Abstract
As the symbol of gemini surfactants, the spacer group connects two surfactant monomers and plays an important role in the self-assembling of gemini surfactants. Recent attention has been paid to the effects of spacer nature and length in aqueous systems. In this study, the aggregation behaviours of quaternary ammonium gemini surfactants 12-s-12 with different spacer length (s = 2, 3, 4, 5, 6, 8, 10, 12) have been investigated in the protic ionic liquid EAN. The micellization behaviour was characterized by surface tension measurement. With the help of small-angle X-ray scattering (SAXS), the size and shape of aggregates were verified. The spacer length effect could be reflected by the changes in the micellization and phase behaviours of 12-s-12/EAN systems. The critical packing parameter of gemini molecules would decrease monotonically with the spacer length, which is supported by SAXS results. Due to the charge screening effect of EAN, the aggregation behaviours of 12-s-12 would be mainly influenced by spacer length. Our results would be the important supplement to the spacer length effect of gemini surfactants in the nonaqueous systems.
Graphical abstract
With the increase of spacer chain length, the reverse hexagonal lyotropic liquid crystal phase would transform into the micellar phase.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The gemini surfactant is composed of two single-chain surfactants connected by a spacer at or near the headgroups [1, 2]. The structure could be modulated in the tail, spacer, headgroup and counterion. The spacer is not only the symbol to differentiate the gemini and single-chain surfactants, but also plays an important role in the aggregation behaviour of the gemini surfactant [3]. The spacer could avoid the separation of headgroups, especially in ionic surfactants, overcoming the electrostatic repulsive interactions. The connected monomers would endow the gemini surfactants with stronger hydrophobic interactions and then better physiochemical properties. Due to its unique molecular structure, the gemini surfactant displays fabulous physiochemical properties, such as low critical micellization concentration (CMC), high surface activity, low Kraft temperature, and rich aggregation behaviours [2, 4].
The spacer length determines the distance between headgroups and has influence on the geometry of the gemini surfactant, resulting in the formation of aggregates of different curvature. In the classical researches of Zana’s group, the behaviours of gemini surfactants 12-s-12 with the methylene spacer were studied at the air/water interface and in the solutions [5,6,7,8]. With the increase of the spacer length, the CMC increases to maximum at s = 6 and then decreases [7]. With the help of Cryo-TEM, wormlike micelles are observed in the 12-2-12 and 12-3-12 solutions, spherical micelles in the 12-4-12, 12-8-12 and 12-12-12 solutions while lamellar structure in 12-16-12 and 12-20-12 solutions [8]. From then on, numerous studies have been carried out [9,10,11,12,13,14,15,16,17,18,19,20]. The structure of aggregates varying from spherical to rodlike micelles formed by 12-s-12 has been confirmed by the small-angle neutron scattering characterization [15]. The spacer length effect has also been explored in the gemini surfactant with the pyrrolidinium headgroup, the CMC of which also reaches a maximum around s = 4 [16]. The gemini surfactants 18-s-18(Et), composed of ethylammonium headgroups and octadecyl alkyl chains, would self-assemble into cylinder micelles with s = 4 and into vesicles with s = 6, 8, 10 [19]. Zheng’s group constructed the gemini supra-amphiphiles with different spacer length [18]. The morphologies of aggregates could be tailored form vesicles to planar lamellar structure with the increase of spacer length.
Except for the spacer length, the nature of the spacer has also an effect on the aggregation behaviours of gemini surfactants. Since the emergence of gemini surfactants, the methylene spacer, which is hydrophobic and flexible, has been well studied [3]. When the spacer is decorated with the hydroxyl groups, the gemini surfactants would behave stronger aggregation ability due to the contribution of hydrogen bonding [21,22,23]. The cationic and anionic gemini surfactants with hydrophilic oligo(oxyetheylene) spacer chains were also synthesized [24,25,26]. The aggregation behaviours of gemini surfactants with partially fluorinated spacer were studied by Wang’s group [27]. The surfactants with spacer of low CF2 to CH2 ratio disfavour aggregation while those with spacer of high ratio favour aggregation. Despite of the flexible spacer, more attention has been paid to the rigid spacer in recent years. Zhao’s group has studied the aggregation behaviours of gemini surfactants with rigid spacer chains. The spacer with the azobenzene group would endow the surfactant to form the photo responsive fluid [28]. With the two phenyl spacer, the large network aggregate and wormlike micelle are formed [29]. Gemini surfactants with other interesting spacer chains, such as phenyl group [30, 31], adamantane group [32], peptides [33, 34], have also been synthesized.
Given the researches on the spacer effect of gemini surfactants in aqueous solutions, it is a wonder how the spacer affects the aggregation behaviours in nonaquesous medium. Zhao’s group has reported such effect of 12-s-12 in cyclohexane with sodium hexanoate or sodium laurate [35]. With the increase of spacer length, the reverse micellar phase transforms to the reverse hexagonal phase and reverse vesicles. We have also carried out studies of spacer length and nature in ionic liquids [36, 37]. In ethanolammonium nitrate (EOAN), the aggregates formed at higher concentrations would change from the less curved lamellar phase to hexagonal phase and then to the highly curved micelles with the increase of spacer length [36]. The hydroxyl group in the spacer would endow the gemini surfactant 12-3OH-12 with a smaller critical micellization concentration and larger critical packing parameter [37].
As ionic liquids consist of ions, the charge screening effect in them is prominent [38, 39]. The spacer length effect could not be influenced by the electrostatic interactions of ionic headgroups of gemini surfactants. Moreover, some interesting aggregation behaviours would be observed in the “weak” self-assembling media. As the continuation of our researches, the aggregation behaviours of classical gemini surfactants 12-s-12 in protic ionic liquid ethylammonium nitrate (EAN) have been studied, in which the spacer length effect would be explored in wide surfactant concentrations, from the air/EAN interface to the aggregates in the bulk. Our results would provide further information on the relationship between structure and aggregation behaviours of gemini surfactants in ionic liquids.
Experimental section
Materials
The Gemini surfactants 12-s-12 and ionic liquid EAN were prepared according to our previous reports [36, 40]. 12-2-12 was synthesized by N, N, N′, N′-tetramethylethylenediamine and excess 1-bromododecane (3 M equiv.) refluxing in ethanol for 48 h while 12-s-12 (s ≥ 3) by α, ω-dibromoalkane, and excess N, N-dimethyldodecylamine (3 M equiv.). The products were recrystallized by the ethyl acetate/ethanol mixture for at least three times and then dried in the vacuum oven. Their purity was verified by 1H NMR measurements (Supporting Information).
Sample preparation and phase diagram mapping
All samples were prepared by mixing the 12-s-12 and EAN at designed compositions (in weight percentage (%), thereinafter). These mixtures were homogenized by repeatedly mixing and centrifugation. Then they were equilibrated for at least 1 month before further characterizations. The composition interval was first selected as 5% for a rough phase mapping and then 1% for the determination of the phase boundaries. And the temperature interval was first selected as 10 °C and then 2 °C.
Characterizations
Small-angle X-ray scattering
The X-ray scattering measurements were performed by a SAXSpace small-angle X-ray scattering instrument (Anton Paar, Austria, Cu-Kα, λ = 0.154 nm), equipped with a Kratky block collimation system and a Mythen detector. The X-ray generator was operated at 40 kV and 50 mA. A standard temperature control unit (TCS 150/300) connected with the SAXSpace was used to control the temperature at a desired value. The LLC samples were transferred to the paste cell while solution samples to the standard quartz capillary with a diameter of 1 mm. The scattering curves of solvents filled in the same capillary were recorded as the background. The solution data were corrected for background scattering of the capillary and solvent. The desmearing process was done with SAXSquant software while the model fitting of the Small-angle X-ray scattering (SAXS) curves with SASfit software (version 0.94.7). The details of SAXS analysis in micelles and LLCs are shown in Supporting Information.
Polarized optical microscopy
Photographs of samples were taken by a DYP-990 polarized optical microscope (POM) (Dianying, Shanghai China) with a DYE-400 thermal stage (± 1 °C) and a CMOS camera (DYS-1000).
Surface tension measurement
A tensiometer QBZY-2 (Fangrui, Shanghai China) was used with a Wilhelmy platinum plate to measure surface tension at 25 °C. The plate was cleaned well and heated briefly with an alcohol burner until it glowed red before each measurement. All measurements were repeated at least twice until the values were reproducible.
Results and discussion
These gemini surfactants (12-s-12) have the same headgroups, alkyl chains, and counterions. The only difference lies in the spacer length (2 ≤ s ≤ 12). Hence, the difference in the aggregation behaviours must result from the spacer length.
Micellization of 12-s-12 in EAN
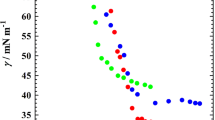
To study the adsorption behaviour at the air/EAN and micellization behaviour in EAN, the surface tension measurements were carried out at 25 °C. As for the high Krafft point of 12-6-12 in EAN, such measurement was not involved. The surface tension results of 12-s-12 (s = 2, 3, 4, 5, 8, 10, 12) are shown in Fig. 1. The surface tension (γ) decreases with the increase of surfactant concentration (C) and then kept almost constant, which are typical of surfactants. The critical micellization concentration (CMC) of 12-s-12 could be obtained from the breakpoint in these curves. It is noticeable the CMC values are around 10 mM, much larger than those in water [5]. This is due the weaker solvophobic interactions in EAN. Another important thing is that the CMC values of 12-s-12 keep decreasing with the increase of spacer length (Fig. 2). As has been reported in the m-s-m type gemini surfactants with various headgroups, the CMC values would generally reach a maximum around the moderate spacer length (s = 5 ~ 6) [2, 5, 16]. The electrostatic interaction between headgroups may hinder the micelle formation. Due to the charge screening effect in EAN, the spacer could provide extra solvophobic interaction along with the alkyl chains, promoting the formation of micelles. Thus, 12-s-12 with the longer spacer has the smaller CMC.
The surface parameters such as the effectiveness of γ reduction (ΠCMC), the surface excess of surfactant (Γmax), the minimum area per surfactant molecule (Amin) adsorbed at the air/IL interface, and the standard Gibbs free energy of micellization (ΔGm) could be calculated according to the equations (S1-S4) and listed in Table 1. The surface activity of surfactants could be reflected by ΠCMC. It is suggested that gemini surfactants with moderate spacer have better surface since ΠCMC arrives at a maximum. The ΔGm becomes larger with the increase of spacer length, for stronger solvophobic interactions of gemini surfactants. The most impressing parameter is Amin. As shown in Fig. 2, the Amin slightly varies from 0.806 to 1.06 nm2 in EAN and goes through a minimum at s = 4. As reported in 12-s-12/water systems, the Amin reaches a maximum around s = 10 ~ 12 [2, 7]. It is suggested that the spacer would take the folded conformation, which is caused by the hydrophobic interactions between spacer and alkyl chains, while electrostatic interaction between headgroups prevents the molecules from getting close. In EAN, the electrostatic interaction could be neglected. The spacer group would be more flexible with the increase of CH2 groups. Thus, the Amin decreases initially as the spacer takes the folded conformation and then increases for longer spacer takes larger area.
General phase behaviours
The phase behaviours of 12-s-12 in EAN could divided into three categories according to the spacer length.
Short spacer
The aggregation behaviours of 12-2-12 and 12-3-12 in EAN have been reported in our previous studies [37, 40]. For better understanding of spacer length effect, the phase behaviours are described briefly here with corresponding phase diagrams shown in Fig. 3a and b. The isotropic micellar phases (L1) are observed in a wide concentration range compared to those in aqueous systems, resulting from the relatively weaker solvophobic interactions in EAN [41]. At the high surfactant concentrations, the reverse hexagonal LLC phases (H2) are formed instead of the normal hexagonal (H1) or lamellar (Lα) lyotropic liquid crystal (LLC) phases in water.
Medium spacer
For the medium spacer (s = 4, 5, 6, 8), the phase behaviours of 12-s-12 have some changes at low concentrations. As shown in Fig. 3c–f, the two coexisting phases (TPs), which is also regarded as coacervation, appear before the L1 and H2 phases [42]. The two coexisting phases have been observed in the 12-3OH-12/EAN and 12-s-12/EOAN systems [36, 37]. It was suggested that the interactions between surfactants and charge screening effect of ILs would be necessary for such phase separation. In the 12-s-12/EAN systems, the phase separation takes place just above CMC as observed during the surface tension measurements. The interface of the up and low phases could be observed by naked eyes. With the increase of the concentration, the volume percentage of the upper phase becomes larger. Finally, two phases transform into one phase. Both of two phases are consisting of micelles as confirmed by the SAXS, freeze fracture TEM, and other characterizations. The only difference is that the number of micelles in the up phase is much larger than that in the low phase. Such an interesting phenomenon would be reported in another work.
Long spacer
When the spacer length reaches 10 and 12, the phase behaviours become much simpler with only micelles formed in the whole concentration range. This is similar with what is observed in water and EOAN [6, 36]. The coexisting two phases appear at low concentrations and transform into the micellar phases at high concentrations. No trace of LLC is detected even at the 90% surfactant concentration.
Phase behaviours of 12-s-12 in EAN
We would take the 12-4-12/EAN system as an example to provide the detailed information of phase behaviours. The two coexisting phases disappeared and transformed into the micellar phase above 40% 12-4-12 concentration. Such micellar solutions are clear, transparent, and viscous, which are dark under POM, corresponding to the isotropic phases. The SAXS characterization was carried out to determine the size and shape of the micelles. As shown in Fig. 4, the strong correlation peak at the medium q range illustrates strong interactions between aggregates. The ellipsoid form factor and hard sphere structure factor were used to resolve the SAXS results, with parameters shown in Table S1. The micelle is prolate, with the equal semi-axis (a) 0.99 nm and the principle semi-axis (b) 1.53 nm. Such parameters correspond to the core of micelles, for the similar scattering length density of surfactant headgoup and EAN. The distance between micelles (3.16 nm) could be evaluated by twice of the hard sphere repulsion radius RHS. Such small distance corresponds to the densely packing of micelles at a rather high surfactant concentration.
The insert ellipsoid image illustrates the shape of micelles. Open circles for experimental data and lines for fitting results. a, the equal semi-axis; b, the principle semi-axis; ε, the axis ratio.
Compared to the L1 phases, the viscoelasticity of the LLC phases increases dramatically, which could hold their weight in the inverted vial test. The POM images in Fig. 5a–c show fan-like texture, indicating the formation of the LLC hexagonal phase. The texture becomes more and more regular with the increase of concentration. The SAXS curves show two obvious peaks, the q values of which follow a 1:2 ratio, corresponding to 1st and 3rd peaks of the hexagonal phases. The 2nd peak could be observed in the SAXS curve at the 80% 12-4-12 concentration. The certification of the H2 phase is discussed in the previous report, detailed structure information of which could be calculated from the SAXS results [40].
As shown in Table 2, the lattice parameter D, corresponding to the distance between two centres of neighbouring cylinders, increases with the 12-4-12 concentration. Such a trend is consistent with what has been observed in the H2 phases but contrary to those in the H1 phases [36, 40]. For other structure parameters, the core radius of the solvent (R2) decreases for the less solvent while the thickness of hydrocarbon domains (d2) increases since the alkyl chain intersects less. As the surfactant molecules pack more and more densely, the area per molecule at the solvophilic/solvophobic interface (S2) decreases.
As for the other gemini surfactants, the main difference in the phase behaviour lies in the domains of various phases. The L1 and H2 phases in these systems were also verified by POM and SAXS characterizations. The SAXS curves of micelles are shown in Fig. S1 with fitting results shown in Table S1. At such high concentrations, the micelles are small in size and prolate in shape. With the increase of spacer length, the micellar parameters keep similar. However, for long spacer (s = 10, 12), the micelle decreases in size and content.
As for the H2 phase, the structure parameters of the H2 phases are calculated and shown in Fig. 6 and Table S2. The comparison is done at different concentrations considering the solubility of 12-s-12 in EAN. Given the conditions at the 70% (Fig. 6a) and 75% (Fig. 6b) 12-s-12 concentrations, it could be concluded that the lattice parameter D goes through a minimum around s = 3 with s ranging from 2 to 8. As the lattice parameter is composed of the solvent core R2 and solvophobic thickness d2, the change of D is mainly caused by d2 while the R2 varies little with the spacer length for the certain solvent content. The initial increase in spacer length may favour the intersection of alkyl chains while further increase would also contribute to the solvophobic domain. Unlike Amin at the air/EAN interface, the most important parameter S2 monotonically increases with the spacer length, for the molecules would adopt much denser packing at such higher concentrations, which is in accordance with the condition of the H1 and Lα phases in water [2, 6]. This means the 12-s-12 molecules would occupy larger area at the solvophobic/solvophilic interface for the longer spacer length. In EAN, the electrostatic repulsion interactions between molecules are weakened and the spacer penetrates into the solvophobic region, leading to the slowly increasing S2.
Spacer length effect in EAN
For a better understanding of the spacer effect, the aggregation behaviour of the monomer of gemini surfactant, dodecyl trimethylammonium bromide (DTAB), was also investigated. The behaviour at the surface was characterized by the surface tension measurements with results shown in Fig. S3 and Table S3. The CMC of DTAB (164 mM) is one order larger than those of 12-s-12, for the weaker solvophobic interactions of single-chain surfactant. It is surprising to see that the Amin of DTAB (0.948 nm2) is comparable with those of 12-s-12, illustrating the densely packing of gemini surfactant. The phase diagram of DTAB in EAN is shown in Fig. S4. As reported by Evans and Varela, DTAB could self-assemble into micelles at low concentrations [43, 44]. In this system, there is no phase separation in the L1 phase. With the increase of DTAB concentration, the hexagonal LLC is formed, which could be confirmed by the fan-like texture in Fig. S5. This hexagonal phase could be indexed as the normal one (H1) for the single-chain surfactant would generally self-assemble into aggregates of positive curvature. The detailed parameter information of the H1 phase could be obtained from the SAXS characterization (Fig. S5). As shown in Table S4, the area at the interface of DTAB is smaller than that of 12-4-12. The aggregates formed by surfactants could be explained by the critical packing parameter (CPP) theory, which is defined as CPP = V/a0lc, where V is the solvophobic volume of surfactants, a0 is the effective headgroup area, and lc is the effective chain length of the surfactant in its molten state [45]. The V and lc could be calculated by the Tanford formula while a0 estimated by S. As the gemini surfactants are consisting of two surfactant monomers, the CPP values of 12-s-12 would be much larger than that of DTAB (Table S4), which accounts for the reverse LLC phase.
Based on the results above, it could be concluded that 12-s-12 would adopt a smaller CPP with the increase of the spacer length. It was also pointed by Boschkova that the CPP value of 12-12-12 is smaller than that of 12-3-12 [46]. Such influence on the phase behaviours is manifested by the expansion of the L1 phase and vanishment of the H2 phase. Actually, the CMC of 12-s-12 decreases with the spacer length. Even though 12-10-12 and 12-12-12 have stronger solvophobic interaction than the others, they could not form the H2 phase even at high concentrations. Such spacer length effect could also be reflected in the 12-8-12/EAN system. As shown in Fig. 3 and Table S5, the critical concentration of the H2 phase is around 65% for 12-s-12/EAN systems (s = 2 ~ 5) while increases to 72% for the 12-8-12/EAN system. It is even more difficult for 12-s-12 with long spacer to self-assemble into the H2 phase.
The phase behaviours of 12-s-12 in aqueous systems have been studied by Zana and Skoulios [4, 6]. As summarized in Table S5, with the spacer length smaller than 8, similar phase behaviours are observed: the L1, H1, and Lα phases are formed successively. When the spacer length increases to 10 or 12, the LLC phases disappear and only the L1 phase remains. Compared to aqueous system, both the micelle and LLC are formed at higher concentrations in EAN. What’s more, such critical concentrations vary less with spacer length in EAN. For instance, the formation of LLC starts around 15% in the 12-2-12/water system while around 40% in the 12-8-12/water system. The electrostatic interactions between headgroups in water are so strong, which makes the increase of headgroup area with spacer length more obvious. The CPP of gemini surfactants decreases with the spacer for the larger headgroup, leading to the expansion of the micellar phase. As reported in our previous research, 12-2-12 has a larger CPP in EAN than that in water, since the charge screening effect of EAN and its possible participation into solvophobic region [40]. These effects would relieve the decrease of CPP with spacer length in EAN, resulting in the smaller changes of phase behaviours. This means the CPP of 12-s-12 takes fewer changes with spacer length in EAN, suggesting that the spacer length effect would be smaller in EAN compared to that in water.
Conclusion
The spacer length effect of cationic gemini surfactants has been investigated in EAN. With the increase of spacer length, the CMC increases monotonically for the stronger solvophobic interaction of 12-s-12. Due to the charge screening effect of EAN, the spacer would take the folded conformation, leading to a minimum of Amin at the air/EAN interface. The spacer length also has influence on the phase behaviours of 12-s-12. When the spacer is short (s = 2, 3), the micellar phase and reverse hexagonal LLC phase are formed. With the increase of spacer length (4 ≤ s ≤ 8), the micellar phase separates into two coexisting micellar phases. Meanwhile, the reverse hexagonal LLC phase disappears when the spacer length reaches 10 and 12. The SAXS results have confirmed the CPP values of 12-s-12 decrease with the spacer length, resulting in the vanishment of the H2 phase. Compared to their monomer DTAB, the gemini surfactants have larger CPP values, leading to the formation of the reverse aggregates. Compared with the aqueous systems, the spacer length effect has smaller influence on phase behaviours in EAN, since the charge screening effect of EAN and its possible participation into solvophobic region would relieve the decrease of CPP with spacer length. The unique aggregation behaviours of gemini surfactants in EAN result from characteristics of surfactant structure and the solvent, which would shed light on the self-assembly in nonaqueous solutions.
References
Menger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Edit 39:1906–1920
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interf Sci 97:205–253
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci 248:203–220
In M, Zana R (2007) Phase behavior of gemini surfactants. J Dispers Sci Technol 28:143–154
Zana R, Benrraou M, Rueff R (1991) Alkanediyl-α, ω-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 7:1072–1075
Alami E, Levy H, Zana R, Skoulios A (1993) Alkanediyl-α,ω-bis(dimethylalky1ammoniumbromide) surfactants. 2. Structure of the lyotropic mesophases in the presence of water. Langmuir 9:940–944
Alami E, Beinert G, Marie P, Zana R (1993) Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 9:1465–1467
Danino D, Talmon Y, Zana R (1995) Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) Surfactants. 5. Aggregation and microstructure in aqueous solutions. Langmuir 11:1448–1456
Borse M, Sharma V, Aswal VK, Goyal PS, Devi S (2005) Effect of head group polarity and spacer chain length on the aggregation properties of gemini surfactants in an aquatic environment. J Colloid Interface Sci 284:282–288
Sikiric M, Primozic I, Talmon Y, Filipovic-Vincekovic N (2005) Effect of the spacer length on the association and adsorption behavior of dissymmetric gemini surfactants. J Colloid Interface Sci 281:473–481
Chen LF, Shang YZ, Liu HL, Hu Y (2006) Effect of the spacer group of cationic gemini surfactant on microemulsion phase behavior. J Colloid Interface Sci 301:644–650
Klijn JE, Stuart MCA, Scarzello M, Wagenaar A, Engberts JBFN (2006) pH-dependent phase behavior of carbohydrate-based gemini surfactants. Effect of the length of the hydrophobic spacer. J Phys Chem B 110:21694–21700
Ao MQ, Huang PP, Xu GY, Yang XD, Wang YJ (2009) Aggregation and thermodynamic properties of ionic liquid-type gemini imidazolium surfactants with different spacer length. Colloid Polym Sci 287:395–402
Yang JP, Xie JY, Chen GM, Chen XC (2009) Surface, interfacial and aggregation properties of sulfonic acid-containing gemini surfactants with different spacer lengths. Langmuir 25:6100–6105
Chavda S, Kuperkar K, Bahadur P (2011) Formation and growth of gemini surfactant (12-s-12) micelles as a modulate by spacers: a thermodynamic and small-angle neutron scattering (SANS) study. J Chem Eng Data 56:2647–2654
Cai B, Dong JF, Cheng L, Jiang Z, Yang Y, Li XF (2013) Adsorption and micellization of gemini surfactants with pyrrolidinium head groups: effect of the spacer length. Soft Matter 9:7637–7646
Sakai K, Nomura K, Shrestha RG, Endo T, Sakamoto K, Sakai H, Abe M (2014) Effects of spacer chain length of amino acid-based gemini surfactants on wormlike micelle formation. J Oleo Sci 63:249–255
Shi LJ, Sun PP, Zheng LQ (2016) Controllable hierarchical self-assembly of gemini supra-amphiphiles: the effect of spacer length. Soft Matter 12:8682–8689
Woch J, Ilowska J, Hordyjewicz-Baran Z, Arabasz S, Kaczmarczyk B, Grabowski R, Libera M, Dworak A, Trzebicka B (2018) Aqueous solution behaviour and solubilisation properties of octadecyl cationic gemini surfactants and their comparison with their amide gemini analogues. Soft Matter 14:754–764
Pal N, Samanta K, Mandal A (2019) A novel family of non-ionic gemini surfactants derived from sunflower oil: synthesis, characterization and physicochemical evaluation. J Mol Liq 275:638–653
Pei XM, Zhao JX, Ye YZ, You Y, Wei XL (2011) Wormlike micelles and gels reinforced by hydrogen bonding in aqueous cationic gemini surfactant systems. Soft Matter 7:2953–2960
Pei XM, Zhang Q, Liu Z, Song BL, Li R, Zhao JX, Cui ZG (2016) Surface dilatational properties of gemini surfactants containing multiple hydroxyl groups. Colloid Polym Sci 294:1405–1412
Tiwari AK, Sonu M, Sowmiya SK (2012) Saha, Micellization behavior of gemini surfactants with hydroxyl substituted spacers in water and water-organic solvent mixed media: the spacer effect. J Mol Liq 167:18–27
Dreja M, Gramberg S, Tieke B (1998) Cationic amphitropic gemini surfactants with hydrophilic oligo(oxyethylene) spacer chains. Chem Commun 13:1371–1372
Liu XP, Feng J, Zhang L, Gong QT, Zhao S, Yu JY (2010) Synthesis and properties of a novel class of anionic gemini surfactants with polyoxyethylene spacers. Colloids Surf A Physicochem Eng Asp 362:39–46
Bhadani A, Endo T, Sakai K, Sakai H, Abe M (2014) Synthesis and dilute aqueous solution properties of ester functionalized cationic gemini surfactants having different ethylene oxide units as spacer. Colloid Polym Sci 292:1685–1692
Li YJ, Li PX, Dong CC, Wang XY, Wang YL, Yan HK, Thomas RK (2006) Aggregation properties of cationic gemini surfactants with partially fluorinated spacers in aqueous solution. Langmuir 22:42–45
Song BL, Hu YF, Zhao JX (2009) A single-component photo-responsive fluid based on a gemini surfactant with an azobenzene spacer. J Colloid Interface Sci 333:820–822
Xie DH, Zhao JX (2013) Unique aggregation behavior of a carboxylate gemini surfactant with a long rigid spacer in aqueous solution. Langmuir 29:545–553
Wang LY, Zhang Y, Ding LM, Liu J, Zhao B, Deng QG, Yan T (2015) Synthesis and physiochemical properties of novel gemini surfactants with phenyl-1,4-bis(carbamoylmethyl) spacer. RSC Adv 5:74764–74773
Zhu DY, Cheng F, Chen Y, Jiang SC (2012) Preparation, characterization and properties of anionic gemini surfactants with long rigid or semi-rigid spacers. Colloids Surf A Physicochem Eng Asp 397:1–7
Fu SQ, Guo JW, Zhong X, Yang Z, Lai XF (2016) Synthesis, physiochemical property and antibacterial activity of gemini quaternary ammonium salts with a rigid spacer. RSC Adv 6:16507–16515
Qi RL, Liu J, Zhang N, Ji XL, Han YC, Wang YL (2019) Assembly and evolution of gemini-type peptide amphiphile with a di-lysine spacer. Langmuir 35:6154–6160
Wang MN, Han YC, Qiao FL, Wang YL (2015) Aggregation behavior of a gemini surfactant with a tripeptide spacer. Soft Matter 11:1517–1524
Deng SL, Zhao J (2018) Self-assembly of cationic gemini surfactants, alkanediyl-bis-(dimethyldodecyl-ammonium bromide), in cyclohexane: effects of spacer length on their association into reverse lyotropic liquid crystalline or reverse vesicles. Soft Matter 14:734–741
Li QT, Yao MH, Yue X, Chen X (2017) Effects of a spacer on the phase behavior of gemini surfactants in ethanolammonium nitrate. Langmuir 33:4328–4336
Li QT, Wang XD, Yue X, Chen X (2015) Unique phase behaviors in the gemini surfactant/EAN binary system: the role of the hydroxyl group. Langmuir 31:13511–13518
Greaves TL, Drummond CJ (2008) Ionic liquids as amphiphile self-assembly media. Chem Soc Rev 37:1709–1726
Greaves TL, Drummond CJ (2008) Protic ionic liquids: properties and applications. Chem Rev 108:206–237
Wang XD, Chen X, Zhao YR, Yue X, Li QH, Li ZH (2012) Nonaqueous lyotropic liquid-crystalline phases formed by gemini surfactants in a protic ionic liquid. Langmuir 28:2476–2484
Greaves TL, Weerawardena A, Fong C, Drummond CJ (2007) Many protic ionic liquids mediate hydrocarbon-solvent interactions and promote amphiphile self-assembly. Langmuir 23:402–404
Wang MN, Wang YL (2014) Development of surfactant coacervation in aqueous solution. Soft Matter 10:7909–7919
Evans DF, Yamauchi A, Roman R, Casassa EZ (1982) Micelle formation in ethylammonium nitrate, a low-melting fused salt. J Colloid Interface Sci 88:89–96
Fernandez-Castro B, Mendez-Morales T, Carrete J, Fazer E, Cabeza O, Rodriguez JR, Turmine M, Varela LM (2011) Surfactant self-assembly nanostructures in protic ionic liquids. J Phys Chem B 115:8145–8154
Israelachvili JN (1992) Intermolecular and surface forces. Academic Press, San Diego
Boschkova K, Feiler A, Kronberg B, Stålgren JJR (2002) Adsorption and frictional properties of gemini surfactants at solid surfaces. Langmuir 18:7930–7935
Funding
The authors received financial supports from the National Natural Science Foundation of China (21703175, 21673129, and 51905158), Xuzhou University of Technology, and the Project National United Engineering Laboratory for Advanced Bearing Tribology, Henan University of Science and Technology (No. 201812).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2992 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Wang, X., Zhuang, W. et al. Spacer length effect on the aggregation behaviours of gemini surfactants in EAN. Colloid Polym Sci 299, 685–692 (2021). https://doi.org/10.1007/s00396-020-04795-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-020-04795-1