Abstract
A novel branched polyether surfactant (TPE) was prepared by anion polymerization with different proportions of propylene oxide (PO) and ethylene oxide (EO) using 1,1,2,2-tetrakis(4-hydroxyphenyl)ethane as a core. The structures and average molecular weight (M n ) of the TPE were characterized by 1H NMR and GPC. The cloud point was determined by turbidimetry in the presence of inorganic salts. Inorganic salts decreased the cloud point of TPE polyether in the following order: Na2CO3 > Na2SO4 > NaCl > CaCl2 > MgCl2. The effects of inorganic salts (NaCl, MgCl2, CaCl2, and NaSCN) and temperature on the surface activity of TPE in aqueous solution were investigated by surface tension measurements. The surface activity parameters and the thermodynamic parameters were calculated from surface tension data. Similar to the effect of increasing temperature, the salting-out inorganic salts such as NaCl, MgCl2, and CaCl2 favor the micellization and increase the maximum surface excess concentration, while the salting-in NaSCN has the opposite effect. The influence of NaCl on the morphology of micelles was investigated by TEM. The micellization is entropy-driven at low temperature and enthalpy-driven at higher temperature. The TPE polyether has large surface activity and can be used as a demulsifier to break up crude oil emulsions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to the flexible design of the branches, different ratios of hydrophobic groups to hydrophilic groups, and the molecular weight of polypropylene oxide (PPO), polyethers comprising PPO–polyethylene oxide (PEO) are studied extensively with various methods and widely used in biomacromolecule separation, drug delivery, hydrogel, controlled release, and nanoparticle preparation [1–5]. In crude oil demulsification, branched polyethers can separate water from crude oil emulsions more effectively [6–9].
Bicyclic aromatic polyethers exceed their monocyclic counterparts in separating water from crude oil emulsions owing to the improved interaction between the bicyclic demulsifiers and the asphaltene molecules located at the oil–water interface, which are polynuclear aromatic compounds [10]. Moreover, the increased number of PPO–PEO chains and sequential polyether (R–PPO–PEO) can also improve the demulsification performance [9–13]. Here we develop a novel polyether based on the above conclusion with four aromatic rings and four number of PPO–PEO chains in sequential structure.
It is well known that crude oil from underground has plenty of inorganic salts [14–17], and the salts greatly influence the surface activity of polyethers [18–22]. Therefore, it is necessary to study the surface activity of polyethers at different temperature and inorganic salts because of the high temperature needed to break up crude oil emulsions.
Experimental Section
Materials
Propylene oxide (PO) 98 %, ethylene oxide (EO) 98 %, Na2CO3, Na2SO4, NaCl, CaCl2, MgCl2, KOH, and NaSCN were purchased from Sinopharm Chemical Reagent Corporation and used as received. 1,1,2,2-Tetrakis(p-hydroxyphenyl)ethane was synthesized in our laboratory according to Ref. [23]. Ultrapure water (18 MΩ cm−1) was used in the experiments.
Synthesis and Characterization of TPE
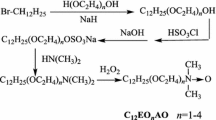
TPE polyether was synthesized by anionic polymerization with PO and EO using 1,1,2,2-tetrakis(p-hydroxyphenyl)ethane as precursor in the presence of potassium hydroxide (Scheme 1). The precursor was first dissolved in PO, and then the reaction was kept at 120–140 °C and pressure of 0.2–0.4 MPa in a pressure steel kettle with a magnetic drive stirrer, an electric heating mantle with a thermocouple inserted in the reactor body, and circulating water.
The structure of TPE was characterized by 1H NMR (Bruker AV-400 spectrometer operating at 400 MHz). 1H NMR (CDCl3), δ: 1.13 (m, CH3, 3H), 3.4–3.8 (m, CHCH2 and CH2CH2), 6.64 (d, ArH, 4H), 7.32 (d, C-ArH, 4H).
Gel Permeation Chromatography (GPC)
The average molecular weight was measured by GPC (Waters binary HPLC pump 1525 and a Waters 2414 refractive index detector) with standard polystyrenes and tetrahydrofuran as eluent.
Cloud Point Measurement
Cloud point measurement is usually performed by visual observation and taken as the temperature for the first appearance of turbidity [24–27]. To eliminate the visual error, a HACH 2100Q turbidimeter was employed to measure the turbidity of TPE polyether at various concentrations. A refrigerated circulator HD120-RT8 (Prima Instrument, UK) was used to control the sample temperature from 4 to 18 °C at a step of 0.5 °C. The turbidity of the sample is a function of nephelometric turbidity units (NTU) and temperature. The cloud point is defined as the temperature at the intersection of the two lines resulting from the fitting of the turbidity curve as a function of temperature before and after the inflection.
Surface Tension Measurements
The surface tension measurements were performed on a K100 processor tensiometer (Germany, Krüss Corporation, precision 0.01 mN m−1) at temperature from 25 to 65 °C within a step of 5 °C.
TEM Observation
The micelles formed in aqueous solutions were observed by transmission electron microscopy (TEM) using a JEM-2010 electron microscope. The sample was dropped onto copper grids followed by a drop of phosphotungstic acid (1 wt% aqueous solution) for staining.
Results and Discussion
Characterization of TPE
The structures of monomer and polymer were confirmed by the 1H NMR spectrum shown in Fig. 1. The weight ratio of PO/EO validated by 1H NMR according to the two signals at 0.9–1.3 and 3.4–3.8 ppm [10, 28, 29] is 2.66. The molecular weight (M n ) and distribution (M w /M n ) of TPE obtained by GPC were 2250 and 1.21, respectively.
Cloud Point
Figure 2 shows the turbidity of TPE polyether at different concentrations as a function of temperature in aqueous solution. The intersection marked in the fittings to the turbidity curve is defined as the cloud point. It is obvious that the cloud point decreased remarkably with the increase in concentration below 20 g L−1 and changed slightly with concentration up to 20–50 g L−1. This behavior indicates that the cloud point tends to keep constant with increasing concentration [30, 31]. The decrease in cloud point is ascribed to the increased micelle–micelle interaction with the increase in micelle concentration [32]. The interactions between micelles, which are influenced by the size and number of micelles, increase rapidly, then become almost constant, and finally affect the cloud point. The change in size and number of micelles with increasing concentration is confirmed by TEM as discussed below.
The cloud point at 50 g L−1 is 8 °C, lower than that reported for EO13PO30EO13 or L64 (55 °C) at the same concentration [33]. Besides the very hydrophobic 1,2,2-tetrakis(p-hydroxyphenyl)ethane and large ratio of PO/EO, the branching, and greater symmetry may contribute to the lower cloud point of TPE polyether [34].
The turbidity curves and effect of several inorganic salts on the cloud points of TPE polyether at 5 g L−1 are presented in Fig. 3. It suggests that the higher the concentration of inorganic salt is, the earlier the turbidity appears. As shown in Fig. 3f, a slight linear decrease in cloud point is seen with increasing concentration of aqueous salt solution. It is clear that the effect of inorganic salts on decreasing cloud point is in the following order: Na2CO3 > Na2SO4 > NaCl and NaCl > CaCl2 > MgCl2. Salts containing divalent anions such as SO4 2− are known to be more effective at salting out the PEO chains than those containing monovalent anions like Cl− [35–37]. The decrease in cloud point with different anion salts is more rapid than that of different cation salts, indicating that the cloud point of a surfactant solution depends on its cation group and anion group. The anion group has greater influence than the cation group, especially with large polyatomic anions [36].
Effect of Inorganic Salts on Surface Activity
Figure 4 displays the surface tension isotherms of TPE aqueous salt (NaCl, MgCl2, CaCl2, and NaSCN) solutions at the same anionic concentration of 1 mol L−1. A steady decrease in surface tension with concentration was observed. Two intersections that appeared at higher concentration were as marked A and B. The surface tension initially decreases sharply before point A and thereafter decreases at a slower rate. This transition has been noted for PPO–PEO polyethers [38–40]. Before point A, the decrease in surface tension indicates the formation of polyether monolayer absorbed at the air–water interface, while point B corresponds to the formation of multimolecular micelles at the critical micelle concentration (cmc) [40]. The intermediate region between A and B is often attributed to the polydispersity of PPO–PEO polyethers or the formation of unimolecular micelles [38]. The addition of NaCl, MgCl2, and CaCl2 known as salting-out salts promotes surface activity by reducing the surface tension, while salting-in salt such as NaSCN decrease surface activity.
The surface-active parameters such as maximum surface excess concentration (Γ max), minimum surface area per molecule (A min), effectiveness of surface tension reduction or surface pressure (π cmc), and the efficiency of surface tension reduction and adsorption (pC 20) can be obtained by the following equations [41, 42]:
where R is the gas constant, N is Avogadro’s number, ∂γ/∂ (logC) is the slope of the surface tension isotherms between A and B, γ 0 is the surface tension of pure water at that temperature T, and C 20 is the reduction of surface tension of pure water by 20 mN m−1. Table 1 displays the parameters calculated from the above equations. The addition of salts has a small influence on the Γ max, γ cmc, and pC 20, but great effects on the cmc. The efficiency expressed as pC 20 means the performance in reducing surface tension of bulk phase concentration by 20 mN m−1, whereas the effectiveness means the minimum surface tension that surfactant can reach. The increasing values of pC 20 on the addition of NaCl, MgCl2, and CaCl2 suggest that the salting-out salts promote the efficiency of surface tension reduction and adsorption, while the NaSCN reflects the opposite effect [35]. The Γ max is a useful measure of the effectiveness of adsorption at the water–air interface [43]. The small increase of Γ max indicates that the salting-out salts slightly favor the adsorption, whereas the salting-in NaSCN slightly inhibits the adsorption. The π cmc has a similar result with Γ max. It can be concluded that the salting-out salts promote both efficiency and effectiveness of surface tension reduction and adsorption.
The other useful parameter to evaluate the effect on micellization and adsorption processes is cmc/C 20. An increase in the cmc/C20 means that adsorption is facilitated more than micellization [43]. Both the larger pC 20 and smaller cmc/C20 on the addition of salting-out salts suggest that these salts facilitate the micellization; on the contrary, the salting-in NaSCN favors the adsorption in accordance with the conclusion made by Zhai [20].
The salting-out salts decrease the cmc in the following order: NaCl > MgCl2 > CaCl2. The monovalent cation is more effective at salting out the polyether molecules than divalent cations such as Ca2+ and Mg2+. It has been reported that anions have a more pronounced effect than cations [44]. For common monovalent anions, the typical order for their effect on the physical behavior of aqueous processes is known as the Hofmeister series [22, 44–47]: F− > Cl− > Br− ~ NO3 − > I− > SCN−. The Cl− has a strong hydration effect compared with SCN− which is classified into poorly hydrated anions. It was originally believed that the Cl− enhanced the water structure by disrupting the hydrogen-bonding network between polyether molecules and water, lowering the solubility of polyether molecules; whereas the poorly hydrated SCN− interferes with the water structure. Deyerle and Zhang [45] proposed the interaction between these ions and polyether molecules. Well-hydrated Cl− interacts with the hydrophilic PEO groups by changing the entropy of hydration water around the polyether, making these water molecules less available to the polyether and therefore decreasing the cmc. In contrast, poorly hydrated SCN− enhances the polyether hydration by binding to hydrophobic PPO groups leading to the increase in cmc.
In accordance with the above results and research from Zhai and Chen [20, 39], the salting-in effect and salting-out effect are illustrated in Fig. 5. The conformation of polyether is strongly affected by inorganic salts. The salting-out salts compress the polyether molecules while the salting-in salts expand these molecules [18, 33].
The standard Gibbs free energy of micellization (\( \Delta G_{m}^{o} \)) and Gibbs free energy of adsorption (\( \Delta G_{\text{ads}}^{o} \)) are determined by [48, 49]
where X cmc is the mole fraction unit of the cmc value. According to the literature [43, 50], for nonionic surfactant, the \( \Delta G_{m}^{o} \) can be given as
where cmc is expressed in molar unit and ω is the number of moles of water per liter.
As would be expected, both micellization and adsorption are spontaneous whether with or without salts as reflected by the negative values of \( \Delta G_{m}^{o} \) and \( \Delta G_{\text{ads}}^{o} \) respectively. These more negative values and the increased cmc/C 20 in the addition of salting-out salts facilitate the micellization more than adsorption. The more negative values of \( \Delta G_{\text{ads}}^{o} \) than the corresponding values of \( \Delta G_{m}^{o} \) indicate that adsorption is more spontaneous than micellization [51, 52], in accordance with the fact that when polyether molecules dissolve in water, these molecules are always adsorbed onto the air–water interface, and then aggregate to form micelles only when the concentration exceeds the cmc.
Morphology of Copolymer Aggregate
TEM micrographs were used to validate the morphology of TPE polyether micelles and the influence of NaCl on these micelles (Fig. 6). The micelles in aqueous solution are spherical, and the size and number of these micelles increase with increasing concentration of TPE polyether. The mean size of the micelles increase rapidly from 40 to 121 nm with concentrations from 10 to 30 g L−1, and change slightly to 159 nm when the concentration reaches 50 g L−1. This behavior affects the interaction between micelles, and finally affects the cloud point. The tendency of the particle size of micelles to increase with concentration of polyether is in accordance with Ref. [53].
It was observed that little inorganic salts like NaCl have no influence on the mean size of micelles. With further increase in concentration of NaCl, the mean size of micelles increases continuously. The phenomenon that the particle size of micelles increases with increasing concentration of TPE polyether or inorganic salt indicates that the micelles are easy to grow and easy to separate into two phase [32]. When the concentration of NaCl is high, the image of NaCl can be seen clearly from TEM as shown in Fig. 6f.
Effect of Temperature on Surface Activity
Figure 7 demonstrates clearly the decrease in surface tension with increasing temperature of TPE polyether with and without NaCl at various temperatures. Table 2 shows surface-active parameters calculated from Eqs. (1), (2), (3), and (4). The effect of temperature on cmc is complex, the value appearing first to decrease and then to increase with further increase in temperature. Temperature increase causes decreased hydration of the hydrophilic PEO groups, resulting in the increase in hydrophobicity which favors micellization. When surfactant molecules dissolve in water, the degree of structured water is increased. However, the structured water surrounding the hydrophobic groups would be lost as the temperature increases, an effect that disfavors micellization. The two opposite effects determine whether the cmc increases or decreases over a particular temperature range [43]. For TPE polyether, as the temperature increases further, the effect of the hydrophobic groups exerts a predominant influence as the cmc reaches a minimum value leading to the increase in cmc [54].
It is obvious that for both TPE solutions the γ cmc decreases continuously as the temperature increases, and the presence of salting-out NaCl promotes the surface activity over the whole temperature range. π cmc also decreases with increased temperature indicating the lower effectiveness in surface tension reduction, which is consistent with previous reports [43, 54].
A min is found to increase with increasing temperature whether in the absence or presence of NaCl in agreement with the previous reports [27, 52]. As reported [43], the A min is mainly determined by PEO groups of polyether. At low temperature, the PEO groups protrude into the aqueous solution. The PEO chains become spread at the air–water interface at an elevated temperature, resulting in larger area of molecules. The presence of salting-out NaCl reduces the number of free water molecules in the bulk solution leading to the compression of the TPE molecules, therefore reducing the area. In addition, increased molecule motion and chain flexibility at elevated temperature resulting in expanding of polyether molecules contribute to the increase in A min [55]. On the other hand, the increased dehydration of the PEO groups as the temperature increases improves the adsorption of polyether molecules as seen from increased pC 20.
The conformational transition of polymer at different temperature with the increase in concentration is illustrated in Fig. 8. With the increase in temperature, the hydrophobicity of the PPO chains increases leading to formation of a more compact hydrophobic core and polyether molecules form micelles more easily [39, 45]. However the PEO chains expand continuously as the temperature increases; as a result the micellar size increases at higher temperature as confirmed by small angle neutron scattering [33]. The conformational transition is also sensitive to salts.
Thermodynamic Parameters of Micellization
The thermodynamic parameters such as enthalpy, entropy, and energy of surface formation can be evaluated from the temperature effect on surface activity. The enthalpy (\( \Delta H_{m}^{o} \)) can be obtained by applying the well-known relations
where the lnX cmc is a 2nd-order polynomial function of temperature [54, 56]. It was reported that a new equation can fit the point excellently by the relation [57]
where a, b, and c are determined by least-squared regression analysis. Once the Gibbs free energy and the enthalpy are obtained, obviously, the entropy can be determined by
Table 2 displays the thermodynamic parameters of TPE at different temperatures with or without NaCl. It is found that all the thermodynamic parameters decrease monotonically as the temperature increases over the whole temperature range from 25 to 65 °C. It is clear that the values of \( \Delta H_{m}^{o} \) and \( \Delta S_{m}^{o} \) are quite sensitive to temperature. The positive values of \( \Delta H_{m}^{o} \) when the temperature is below 45 °C indicate that the transfer of unimers from solution to micelle is an endothermic process at low temperature, whereas the process transforms into exothermic at higher temperature. The thermal transformation of micellization is in accordance with Refs. [58, 59]. The smaller \( \Delta S_{m}^{o} \) in aqueous NaCl solution than the corresponding value in aqueous solution indicates that well-hydrated Cl− interacts with PEO groups, disrupting the hydrogen bonds between TPE polyether molecules and water, decreasing the order of structured water and resulting in the increase in entropy.
Figure 9 shows the plot of these thermodynamic parameters as a function of temperature. In order to make the entropic contribution to \( \Delta G_{m}^{o} \) clear, \( - T\Delta S_{m}^{o} \) is employed instead of \( \Delta S_{m}^{o} \). The \( \Delta G_{m}^{o} \) is the sum of the enthalpic (\( \Delta H_{m}^{o} \)) and entropic (\( - T\Delta S_{m}^{o} \)) contributions. It is clear that with the increase in temperature \( \Delta H_{m}^{o} \) decreases from positive to negative, whereas \( - T\Delta S_{m}^{o} \) increased from negative toward positive in both samples leading to the weak effect of temperature on \( \Delta G_{m}^{o} \). The behavior of these thermodynamic parameters is in agreement with previous reports [58–60]. It means that in the low temperature region, the entropic contribution predominates the micellization process; whereas, at higher temperature the enthalpic contribution plays a significant role to keep the change of free energy small [60].
It is noted that as temperature increases, the \( \Delta H_{m}^{o} \) becomes smaller. The enthalpy gain from the interaction of hydrocarbon chains will overcome the enthalpy loss due to the release of water molecules at low temperature, and the enthalpy loss becomes dominant gradually leading to the negative \( \Delta H_{m}^{o} \) at elevated temperature. The interaction between polyether molecules and water induces the formation of an iceberg-like ordered structure of water molecules causing a significant decrease in entropy. This structure of water would be lost at elevated temperature leading to the increase in entropy. Finally, the entropy loss will exceed the entropy gain, and thus, \( - T\Delta S_{m}^{o} \) goes toward positive with increasing temperature.
It is observed that the entropy change is always positive over the whole temperature range. The increase in entropy has been attributed to the rupture of structured water molecules surrounding the polyether molecules and increased configurational entropy of hydrophobic chains transformed from the aqueous solution to micelle [54].
Enthalpy–Entropy Compensation
The phenomenon of a linear relation between the enthalpy changes and entropy changes in surfactant solution is observed in many studies and is known as enthalpy–entropy compensation [25, 26, 61, 62].
Figure 10 demonstrates that the enthalpy change varies linearly with entropy change and compensates each other, and the two lines almost having the same slope and intercepts. These linear changes are expressed in the form
The slope of these plots corresponds to the compensation temperature (T c) representing the interaction between solvent–solvent and solvent–solute. The larger the value of T c is, the greater the interaction is [25, 58]. The intercept, \( \Delta H_{\text{m}}^{ * } \), provides information on the solute–solute interactions and is considered as a measurement of the stability of micelle under the condition \( \Delta S_{m}^{o} = 0 \). The smaller the value of \( \Delta H_{\text{m}}^{ * } \) is, the more stable the micelle is. Sugihara and Hisatomi [63] attributed the intercept to the aggregation number of polyether depending on the polyether species. The T c values in both samples are 317.5 K which is in agreement with the range of 300–325 K for nonionic surfactants [19, 39, 54, 60]. These values are larger than those reported for structurally similar linear Tetronics polyether (280–298 K) and branched Tetronics polyether (307.9 and 309.3 K).
The similar values of slope and intercept in the absence and presence of NaCl indicate that the salt has little influence on the enthalpy-entropy compensation of TPE polyether.
Conclusions
We prepared a novel PPO–PEO polyether with four aromatic rings and four branches. Inorganic salts can decrease the cloud point of the polyether as a result of the salting-out effect, especially on the addition of salts containing divalent anions such as SO4 2− and CO3 2−. As a surfactant, the polyether has high surface activity at room temperature and the activity is promoted by the increase in temperature as well as the addition of salting-out salts such as NaCl, MgCl2, and CaCl2. Not only the activity but also micellization is inhibited by the addition of salting-in NaSCN. The addition of inorganic salts causes great changes in the efficiency of surface tension reduction and adsorption, small changes in effectiveness of surface tension, but has little influence on the effectiveness of adsorption. The mean size and the number of micelles increase with increasing concentration as observed from TEM micrographs. Low concentrations of NaCl have no influence on the mean size of micelles, but the mean size of micelles increase with further increase in the concentration of NaCl. The cmc decreases first and then increases as temperature increases, indicating an increased dehydration of hydrophilic PEO groups with water at low temperature which favors the micellization and the increasing breakdown of ordered water surrounding the hydrophobic groups which disfavors micellization at higher temperature. The micellization is confirmed to be endothermic at low temperature and exothermic at higher temperature indicating that the micellization is entropy-driven and enthalpy-driven, respectively. The correlation between enthalpy and entropy change in micellization exhibits an excellent linearity. In addition, it is helpful for predicting the properties of polyether in breaking up crude oil emulsions or other uses.
References
Wu C, Liu T, Chu B, Schneider DK, Graziano V (1997) Characterization of the PEO–PPO–PEO triblock copolymer and its application as a separation medium in capillary electrophoresis. Macromolecules 30:4574–4583
Bae KH, Lee Y, Park TG (2007) Oil-encapsulating PEO–PPO–PEO/PEG shell cross-linked nanocapsules for target-specific delivery of paclitaxel. Biomacromolecules 8:650–656
Khullar P, Mahal A, Singh V, Banipal TS, Kaur G, Bakshi MS (2010) How PEO–PPO–PEO triblock polymer micelles control the synthesis of gold nanoparticles: temperature and hydrophobic effects. Langmuir 26:11363–11371
Jia L, Guo C, Xiang J, Wang N, Yang L, Tang Y, Liu H (2012) Interaction between PEO–PPO–PEO copolymers and a hexapeptide in aqueous solutions. Langmuir 28:1725–1732
White JC, Saffer EM, Bhatia SR (2013) Alginate/PEO–PPO–PEO composite hydrogels with thermally-active plasticity. Biomacromolecules 14:4456–4464
Atta AM, Ismail HS, Elsaeed AM, Fouad RR, Fada AA, Abdel-Rahman AAH (2013) Preparation and application of nonionic polypropylene oxide-graft-polyethylene glycol copolymer surfactants as demulsifier for petroleum crude oil emulsions. J Dispers Sci Technol 34:161–172
Wang J, Hu F-L, Li C-Q, Li J, Yang Y (2010) Synthesis of dendritic polyether surfactants for demulsification. Sep Purif Technol 73:349–354
Zhang Z, Xu G, Wang F, Dong S, Chen Y (2005) Demulsification by amphiphilic dendrimer copolymers. J Colloid Interface Sci 282:1–4
Kailey I, Feng X (2013) Influence of structural variations of demulsifiers on their performance. Ind Eng Chem Res 52:785–793
Abdel-Azim AAA, Zaki NN, Maysour NES (1998) Polyoxyalkylenated amines for breaking water-in-oil emulsions: effect of structural variations on the demulsification efficiency. Polym Adv Technol 9:159–166
Wu J, Xu Y, Dabros T, Hamza H (2005) Effect of EO and PO positions in nonionic surfactants on surfactant properties and demulsification performance. Colloids Surf A 252:79–85
Zhang Z, Xu GY, Wang F, Dong SL, Li YM (2004) Characterization and demulsification of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) copolymers. J Colloid Interface Sci 277:464–470
Berger PD, Hsu C, Arendell JP (1988) Designing and selecting demulsifiers for optimum field performance on the basis of production fluid characteristics. SPE Prod Eng 3:522–526
Liu G, Xu X, Gao J (2003) Study on the compatibility of high-paraffin crude oil with electric desalting demulsifiers. Energy Fuels 17:625–630
Binks BP, Murakami R, Armes SP, Fujii S (2006) Effects of pH and salt concentration on oil-in-water emulsions stabilized solely by nanocomposite microgel particles. Langmuir 22:2050–2057
Ye G, Lu X, Han P, Shen X (2010) Desalting and dewatering of crude oil in ultrasonic standing wave field. J Petrol Sci Eng 70:140–144
Vafajoo L, Ganjian K, Fattahi M (2012) Influence of key parameters on crude oil desalting: an experimental and theoretical study. J Petrol Sci Eng 90–91:107–111
Ohashi K, Hashizaki K, Taguchi H, Saito Y (2009) Effects of inorganic salts on micellization and solubilization in an aqueous solution of poly(ethylene oxide)/poly(propylene oxide)/poly(ethylene oxide) triblock copolymer. J Dispers Sci Technol 30:720–724
Kadam Y, Singh K, Marangoni DG, Ma JH, Aswal VK, Bahadur P (2010) Thermodynamic of micelle formation of nonlinear block co-polymer Tetronic® T904 in aqueous salt solution. Colloids Surf A 369:121–127
Zhai X, Xu G, Chen Y, Liu T, Zhang J, Yuan J, Tan Y, Zhang J (2013) Effect of inorganic salts on the aggregation behavior of branched block polyether at air/water and n-heptane/water interfaces. Colloid Polym Sci 291:2825–2836
Saien J, Asadabadi S (2014) Salting out effects on adsorption and micellization of three imidazolium-based ionic liquids at liquid–liquid interface. Colloids Surf A 444:138–143
Zajforoushan Moghaddam S, Thormann E (2015) Hofmeister effect of salt mixtures on thermo-responsive poly(propylene oxide). PCCP 17:6359–6366
Li Simon MK (1991) Process for preparing tetraphenolic compounds. US Patent 5,012,016, 30 Apr 1991
Patel K, Bahadur P, Guo C, Ma JH, Liu HZ, Yamashita Y, Khanal A, Nakashima K (2007) Salt induced micellization of very hydrophilic PEO–PPO–PEO block copolymers in aqueous solutions. Eur Polym J 43:1699–1708
Sanan R, Mahajan RK (2013) Polyethylene glycol assisted micellar, interfacial and phase separation studies of triblock copolymer–nonionic surfactant mixtures. Colloids Surf A 433:145–153
Molina-Bolívar JA, Hierrezuelo JM, Carnero Ruiz C (2013) Energetics of clouding and size effects in non-ionic surfactant mixtures: the influence of alkyl chain length and NaCl addition. J Chem Thermodyn 57:59–66
Sayed GH, Ghuiba FM, Abdou MI, Badr EAA, Tawfik SM, Negm NAM (2012) Synthesis, surface, thermodynamic properties of some biodegradable vanillin-modified polyoxyethylene surfactants. J Surfact Deterg 15:735–743
Nordon A, Meunier C, Carr RH, Gemperline PJ, Littlejohn D (2002) Determination of the ethylene oxide content of polyether polyols by low-field 1H nuclear magnetic resonance spectrometry. Anal Chim Acta 472:133–140
LeBas CL, Turley PA (1984) Primary hydroxyl content in polyols-evaluation of two nuclear magnetic resonance (NMR) methods. J Cell Plast 20:194–199
Batıgöç Ç, Akbaş H, Boz M (2011) Thermodynamics of non-ionic surfactant Triton X-100-cationic surfactants mixtures at the cloud point. J Chem Thermodyn 43:1800–1803
Li JL, Bai DS, Chen BH (2009) Effects of additives on the cloud points of selected nonionic linear ethoxylated alcohol surfactants. Colloids Surf A 346:237–243
Rao W, Wang Y, Han J, Wang L, Chen T, Liu Y, Ni L (2015) Cloud point and liquid-liquid equilibrium behavior of thermosensitive polymer L61 and salt aqueous two-phase system. J Phys Chem B 119:8201–8208
Mata JP, Majhi PR, Guo C, Liu HZ, Bahadur P (2005) Concentration, temperature, and salt-induced micellization of a triblock copolymer Pluronic L64 in aqueous media. J Colloid Interface Sci 292:548–556
Schott H (1969) Hydrophile–lipophile balance and cloud points of nonionic surfactants. J Pharm Sci 58:1443–1449
Pandit N, Trygstad T, Croy S, Bohorquez M, Koch C (2000) Effect of salts on the micellization, clouding, and solubilization behavior of pluronic F127 solutions. J Colloid Interface Sci 222:213–220
Jain NJ, George A, Bahadur P (1999) Effect of salt on the micellization of pluronic P65 in aqueous solution. Colloids Surf A 157:275–283
Batıgöç Ç, Akbaş H (2011) Spectrophotometric determination of cloud point of Brij 35 nonionic surfactant. Fluid Phase Equilib 303:91–95
Dong J, Chowdhry BZ, Leharne SA (2003) Surface activity of poloxamines at the interfaces between air–water and hexane–water. Colloids Surf A 212:9–17
Chen Y, Liu T, Xu G, Zhang J, Zhai X, Yuan J, Tan Y (2015) Aggregation behavior of X-shaped branched block copolymers at the air/water interface: effect of block sequence and temperature. Colloid Polym Sci 293:97–107
Alexandridis P, Athanassiou V, Fukuda S, Hatton TA (1994) Surface activity of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) copolymers. Langmuir 10:2604–2612
Rosen M (1974) Relationship of structure to properties in surfactants: II. Efficiency in surface or interfacial tension reduction. J Am Oil Chem Soc 51:461–465
Rosen MJ, Aronson S (1981) Standard free energies of adsorption of surfactants at the aqueous solution/air interface from surface tension data in the vicinity of the critical micelle concentration. Colloids Surf 3:201–208
Rosen MJ, Kunjappu JT (2004) Surfactants and interfacial phenomena. Wiley, New Jersey
Zhang Y, Cremer PS (2010) Chemistry of Hofmeister anions and osmolytes. Annu Rev Phys Chem 61:63–83
Deyerle BA, Zhang Y (2011) Effects of Hofmeister anions on the aggregation behavior of PEO–PPO–PEO triblock copolymers. Langmuir 27:9203–9210
Kunz W, Lo Nostro P, Ninham BW (2004) The present state of affairs with Hofmeister effects. Curr Opin Colloid Interface Sci 9:1–18
Li X, Huang K, Xu Y, Liu H (2014) Interaction of sodium and potassium ions with PEO-PPO copolymer investigated by FTIR, Raman and NMR. Vib Spectrosc 75:59–64
Al Sabagh AM, Kandil NG, Badawi AM, El-Sharkawy H (2000) Surface activity and thermodynamic of micellization and adsorption for isooctylphenol ethoxylates, phosphate esters and their mixtures with N-diethoxylated perfluorooctanamide. Colloids Surf A 170:127–136
Rosen MJ, Cohen AW, Dahanayake M, Hua XY (1982) Relationship of structure to properties in surfactants. 10. Surface and thermodynamic properties of 2-dodecyloxypoly(ethenoxyethanol)s, C12H25(OC2H4)xOH, in aqueous solution. J Phys Chem 86:541–545
Mańko D, Zdziennicka A, Jańczuk B (2014) Thermodynamic properties of adsorption and micellization of n-oktyl-β-d-glucopiranoside. Colloids Surf B 114:170–176
Liu T, Xu G, Gong H, Pang J, He F (2011) Effect of alcohols on aggregation behaviors of branched block polyether Tetronic 1107 at an air/liquid surface. Langmuir 27:9253–9260
Abdel-Raouf M-S, Abdul-Raheim A-R, Abdel-Azim A-A (2011) Surface properties and thermodynamic parameters of some sugar-based ethoxylated amine surfactants: 1—synthesis, characterization, and demulsification efficiency. J Surfact Deterg 14:113–121
Xin X, Xu G, Zhang Z, Chen Y, Wang F (2007) Aggregation behavior of star-like PEO–PPO–PEO block copolymer in aqueous solution. Eur Polym J 43:3106–3111
Chen LJ, Lin SY, Huang CC, Chen EM (1998) Temperature dependence of critical micelle concentration of polyoxyethylenated non-ionic surfactants. Colloids Surf A 135:175–181
Islam MN, Kato T (2003) Temperature dependence of the surface phase behavior and micelle formation of some nonionic surfactants. J Phys Chem B 107:965–971
Jolicoeur C, Philip PR (1974) Enthalpy–entropy compensation for micellization and other hydrophobic interactions in aqueous solutions. Can J Chem Eng 52:1834–1839
Kim HU, Lim KH (2004) A model on the temperature dependence of critical micelle concentration. Colloids Surf A 235:121–128
Chen L-J, Lin S-Y, Huang C-C (1998) Effect of hydrophobic chain length of surfactants on enthalpy–entropy compensation of micellization. J Phys Chem B 102:4350–4356
Mehta SK, Bhasin KK, Chauhan R, Dham S (2005) Effect of temperature on critical micelle concentration and thermodynamic behavior of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media. Colloids Surf A 255:153–157
Inoue T (2009) Micelle formation of polyoxyethylene-type nonionic surfactants in bmimBF4 studied by 1H NMR and dynamic light-scattering. J Colloid Interface Sci 337:240–246
Inoue T, Ohmura H, Murata D (2003) Cloud point temperature of polyoxyethylene-type nonionic surfactants and their mixtures. J Colloid Interface Sci 258:374–382
Kumar B, Tikariha D, Ghosh KK, Barbero N, Quagliotto P (2013) Effect of polymers and temperature on critical micelle concentration of some gemini and monomeric surfactants. J Chem Thermodyn 62:178–185
Sugihara G, Hisatomi M (1999) Enthalpy–entropy compensation phenomenon observed for different surfactants in aqueous solution. J Colloid Interface Sci 219:31–36
Acknowledgments
The authors gratefully acknowledge the financial support from National Science and Technology Major Project, China (Grant No. 2016ZX05025-003-02).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, Z., Shi, Z., Zhao, S. et al. Synthesis and Properties of a Novel Branched Polyether Surfactant. J Surfact Deterg 19, 1107–1120 (2016). https://doi.org/10.1007/s11743-016-1879-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1879-7