Abstract
A novel series of ferrocenyl surfactants was synthesized by the reaction of ferrocene disulfonic acid with different primary and tertiary fatty amines to produce the corresponding ammonium salts Fc[SO3 − +NH3(CH2) n CH3]2, where n = 9, 11, or 15 and Fc[SO − +3 NH(CH3)2(CH2) n CH3]2, where n = 7 or 11, respectively, and where Fc = ferrocene. Chemical structures were confirmed by microelemental analysis, FTIR, and 1H NMR spectroscopy. The critical micelle concentration of each prepared surfactant was determined using equilibrium surface tension. Furthermore, air/water interface parameters including effectiveness (π CMC), efficiency (Pc20), maximum surface excess (Гmax), and minimum surface area (A min) were determined at 30, 40, and 50 °C. Thermodynamic parameters (ΔG°, ΔS°, and ΔH°) for both micellization and adsorption processes were recorded. The new synthesized surfactants were screened as antimicrobial agents against different bacterial and fungal organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In designing a metal-containing surfactant system, the metal may be directly bound to the head group [1–3] or to the counterion of the cationic surfactant [4–6].
In addition, surfactants containing ferrocene (Fc) moiety have been of considerable interest for several years [7]. Fc(CH2)11SO3 − can be reversibly oxidized to the zwitterionic state Fc+(CH2)11SO3 −. The surface and bulk properties of Fc(CH2)11SO3 − and Fc+(CH2)11SO3 − in aqueous solutions have been reported [8].
Recent studies have demonstrated that Fc(CH2)11N+(CH3)3Br− can be studied with electrochemical methods to form the basis of an experimental system that permits spatial and temporal control over the surface tensions of aqueous solutions [9].
This paper reports the synthesis, surface, and antimicrobial properties of novel compounds of ferrocene-based cationic surfactants.
Experimental
Materials
-
1.
Hexadecyl amine and dodecyl amine were obtained from Acros Organics and decyl amine from MP Biomedicals.
-
2.
Ferrocene, N,N-dimethyloctyl amine, and N,N-dimethyldodecyl amine were purchased from Aldrich.
-
3.
Chlorosulfonic acid was purchased from Fluka.
-
4.
Chloroform, ethanol, and 1,4-dioxane were purchased from ADWIC.
Analysis
-
1.
Elemental analyses were carried out at the Micro Analytical Center (Cairo University).
-
2.
A FTIR spectrophotometer (ATI Mattson Genesis Series) was used with KBR pellets.
-
3.
1H-NMR was carried out using a Varian Gemini 200 MHz spectrophotometer. The samples were dissolved in DMSO and TMS. An internal standard was used.
-
4.
The melting points were determined using an electrothermal MEL-TEMP 3.0 apparatus.
Synthesis of Ferrocene Disulfonic Acid (I)
Ferrocene disulfonic acid was prepared through dropwise addition of chlorosulfonic acid (0.05 mol) to a rapidly stirred solution of ferrocene (0.025 mol) in acetic anhydride (75 ml) over 3 min. The temperature was raised from 25 to 40 °C. The mixture was stirred for 16 h and set aside for a further 6 h. The precipitated ferrocene disulfonic acid was filtered off and washed with acetic anhydride (15 ml) (yield % = 83) [10].
Synthesis of Fatty Ammonium Ferrocene Disulfonates (Ia, b, c, d, e)
The required fatty ammonium salts were obtained by dissolving 0.1 mol of each fatty amine in ethanol, then adding the solution to ferrocene disulfonic acid (0.05 mol) dissolved in ethanol and stirring for 1 h. The products were decyl, dodecyl, and hexadecyl primary ammonium salts of ferrocene disulfonic acid, as well as N,N-dimethyloctyl and N,N-dimethyl dodecyl tertiary ammonium salts of ferrocene disulfonic acid.
The specifications and the elemental analyses of the synthesized ammonium salts (Ia, b, c, d, e) are shown in Table 1.
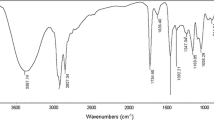
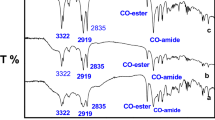
The purity of the investigated compounds was confirmed by the absence of minima near the critical micelle concentration (CMC) in the surface tension plots [7, 11, 12]. The chemical structures of the novel surfactants were confirmed using elemental analyses and FTIR spectroscopy (Table 2; Figs. 1, 2). In addition the chemical structures of Ib and Ie as examples for both primary and tertiary ammonium salts respectively were further confirmed using 1H-NMR spectra (Table 3; Figs. 3, 4).
Surface Tension Measurements
The determination of the surface tension was carried out using a Krüss-type K6 tensiometer equipped with a platinum–iridium Du Nouy ring. The solutions were transferred slowly into the double-walled vessel around which a thermostated liquid was circulated to maintain a constant temperature (30, 40, and 50 °C). Distilled water with a surface tension of 69 dyne/cm at 30 °C was used to prepare all solutions in the concentration range of 5 × 10−3 to 1 × 10−6 mol/L.
Antimicrobial Activities
The antibacterial activity of the synthesized surfactants was evaluated at the Micro Analytical Center (Cairo University) according to the diffusion disc method [13–16] against Pseudomonas aeruginosa and Staphylococcus aureus, while the fungicidal activity was evaluated against Aspergillus flavus and Candida albicans.
Results and Discussion
Primary and tertiary alkyl ammonium salts of ferrocene disulfonic acid may be denoted by

where R = NH3 and R′ = (CH2) n CH3, with n = 9, 11, 15 (Ia, Ib, Ic); or R = NH(CH3)2 and R′ = (CH2) n CH3 with n = 7, 11 (Id, Ie) and
- Ia :
-
= 1,1′-Di-decyl ammonium ferrocene disulfonate
- Ib :
-
= 1,1′-Di-dodecyl ammonium ferrocene disulfonate
- Ic :
-
= 1,1′-Di-hexadecyl ammonium ferrocene disulfonate
- Id :
-
= 1,1′-Di-N,N-dimethyloctyl ammonium ferrocene disulfonate
- Ie :
-
= 1,1′-Di-N,N-dimethyldodecyl ammonium ferrocene disulfonate
1H-NMR spectra of the compound Ib showed that in the N+H3 group, the proton exchange is suppressed and the signal disappeared, due to coupling with 14N with JNH = 50 Hz [17].
Surface Parameters
Surface Tension (γ) and Critical Micelle Concentration (CMC)
Figures 5 and 6 represent the relationship between the surface-tension values and concentration of 1,1′-didecyl ammonium ferrocene disulfonates (Ia) and 1,1′-di-N,N-dimethyloctyl ammonium ferrocene disulfonates (Id) at 30, 40, and 50 °C. The surface tension values of the surfactant 1,1′-dihexadecyl ammonium ferrocene disulfonate (Ic) could not be determined due to its low solubility in an aqueous medium.
The CMC values of both primary and tertiary disubstituted series decreased with increasing alkyl chain length as shown in Table 4, which increases the hydrophobicity of the molecules [18], leading to increased repulsion between the polar medium (H2O) and the nonpolar chains so that the molecules tend to aggregate at lower concentrations [19].
In general, increasing the temperature has a lowering effect on the CMC values of the investigated surfactants because increasing the temperature decreased the hydration of the hydrophilic group, favoring micellization [20].
Effectiveness (π CMC)
The effectiveness determines the surface activity of the surfactant molecules at their CMC. The effectiveness values are listed in Table 4 and may be attributed to the difference in aggregation type of each of the prepared surfactants as well as their hydrophobicity. Increasing the hydrophobic chain length along the prepared amine disulfonic acid salts increases their effectiveness.
The most effective surfactant is the one producing the lowest surface tension at CMC. According to the results of π CMC, Ib and Ie are found to be more effective as shown in Table 4.
Efficiency Pc20
From Table 4, it is clear that the efficiency of adsorption (Pc20) increases increasing numbers of the carbon atoms of the hydrophobic group.
For all the prepared surfactants, the efficiency also increased with increasing temperature. This increase was due to the decrease in the surface tension values by heating, leading to a decrease in the concentration, resulting in a decrease in the surface tension of the solvent decreased by 20 mN m−1 cm−1.
Maximum Surface Excess (Гmax)
Increasing the temperature increases the interaction between the polar solvent and the surfactant molecules, which directs them towards the bulk of the solution leading to a decrease in the surface excess. Also increasing the hydrophobic chain length leads to the complete coverage of the surfactant solution by adsorbed molecules at lower concentrations (shifts Гmax to lower concentrations).
Minimum Surface Area (Amin)
The minimum area occupied by classical surfactants [P(CH2) n CH3, where P is a polar head group, e.g., N+(CH3)3 or SO −24 ] at the surface of water is determined, in part, by a competition between Van der Waals forces among aliphatic chains and repulsive interactions (e.g., electrostatic or hydration) between polar head groups. Therefore, an increase in aliphatic chain length results in a decrease in the minimum area per molecule at the surface of an aqueous solution [21].
In our case, A min increased with the length of the aliphatic chain of the surfactant. The balance of forces leading to the organization of the ferrocenyl surfactants on the surfaces of aqueous solutions is, therefore, different from the balance of forces governing the assembly of classical surfactants on the surfaces of aqueous solutions. This result agrees with a previously reported work in ferrocene surfactants [22].
Increasing the temperature increased the surface coverage area of the molecules, which can be explained by the increase in the number of adsorbed molecules at the interface; hence the molecules occupied a larger area (Table 4).
By comparing the A min values of Ib and Ie, we observed that A min of Ie is slightly greater than that of Ib. This could be attributed to the fact that branching has a small effect and a small increase in the area per molecule at the interface [20].
Thermodynamic Parameters of Micellization and Adsorption of the Prepared Surfactants
According to Gibbs’ adsorption equations (Eqs. 1–6) and following the methodology of [21], the thermodynamic parameters of micellization and adsorption of the synthesized surfactants were calculated at 30, 40, and 50 °C. The results are listed in Table 5. For micellization:
For adsorption:
The standard free energies of micellization and adsorption, ΔG°(mic) and ΔG°(ads), are always negative, indicating that these are spontaneous processes. There is more decrease in the negativity of ΔG°(ads) than of micellization, showing the increasing tendency of surfactant molecules to be adsorbed at the interfaces. The preference for adsorption relates to the fact that the repulsion forces occurring between the hydrophobic molecules and the aqueous phase reach their minimum value when surfactant molecules are located at the interface (air/water).
The values of standard entropy changes of adsorption (ΔS°(ads)) show a greater increase than those of micellization [ΔS°(mic)] (Table 5), indicating greater randomness of the molecules in the adsorbed state than in the micellized one. This may be due to the compactness of the hydrophobes within the micelles, which offers a higher degree of constraint of molecules [23].
ΔH°(mic) are all positive values indicating the endothermic nature of the micellization process with the surfactants under study. The values of the standard heat enthalpy of micellization, ΔH°(mic), decreased with increasing hydrophobic chain length.
Antimicrobial Activity
The synthesized surfactants were evaluated as biocides for Gram-positive bacteria, Gram-negative bacteria, and fungi. The data are shown in Table 6.
In general, the inhibition-zone diameter values (mm) are classified as follows [24]: >15 mm = significant activity, 7–14 mm = moderate activity, and <7 mm = weak activity.
According to Table 6, most of the compounds show moderate antibacterial and antifungal activities, while only Ia and Ib show significant antibacterial activity.
It is clear from the data that the antibacterial activities of the compounds decrease with increasing chain length in Ia, Ib, and Ic, which may be attributed to the greatly increased lipophilicity of the molecules resulting from both the chain and the counterion (ferrocene sulfonates), leading them to take more time to cross the cell membrane, so activity decreases [25]. The activities of Id and Ie are not affected by the difference in the chain length.
The wall of Gram-positive bacterial cells is composed of a peptidoglycan chain of polysaccharide, teichonic acid, and phosphated sugar. Teichonic acids gave the Gram-positive bacterial cell wall a negative charge, which may be important in determining the types of substances attracted to the cell membrane [26].
Data in Table 6 show that these surfactants have approximately the same activity against Pseudomonae aeruginosa and Staphylococcus aureus. This means that their mechanism of action may depend on the counterion penetrating into the cytoplasm of the cell, where it inactivates essential metabolic proteins. The inactivation proceeded via oxidation of these proteins resulting in the bacterial cell death [27], while the cation portion of the molecule is attracted to the negatively charged cell membrane, resulting in neutralizing its charge and distorting its selective permeability as well [28].
References
Menger FM, Gan LH, Johnson E, Durst DH (1987) Phosphate ester hydrolysis catalyzed by metallomicelles. J Am Chem Soc 109:2800–2803
Bunton CA, Scrimin P, Tecilla PJ (1995) Source of catalysis of dephosphorylation of p-nitrophenylphosphate by metallomicelles. Chem Soc Perkin Trans 2:419–425
Hafiz AA (2005) Metallosurfactants of Cu(II) and Fe(III) complexes as catalysis for the destruction of paraoxon. J Surfact Deterg 8:359–363
Badawi AM, Hafiz AA, Ibrahim HA (2003) Catalytic destruction of malathion by metallomicelle layers. J Surfact Deterg 6:239–241
Hafiz AA, El-Awadi MY, Mokhtar SM, Badawi AM (2005) Catalytic destruction of paraoxon by metallomicelle layers of Co(II) and Cr(III). J Surfact Deterg 8:203–206
Hafiz AA (2008) Derivatives crystal structure of benzyl triphenyl phosphonium chlorometalate: some surface and biological properties of their metallosurfactant. J Iranian Chem Soc 5:106–114
Tajima K, Huxur T, Imai Y, Motoyama I, Nakamura A, Koshinuma M (1995) Surface activities of ferrocene surfactants. Colloids Surf A 94:243–251
Aydogan N, Abbott NL (2002) Effect of electrolyte concentration on interfacial and bulk solution properties of ferrocenyl surfactants with anionic head groups. Langmuir 18:7826–7830
Bai G, Graham MD, Abbott NL (2005) Role of desorption kinetics in determining Marangoni flows generated by using electrochemical methods and redox-active surfactants. Langmuir 21:2235–2241
Knox GR, Pauson PL (1958) Ferrocene derivatives. Part VII. Some sulphur derivatives. J Chem Soc 1958:692–696
Gallardo BS, Abbott NL (1997) Active control of interfacial properties: a comparison of dimeric and monomeric ferrocenyl surfactants at the surface of aqueous solutions. Langmuir 13:203–208
Mata J, Varade D, Bahadur P (2005) Aggregation behavior of quaternary salts based cationic surfactants. Thermochimica Acta 428:147–155
Jawetz E, Melnick JL, Adelberg EA (eds) (1974) Review of medical microbiology. Lange Medical Publications, Los Altos
Grayer RJ, Harbone JB (1994) A survey of antifungal compounds from higher plants. Phytochemistry 37:19–42
Muanza DN, Kim BW, Euler KL, Williams L (1994) Antibacterial and antifungal activity of nine medical plants from Zaire. Int J Pharmacol 32:337–345
Irob ON, Moo-Young, Anderson WA (1996) Antimicrobial activity of Annatto (Bixa orellana) extract. Int J Pharmacol 34:87–90
Kemp W (1987) Organic spectroscopy, 2nd edn. ELBS Publication, Hong Kong, p 136
Santhakumar K, Kumaraguru N, Arumugham MN, Arunachalam S (2006) Metallomicelles of Co(III) coordination complexes: synthesis, characterization and determination of CMC values. Polyhedron 25:1507–1513
Bunton CA, Ohmenzetter K, Sepulveda L (1986) Binding of hydrogen ions to anionic micelles. J Phys Chem 90:2000–2004
Rosen MJ (1978) Surfactants and interfacial phenomena. Wiley, New York
Rosen MJ (1989) Surfactants and interfacial phenomena, 2nd edn. Wiley, New York, p 431
Gallardo BS, Metcalfe KL, Abbott NL (1996) Ferrocenyl surfactants at the surface of water: principles for active control of interfacial properties. Langmuir 12:4116–4124
Gad EAM, El-Sukkary MMA, Ismail DA (1997) Surface and thermodynamic parameters of sodium N-acyl sarcosinate surfactant solution. J Am Oil Chem Soc 74:43–47
Chohan ZH, Supuran CT (2005) Organometallic compounds with biologically active molecules: in vitro antibacterial and antifungal activity of some 1, 1′-(dicarbohydrazono) ferrocenes and their cobalt II, copper II, nickel II and zinc II complexes. Appl Organometallic Chem 19:1207–1214
Badawi AM, Hafiz AA (2007) Synthesis and immunomodulatory activity of some novel amino acid germinates. J Iranian Chem Soc 4:107–113
Negm NA, Morsy SMI, Said MM (2005) Biocidal activity of some Mannich base cationic derivatives. Bioorganic Med Chem 13:5921–5926
Postgate JR (1984) The sulphate reducing bacteria, 2nd edn. Cambridge University Press, Cambridge
Lechevallier MW, Lowry CD, Lee RG (1990) Disinfecting biofilms in a model distribution system. J Am Water Works Assoc 82:87–99
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hafiz, A.A., Badawi, A.M., El-Deeb, F.I. et al. Ferrocene-Based Cationic Surfactants: Surface and Antimicrobial Properties. J Surfact Deterg 13, 165–172 (2010). https://doi.org/10.1007/s11743-009-1140-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1140-8