409
Huh C (1983) Equilibrium of microemulsion that coexist with the oil and brine. Soc Pet Eng J 23:829
Tolosa LI, Forgiarini A, Moreno P, Salager JL (2006) Combined effects of formulation and stirring on emulsion drop size in the vicinity of three-phase behavior of surfactant-oil-water systems. Ind Eng Chem Res 45:3810–3814
Wasan DT, McNamara JJ, Shaw SM, Sampath K, Aderangi N (1979) The role of coalescence phenomena and interfacial rheological properties in enhanced oil recovery: an overview. J Rheol 23(2):181–207
Flumerfelt RW, Catalano AB, Tong CH (1981) The coalescence characteristics of low tension oil-water-surfactant systems. Proceeding of Symposium on Surface Phenomena for Enhanced Oil recovery, pp 571
Kazlov MM, Helfrich W (1992) Effects of a cosurfactant on the stretching and bending elasticities of a surfactant monolayer. Langmuir 8:2792–2797
Gradzielski M (1998) Effect of the cosurfactant structure on the bending elasticity in nonionic oil-in-water microemulsions. Langmuir 14:6037–6044
Meglio JM, Dvolaitzky M, Taupin C (1985) Determination of the rigidity constant of the amphiphilic film in birefringent microemulsions: the role of the cosurfactant. J Phys Chem 89:871–874
Hazlett RD, Schecter RS (1988) Stability of macroemulsions. Colloids Surf 29:53–69
Rosen MJ (2004) Surfactants and interfacial phenomena. Wiley, New Jersey
Acknowledgments
Funding for this research was provided by industrial sponsors of the Institute for Applied Surfactant Research at University of Oklahoma: Akzo Nobel, Clorox, Conoco-Philips, Church and Dwight, Dow Chemical, Ecolab, Halliburton, Huntsman, Oxiteno, Procter & Gamble, Sasol North America, S.C. Johnson & Son, and Shell Chemical. The authors would like to express their gratitude to Geoff Russell and Victoria Stolarski, Sasol North America (Lake Charles, LA) for providing extended surfactant samples. Final thanks go to Prof. Jean-Louis Salager and Dr. Antonio Cardenas for useful discussions and literature suggestion.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Witthayapanyanon, A., Phan, T.T., Heitmann, T.C. et al. Interfacial Properties of Extended-Surfactant-Based Microemulsions and Related Macroemulsions. J Surfact Deterg 13, 127–134 (2010). https://doi.org/10.1007/s11743-009-1151-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1151-5