Abstract
An effective and economic two-step route was developed to synthesize the long alkyl chain betaine zwitterionic surfactant directly from natural fatty acids. The optimal processing conditions for synthesizing the intermediate and final product were probed and the yields of 96.4% and 88.3% were obtained for each step, respectively. The surface active behavior of the synthesized decylbetaine surfactant was investigated using the surface tension method. The related thermodynamic parameters were calculated and discussed. The fluorescence probe technology was applied to determine the micropolarity of decylbetaine micelles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zwitterionic surfactants are increasingly attracting the interest of researchers both in industrial applications and within academic fields owing to their unique properties [1–5], such as excellent water solubility, insensitivity to the presence of salts and to temperature, good biodegradability, biological safety due to their mildness to the skin and eyes, high foam stability, and a synergistic effect with a wide variety of ionic and nonionic surfactants [6, 7]. Many zwitterionic surfactants have been synthesized [8] and the increasing demand for this kind of surfactant has already enabled them to achieve well above average growth. However, in comparison with other classes of surfactants, the production cost of zwitterionic surfactants is high and thus limits their more wide industrial uses. Recently the synthesis and the study on the applications and properties of zwitterionic surfactants has become a scientific topic [9–11]. Nevertheless, little attention has been paid to the economic factor of preparation of the zwitterionic surfactants.
Alkylbetaines and their derivatives represent a class of zwitterionic surfactants with a positive charge on the nitrogen atom and a negative charge on the carboxyl group, which exists as electro-neutral internal salts within a wide pH range [12]. In this work, an attempt was made to prepare a long-chain alkylbetaine surfactant from natural fatty acids by an economic pathway. The synthesis involved a two-step procedure. First, carboxylic acid was selectively chlorinated at the alpha position with molecular chlorine to afford the product α-chlorocarboxylic acid. This intermediate was then reacted with trimethyllamine in a hermetic reactor and the product was obtained (Scheme 1). This synthesis route is a desirable one due to the great decrease in the cost of the alkylbetaine surfactants.
The decylbetaine surfactant was prepared according to Scheme 1 in the present study. The surface active behavior of the surfactant and the micropolarity of its micelles were investigated by surface tension and fluorescence probe technology, respectively.
Experimental Procedures
Materials and Equipment Setup
Dodecanoic acid and chlorosulfuric acid were obtained commercially (Sinopharm Chemical Reagent Co. Ltd, Shanghai, China), both were 99% pure and used as received. The fluorescence probe pyrene, 99% pure, from Sigma, was also used without further treatment. The synthesis of intermediate α-chlorondodecanoic acid was carried out in a cylindrical glass reactor (RAT-5D, Shanghai Shenshun Bio-tech Co.) equipped with a stirrer. The temperature was controlled by circulating oil in the external jacket vessel. The ammonolysis reaction was performed in a 1-L reaction kettle (WHF-1, Weihai Automatically-controlled Reaction Kettle Co. Ltd., Peoples Republic of China).
Synthesis and Analysis
Preparation of α-Chlorododecanoic Acid
The intermediate α-chlorododecanoic acid was synthesized according to the pathway shown in Scheme 1(1). A weighed amount (1,000 g, 5 mol) of melted dodecanoic acid was added to the 1.5-L cylindrical glass reactor equipped with a stirrer. Chlorosulfuric acid was added dropwise with stirring and the temperature was increased gradually to a given temperature. The mixture of chlorine and oxygen in a molar ratio of 2:1 was bubbled into the liquid through a glass sinter. Oxygen acted as a radical scavenger in the reaction system. The flow rates of both gases were controlled and metered by means of rotameters. After the reaction, chlorine was removed by introducing nitrogen and the product was cooled to room temperature. The samples of the product were methylated with trifluoro boron–methanol and then analyzed by gas chromatograph (FULI 9790, Fuli Analytical Instrument Co., Ltd) equipped with an SE54 capillary column and FID detection. The catalyst in the product was separated through extraction with water. Then the upper liquid was dried in a vacuum system for future use.
Preparation of Decylbetaine Surfactant
The intermediate α-chlorododecanic acid (234.5 g, 1 mol) was highly dispersed in aqueous solution in a 1-L hermetic reactor which was equipped with a stirrer, cooling coil, and thermocouple temperature controller. And then three times of the amount of (calculated by molar ratio, i.e. 3 mol) trimethylammonium gas were added and the reaction continued for 8 h at 80 °C. A yield of 88.3% of decylbetaine was achieved. After the reaction, trimethylamine was driven from the solution by boiling. The residual α-chlorododecanic acid was extracted with ethyl acetate and the salt was removed by mini electrodialysis equipment. The dried crude product was obtained by evaporation of the water. A colorless crystal was obtained by recrystallization of the crude product from acetone several times. The structure and purity of the final product was confirmed by IR (FTLA 2000-104, ABB Inc., Canada), elemental analysis (Vario EL III, Elementar Co., Germany, about 4.5 mg sample was examined each time) and 1H NMR (Bruker ARX-300 NMR spectrometer, Switzerland, 300.13 MHz. All agents were dissolved by D2O, using D2O (δ = 4.70 ppm) as an internal reference.).
Surface Tension Method
Surface tension (γ) was measured at constant ionic strength (0.1 M NaCl ) and at 25 ± 0.02 °C by the drop volume method [13]. The measurement error for surface tension is within ±0.1 mJ m−2.
Steady-State Fluorescence Measurements
All the fluorescence spectra of Pyrene (Py) were measured on an RF-5301 PC spectrofluorometer (SCHIMADZU, Japan). Emission spectra of Py were obtained by exciting the samples at 335 nm. About 2 mL of the sample solution was placed in a 4-mL quartz cell, which was temperature-controlled at 30 °C. The pyrene spectrum was scanned at wavelengths from 350 to 450 nm. Intensities of the first vibronic band I 1 and the third vibronic band I 3 were taken from the emission intensities at 373 and 384 nm, respectively.
Results and Discussion
Optimizing the Reaction Conditions
The α-chlorocarboxylic acid is a potential intermediate in the fine chemical industry due to its high reactivity, which is based on the charge shift caused by the electronegative chlorine atom in the alpha position. The carbon atom attached to chlorine gets a partially positive charge and it readily reacts with a nucleophile. The synthesis of α-chlorocarboxylic acids catalyzed by strong acidic agents was developed in a previous work [14]. Nevertheless, a large amount of catalyst (mol fraction \( y_{{{\text{ClSO}}_{3} {\text{H}}}} = 0.0670 \)) is needed to obtain high yields in a reasonable reaction time owing to the relative low activity of long alkyl chain carboxylic acid. The conditions for preparing long chain α-chlorocarboxylic acid were not yet developed for industrial practice. The present work was carried out in order to develop practical reaction conditions. Temperature, the dosage of catalyst and the ratio of the amount of oxygen to chlorine were found to be the key factors influencing the yield of α-chlorododecanoic acid. The lowest fraction of catalyst chlorosulfonic acid is \( y_{{{\text{ClSO}}_{3} {\text{H}}}} = 0.0343 \) to guarantee a high yield of the product. A yield of 96.4% of α-chlorododecanoic acid was obtained in 3 h under the following optimized conditions: temperature 120 °C; Cl2 feed 40 L/h; O2 feed 20 L/h; amount of catalyst added \( y_{{{\text{ClSO}}_{3} {\text{H}}}} = 0.0343; \) stirring rate 1,000 rpm (see Fig. 1).
Changes in the concentration of dodecanoic acid (filled circles) and α-chlorododecanoic acid (filled squares) with reaction time at optimal condition: temperature 120 °C; Cl2 feed 40 L/h; O2 feed 20 L/h; amount of catalyst added \( y_{{{\text{ClSO}}_{3} {\text{H}}}} = 0.0343; \) stirring rate 1,000 rpm
Although α-alkylchain betaine surfactants have been successfully synthesized by α-bromo-fatty acids [15], the study has drawn little attention because of its high cost and low yield. In this study, a simple route of synthesizing long alkyl chain betaine surfactant is explored by the substitute reaction of α-chloro-fatty acid with trimethylamine. The synthesis of decylbetaine was carried out in a hermetic reactor to prevent the volatilization of trimethylammonia. The effects of the molar ratio of the reactants, concentration and temperature on the yield of long alkyl betaine were studied. Experimental results show that the reaction can give a good yield (ca. 88.3%) as the molar ratio of trimethylammonia to α-chlorododecanoic acid is ca. 3:1 at 80 °C for 8 h (Fig. 2).
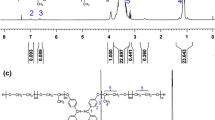
The structural characterization and purity were studied in detail. The infrared spectrum of decylbetaine showed a carbonyl band of carboxylate at 1,640 cm−1 and a weak C–N stretching vibration bond of the quaternary ammonium salt at 960 cm−1while the infrared spectrum of the reactant α-chlorododecanoic acid showed a carbonyl band of a carboxyl group at 1,726 cm−1. The derived 1H-NMR spectrum was analysed and assigned as follows: 0.73(triplet, 3H, –CH 3 ); 1.23–1.60 [multiplet, 16H, –(CH 2)8–]; 1.72 [multiplet, 2H, –CH 2–CH–N+(CH)3)3]; 3.08 [single, 9H, –N+((CH 3)3)]; 3.48–3.52 (multiplet, 1H, α-H –CH2–CH–N+(CH)3)3.). The α-H chemical shift of the product is obviously different with 4.25 of the α-chloroalkyl acid [14], and the value of the chemical shift of decylbetaine is similar to that of the N-dodecyl betaine reported in reference [16]. Analysis results and physical properties of compounds found in this work are summarized in Table 1.
Surface-active Properties
Plots of surface tension versus concentration for decylbetain are shown in Fig. 3. From the plot we obtained the critical micelle concentration (cmc) and the minimum surface tension γ cmc of decylbetaine surfactant as 8.1 × 10−3 mol/L and 38.2 mN/m, respectively.
Using cmc and γ cmc, the maximum surface excess, the area per molecule and the standard free energy of micellization in aqueous solution are calculated by Gibbs adsorption equation [17]
where R is the gas constant, N a is the Avogadro constant and Q is the stoichiometric parameter for a salt. Most researchers deal with the zwitterionic surfactants as the nonionic ones [17–19] for their formally neutral structure and this was also the case in this study, that is, n = 1 and Q = 1. The results obtained are listed in Table 2, where the values of typical anionic, cationic and nonionic surfactants with the same chain length are also included in order to compare their properties with that of decylbetaine.
Table 2 shows that the minimum area per molecule of decylbetaine, A min, is slightly smaller than that of C10H21SO4Na, along with a larger surface excess adsorption amount Γ max. This can be attributed to the argument that the zwitterionic surfactant with a formally net charge results in smaller intermolecular repulsions and thus a more closely packed adsorption layer at the aqueous-air interface is formed. The detected increasing order of cmc and the standard Gibbs energy of micellization \( \Updelta G^{o}_{{{\text{mic}}}} \) is C10H21N+(CH3)3Br− > C10H21SO4 −Na+ > C10H21CH N+(CH3)3(COO−) > C10H21O(C2H4)8OH for the four surfactants containing equivalent hydrophobic groups. The results may be due to the intramolecular repulsive interaction between the headgroups with the same charge for ionic surfactants at the micelle interface. And thus zwitterionic and nonionic surfactants with the neutral headgroup tend to form micelles at lower concentrations than ionic surfactants accompanied by the decreasing \( \Updelta G^{o}_{{{\text{mic}}}} . \)
The fluorescence probe technology is applied to investigate the micropolarity of decylbetaine surfactant micelles based on the sensitivity of the ratio I 1/I 3 of Py to its environmental polarity [22]. The results are shown in Fig. 4.
As can be seen in Fig. 4, the value of I 3 /I 1 increases with the surfactant concentration and an abrupt change occurs in the vicinity of cmc. Then the plateau value 0.875 is reached. This value is equivalent to the micropolarity of ethanol reported in Ref. [23], which is much larger than that of water (0.53). This indicates that although decylbetaine has good solubility in water, the micropolarity of its micelle is much smaller than in an aqueous environment.
References
Kamenka N, Chevalier Y, Zana R (1995) Aqueous-solutions of zwitterionic surfactants with varying carbon number of the intercharger group: 1.micelle aggregation numbers. Langmuir 11:3351–3355
Szule R, Michael RF, Daniel H (1998) Solution behavior of the zwitterionic surfactant octadecyldimethylbetaine. J Phys Chem B 102:6487–6492
Doussin S, Birlirakis N, Georgin D, Taran F, Berthault P (2006) Novel zwitterionic reverse micelles for encapsulation of proteins in low-viscosity media. Chem Eur J 12:4170–4175
Yaseen M, Lu JR, Webster JRP, Penfold J (2006) The structure of zwitterionic phosphocholine surfactant monolayers. Langmuir 22:5825–5832
El-Aila HJY (2005) Effect of urea and salt on micelle formation of zwitterionic surfactants. J Surfact Deterg 8:165–168
Zajac J, Chorro C, Lindheimer M, Partyka S (1997) Thermodynamics of micellization and adsorption of zwitterionic surfactants in aqueous media. Langmuir 13:1486–1495
Broze G (1999) Handbook of detergents, part A: properties, 1st edn, chap 2. Marcel Dekker, New York
Lomax EG (1996) Amphoteric surfactants, 2nd edn. In: Surfactant science series, vol. 59; CRC Press, West Palm Beach
Yoshimura T, Nyuta K, Esumi K (2005) Zwitterionic heterogemini surfactants containing ammonium and carboxylate headgroups. 1. Adsorpt mMicellization Langmuir 21:2682–2688
Yaseen A, Wang Y, Su TJ, Lu JR (2005) Surface adsorption of zwitterionic surfactants: n-alkyl phosphocholines characterised by surface tensiometry and neutron reflection. J Colloid Interf Sci 288:361–370
Zhai LM, Tan XJ, Li T, Chen YJ, Huang XR (2006) Influence of salt and polymer on the critical vesicle concentration in aqueous mixture of zwitterionic/anionic surfactants. Colloid Surfaces A 276:28–33
Delgado C, Dolores Merch´an M, Mercedes Vel´azquez M, Anaya J (2006) Effect of surfactant structure on the adsorption of carboxybetaines at the air–water interface, colloids and surfaces A: physicochem. Eng Aspects 280:17
Zhao GX, Zhu BY (1991) Physical chemistry of surfactant. Peking University Press, Beijing, pp 13–18
Paatero E, Salmi T, Fagerstolt K (1992) Selective synthesis of α-chlorocarboxylic acids. Ind Eng Chem Res 31:2425–2437
Xia YM, Fang Y, He JR, Jiang WZ (1994) The preparation and property studies of betaine amphoteric surfactant based natural fatty acid III. The synthesis and property studies of long alkyl betaine surfactant. Household Pers Care Chem Ind 166:1–3
Weers JG, Rathman JF, Axe FU, Crichlow CA, Foland, LD, Scheuing, DR Wiersema, RJ, Zielske AG (1991) Effect of the intramolecular charge separation distance on the solution properties of betaines and sulfobetaines. Langmuir 7:854–867
Blandamer MJ, Cullis PM, Soldi LG, Engberts JBFN, Kacperska A, Van Os NM, Subha MCS (1995) Thermodynamics of micellar systems: comparison of mass action and phase equilibrium models for the calculation of standard Gibbs energies of micelle formation. Adv Colloid Interf Sci 58:171–209
Seredyuk V, Alami E, Nyde M, Holmberg K (2001) Micellization and adsorption properties of novel zwitterionic surfactants, Langmuir 17:5160–5165
Chevalier Y, Storet Y, Pourchet S, Perchec PL (1991) Tensioactive properties of zwitterionic carboxybetaine amphiphiles. Langmuir 7:848–853
Rosen MJ (1989) Surfactants and interfacial phenomena. 2nd edn. Wiley, New York
Van Os NM, Haak JR, Rupert LAM (1993) Physico-chemical properties of selected anionic, cationic and nonionic surfactants. Elsevier, Amsterdam
Turro NJ, Kuo PL (1986) A fluorescence probe investigation of the effect of alkali metal ions on the micellar properties of a crown ether surfactant. J Phys Chem 90(5):837–841
Xu GY, Luan Y Xia, Liu J, Yu L (2005) Study on surfactant/macromolecule interaction by static fluorescence technology. Acta Phys Chim Sin 21(5):577–582
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Qi, L., Fang, Y., Wang, Z. et al. Synthesis and Physicochemical Investigation of Long Alkylchain Betaine Zwitterionic Surfactant. J Surfact Deterg 11, 55–59 (2008). https://doi.org/10.1007/s11743-007-1054-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-007-1054-2