Abstract
A new polymerizable nonionic surfactant with reactive vinyl groups has been synthesized from N-methylol acrylamide using a two-step procedure. The structure of the surfactant molecule was characterized by Fourier transform infrared, 1H nuclear magnetic resonance and mass spectroscopy. The surface active properties alongside its self-assembly properties were investigated by surface tension, electrical conductivity, and fluorescence spectroscopy measurements. As compared with other nonionic surfactants, this study showed that this polymerizable surfactant possesses slightly a higher critical micelle concentration (CMC) value and the surface tension value at CMC. The obtained CMC values were compatible among measurements, ca. 0.02–0.038 M. The evidence of micelle formation also provided by the zeta potential measurements and the obtained zeta potential values showed that the polymerizable surfactant solutions had limited stability. The hydrolysis stability and solubility of the polymerizable surfactant were also investigated. The solubility results have shown that it was soluble in polar solvents while insoluble in nonpolar solvents both at room temperature and 40 °C. The acidic and basic hydrolysis of the surfactant increased as the temperature increased and the hydrolysis stability was 180 min (basic medium) and 55 min (acidic medium) at 80 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants, consisting of a hydrophilic head and a hydrophobic tail unit in their molecules, play an essential and versatile role in obtaining stable latexes with desired properties in free-radical emulsion polymerization [1–3]. In many applications lower molecular weight surfactants are used, and they are adsorbed on particle surface with weak H or σ bonding interactions during the polymerization in order to control both the size of the particles and the stability of the latexes [4, 5]. However, these physically bound surfactants tend to migrate from the particle surface during polymerization and film formation process and create a separate phase. This causes some problems with latex and film properties such as the lack of stability, water sensitivity, high ionic strength, or lack of gloss and adhesivity [6–10]. Those effects are major disadvantages of emulsion applications like paints, adhesives, and other protective coatings. In order to overcome these difficulties, polymerizable surfactants, which have a further reactive group compared to conventional surfactants, can be used due to their ability to undergo polymerization. Since polymerizable surfactants are covalently incorporated into the polymer chains by copolymerizing with the main monomers, they connect to the particle surface and reduce the surface tension effectively, thereby improving latex properties like particle size, colloidal stability, etc. [11–13]. The use of polymerizable surfactants in emulsion polymerization has drawn much attention since the first discovery of them in 1958 [14]. Chen et al. reported that when a surfmer is used, the surfactant migration is reduced and the water resistance and surface adhesion are improved [15].
Typical reactive groups such as vinyl, allyl, or acrylic groups are located at the hydrophobic tail or hydrophilic head group of the surfactant molecule [16]. To obtain novel latex properties distinct from conventional surfactants, different types of polymerizable surfactants, cationic, anionic, and nonionic have been synthesized [5, 17, 18]. Among them, the nonionic ones bearing different reactive parts (i.e., methacrylic, allylic, maleic, and vinylic) show excellent surface activity and are not easily affected by change of pH values [19].
N-methylol acrylamide (NMA) is a water-soluble bifunctional nonionic monomer with polymerizable vinyl and cross-linkable methylol groups. The condensation reactions occur between its functional groups, and a vinyl group bearing polymerizable surfactant is obtained. The free vinyl groups retain a pendant from the polymer chain and later undergo crosslinking reactions with groups of other polymers or monomers [20, 21]. Because of these important properties, thermoplastic polymers can be formed by copolymerization of N-methylol acrylamide with a variety of vinyl monomers by emulsion polymerization, and they are widely used in industrial applications such as coatings, textile, latex paints, adhesives, and binders [22, 23]. The use of small amounts of NMA in emulsion polymerization may enhance the durability of latexes and may give films with increased wrinkle resistance, impact resistance, abrasion resistance, tensile strength, peel strength, water, alkali, and solvent resistance and gloss [24]. In the former studies, NMA was used as a comonomer in emulsion polymerization of vinyl acetate and several other monomers. It provided superior properties to the latexes such as colloidal stability, adhesion, polarity, and improved the mechanical properties of the films [25, 26]. In our previous study, NMA was used as a cosurfactant in semi-batch emulsion polymerization of vinyl acetate and butyl acrylate with different anionic and nonionic emulsifiers. It was able to cause high conversion of the monomer in small polymer particles, and the obtained latexes were quite stable during and after the polymerization process [20].
The purpose of this study was to synthesize a new surfactant that can contribute to the increase in stability of the emulsion polymerizations and the final latexes, and also to the improvement of the latex and film properties of the emulsion polymers. Polymerizable nonionic surfactant N-methylol acrylamide (p-NMA) containing a reactive vinyl group was prepared by condensation reactions. In this study, we focused on characterizing the p-NMA structure and investigating the physicochemical properties by the surface tension method, electrical conductivity, and fluorescence spectroscopy. In addition, other measurements such as zeta potential, hydrolysis stability, and solubility were performed to verify the micelle formation and their charge properties.
Experimental
Materials
Acrylamide, formaldehyde, 4-methoxyphenol, sodium hydroxide (NaOH), hydrochloric acid (HCl), sulfuric acid (H2SO4), pyrene, acetone, ethyl acetate, hexane, diethyl ether, methanol, ethanol, and chloroform were obtained from Merck. All chemicals were reagent grade quality and used without further purification. Deionized water was used in all studies.
Synthesis of Polymerizable N-Methylol Acrylamide (p-NMA)
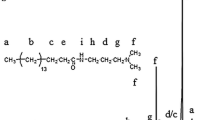
NMA gives condensation reactions between its own functional groups, and by using this property, an oligomeric polymerizable surfactant was obtained. In a typical experiment, 4-methoxyphenol (0.02 g) was mixed with 30 mL water and was heated to 60 °C, and then 42 g acrylamide was added. After the solution was cooled to room temperature, a 25 g formaldehyde solution was added. The pH value of the solution was set to 10.5 by adding 2.5 M sodium hydroxide (NaOH), and the solution was stirred for 1 h. The temperature spontaneously increased to 35–40 °C while preparing this solution. N-methylol acrylamide and N,N-dimethylol acrylamide were formed from the methylol reactions between acrylamide and formaldehyde (Fig. 1). 4-Methoxyphenol was added to the reaction medium to prevent the polymerization of acrylamide with vinyl groups and allow the reactions between the NH2 group of acrylamide and the carbonyl group of formaldehyde. In the second step, the pH value was adjusted to 5.5 with 2.5 M sulfuric acid (H2SO4) and 0.11 g 4-methoxyphenol was added into the reaction medium, and the final solution was stirred for 1 h. Condensation reactions occurred both between the functional groups of NMA and N,N-dimethylol acrylamide. Polymerizable N-methylol acrylamide (p-NMA) was obtained in 48 % aqueous solution.
Characterization of Polymerizable N-Methylol Acrylamide (p-NMA)
The surfactant structure was confirmed by recording FTIR spectra with a Perkin Elmer Spectrum One FTIR (ATR sampling accessory) spectrophotometer. 1H NMR spectra were taken using a Bruker AV 400 MHz spectrometer in DMSO. Mass spectrum was obtained by a MALDI-TOF-MS system equipped with a 337 nm nitrogen UV-Laser from Bruker Daltonics 67 Microflex (Germany) without using a matrix.
The surface tension measurements were performed with a tensiometer (KSV instruments, Sigma 701 model) using the Wilhelmy plate method. The plate was carefully flamed before measurement. The surfactant solutions prepared with distilled water and the instrument were checked by measuring the surface tension of distilled water. Five readings were taken for each measurement to assure their reproducibility. The conductivity measurements of surfactant solutions were taken using a Eutech/Oakton PC 510 model conductometer, and the average of three measurements was taken. The fluorescence spectrum was recorded on a Jobin Yvon-Horiba Fluoromax-P fluorescence spectrophotometer at 25 °C using pyrene as a probe. The emission spectra were scanned from 300 to 350 nm using a 373 nm excitation wavelength. The intensity ratio (I 1/I 3) of the first (I 1) over the third (I 3) vibronic band of the emission spectrum of pyrene, was used to detect the micropolarity of hydrophobic microdomains [27, 28]. A series of surfactant solutions with the same pyrene concentration (1.0 × 10−7 M) was prepared, and the solutions were stirred at room temperature overnight to reach the equilibrium of pyrene in the aqueous phase. The zeta potential of p-NMA was measured with a Brookhaven 90 Plus/BI-MAS on Zeta PLUS mode. All measurements were taken at 25 °C.
The solubility of p-NMA in different solvents was estimated by placing 50 mg surfactant and 1 mL solvent. The dissolution property was determined visually both at room temperature and 40 °C. For hydrolysis stability, a mixture of 10 mmoL polymerizable surfactant and 10 mL 0.1 N NaOH and 0.1 N HCl were separately placed in a water bath at 40 and 80 °C, and the time required for the solutions to be clouded was measured.
Results and Discussion
Spectral Analysis of the Polymerizable Surfactant
P-NMA was synthesized in two steps, and it was obtained as a 48.0 % wt aqueous solution. The water in aqueous p-NMA solution was partially evaporated and dried under vacuum at 60 °C and white crystals were obtained. This crystal form was structurally characterized using IR-spectroscopy, 1H NMR and MALDITOF-MS techniques.
In the FTIR–ATR spectra (Fig. 2) of p-NMA, characteristic peaks were observed. IR (cm−1): 3306 (N–H stretch), 3066, 2956 (C–H stretch), 1655 (C=O stretch), 1300–1450 (C–H bend), 1223 (C–O stretch). The 1H NMR spectrum of p-NMA was as follows: (DMSO, 400 MHz): δ = 6.10–6.20 (m, 8H, CH2), 5.50–5.60 (m, 8H, CH2), 6.18–6.31 (m, 8H, CH), 8.11 (s, 1H, NH), 4.40–4.61 (m, 4H, NCH2OCH2N), 4.55 (m, 2H, NCH2O), 3.65 (t, 1H, OH).The other peaks are due to the unreacted monomers and residual substances (Fig. 3).
In the stick diagram (Fig. 4) showing the mass spectrum of p-NMA, the peaks were separated by 196 mass units, corresponding to the molecular weight of the repeating unit. The line produced by the heaviest ion passing through the machine at m/z = 782.8 is due to the molecular ion peak (M+). By analyzing this peak, the average degree of polymerization (DP n ) was calculated to be 4. Fragmentation of C–C bonds occurs because they are usually weaker than C–H bonds, and this produces a mixture of vinyl, methylene, carbonyl, nitrogen groups, and NMA monomer. The low intensity peak at m/z = 573.3 was obtained by fragmentation of one repeating unit and a CH2 group from the molecular ion peak. The peak at m/z = 544.9 having a spacing of 28 reflected the C2H4 + unit. Elimination of an CO+ molecule resulted in a molecular weight difference of 28 mass units and the most intense peak in the spectrum called the base peak was obtained (m/z = 516.8). The analysis results confirmed that the polymerizable surfactant, p-NMA was synthesized as shown in Fig. 1. This structure is composed of four repeating units and H– and HO– groups are located at the end of the molecule and the molecular weight was determined to be 802 g/mol.
Determination of CMC
Like other conventional low molecular weight surfactants, polymerizable surfactants also form micelles above a certain concentration called the critic micelle concentration (CMC). The CMC of surfactants can be determined by measuring some physicochemical properties of their aqueous solutions such as conductivity, surface tension, and viscosity, and by using different methods such as light scattering, calorimetry, fluorescence spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy as a function of surfactant concentrations [29, 30].
Surface Tension
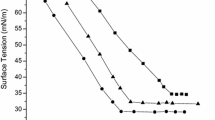
The obtained surface tension values of surfactant solutions were plotted against the logarithm of the solution concentration, and the resulting curve showed two distinct parts. First, the surface tension of aqueous solutions of the surfactant decreased rapidly with addition of surfactant, and then stayed constant. The CMC was determined from the breakpoint or slope change between two regions of the curve (Fig. 5). At lower surfactant concentrations, surfactant molecules were adsorbed at the air/water interface until the solution surface was completely filled. The excess amount of molecules created micelles and the surface tension became constant at higher concentrations [5, 30].
The polymerizable nonionic surfactant prepared in this study has an amphipathic structure different from traditional surfactants that usually have a distinct hydrophobic tail. The CMC value of this surfactant depends on both the hydrophobic and hydrophilic parts of the molecule. The CMC decreases and micelle formation becomes easier with increasing length of the hydrophobic moiety [30]. The polymerizable C=C double bond remains on a phase surface or even in water phase during the formation of micelles. All of them influence the total surface activity of the polymerizable surfactant [19, 29].
Due to the short-chain alkyl groups and presence of extra hydroxyl and amino groups, the p-NMA may be able to interact more strongly with water molecules, via H-bonding, and micelle formation occurs at higher concentrations. The CMC value was 0.02 M, and the surface tension value at the CMC was 44 mN m−1 that indicated the extent of interaction between water and p-NMA molecules at the interface. As expected, short hydrophobic chain length led to an increase of the CMC value, which means that the hydrophobic domain was the predominant driving force for micellization in aqueous solution [31]. Also, the lower surface tension value at the CMC indicated rather good surface activity [32].
Conductivity
Conductivity is an easy method for determining the micellar properties of surfactants [32]. Figure 6 shows the electrical conductivity values against increasing surfactant concentration and the break point of two straight lines gave the CMC value of p-NMA. Below CMC value, the conductivity value rose with an increase in surfactant concentration due to the free surfactant ions in the medium. The surfactant molecules formed micelles when the surfactant concentrations came to the intersection point. The micelle mobility was lower than the free surfactant ions; therefore, the conductivity increase was smaller at the concentrations above the CMC [33].
The conductance data of p-NMA clearly showed a broader inflection in the curve. The CMC value of the surfactant was approximated as 0.025 M, and this was very close to the value obtained from the surface tension measurements.
Fluorescence Measurements
To confirm the CMC value of p-NMA, fluorescence measurements were carried out. Pyrene is the most commonly used fluorescence probe and the characteristics of its emission spectrum could be used to predict the polarity of its environment [34, 35]. Before occurrence of micelles, the pyrene molecule is situated in aqueous phase, but it is located in a highly hydrophobic micelle core above CMC. This polarity change in the environment reflects the spectral characteristics of pyrene and the intensity ratio of the first peak (~373 nm) to the third peak (~384 nm) (I 3/I 1) in the steady state emission spectra shifts while the environment of pyrene changes. When the I 3/I 1 intensity ratio is plotted versus surfactant concentration, the CMC value can be obtained from the midpoint of the plot [33, 36]. As shown in Fig. 7, the constant I 3/I 1 ratio began to fall by an increase in concentration to reach equilibrium, then surfactant molecules formed micelles by surrounding the pyrene, and the intensity ratio became constant. The CMC value of the surfactant was approximated as 0.038 M, and this value was slightly higher than those obtained from surface tension and conductivity measurements. This was because it was very difficult to detect the amount of probe solubilized in the micelles when the CMC value was very low. This is a common feature for nonionic surfactants with very low CMC [37].
It was seen that the CMC values of p-NMA obtained from three different methods were consistent with each other. The CMC values of nonionic surfactants are generally lower compared to ionic surfactants. For example, two kinds of polysorbate surfactants, Tween 20 and Tween 80, have CMC values of 0.05 and 0.01 mM, respectively [38]. As compared with other nonionic surfactants, the CMC value of p-NMA was rather high due to the stronger interaction of p-NMA having extra hydroxyl and amino groups with water molecules via H-bonding. Also, the terminal hydroxyl group of p-NMA could cause an increase of the CMC value. The micelle formation occurred at higher concentrations because of the short hydrophobic chain length.
Zeta Potential
The zeta potential of p-NMA solutions was measured as a function of surfactant concentration (Fig. 8). The zeta potential exhibited a value of nearly zero below 0.003 M. After that, the zeta potential values increased as the concentration increased and finally reached a stable value (ca. −24 mV) at 0.0374 M. The obtained values all were under 30 mV and showed that the p-NMA solutions have limited stability [39]. In addition, the concentration value at the midpoint of the zeta potential curve was overlapped with the CMC values obtained from other measurements. These results showed that the solution became stable and higher zeta potential values were obtained when the micelles formed. As seen from Fig. 8, the zeta potential results were obtained in negative values for all concentrations. These negative values supported that the p-NMA molecules formed micelles with the hydrophobic tail groups towards the inside and the hydrophilic head groups (–OH) towards the outside. It can be said that the end groups on the outer surface of p-NMA and hydroxyl ions released by the dissociation–association equilibrium of the water molecules contributes to give a negative charge.
Solubility of p-NMA
The solubility test of the nonionic p-NMA molecule was evaluated visually in various organic solvents having different polarities both at room temperature (RT) and 40 °C. The solubility results of the surfactant molecule are shown in Table 1 and Fig. 9. The surfactant molecule can be dissolved easily in a variety of polar solvents such as methanol, ethanol, acetone, and ethyl acetate due to its own amphiphilic nature. P-NMA showed the lack of solubility in chloroform, diethyl ether, and hexane, as expected. This was consistent with the structure of this molecule, which had a short hydrocarbon tail and was very soluble in water.
Stability to Hydrolysis
The hydrolysis stability of p-NMA was measured against basic (0.1 N NaOH) and acidic (0.1 N HCl) media at two different temperatures, 40 and 80 °C. While the p-NMA solution showed the resistance to hydrolysis with both basic and acidic treatment at 40 °C, it became cloudy due to the hydrolysis in both cases at 80 °C (Fig. 10). At this temperature, the hydrolysis stability of p-NMA was found to be 180 and 55 min in the basic and acidic media, respectively. In other words, the acidic and basic hydrolysis of p-NMA increased with increasing temperature, and p-NMA was found to be more resistant against basic hydrolysis.
Furthermore, the cloud point is a significant factor when the non-ionic surfactants are used in emulsion polymerization. The value of this point must be at polymerization temperature or higher. If this condition cannot be obtained when the non-ionic surfactants are used alone, they must be used along with ionic surfactants in emulsion polymerizations [19]. Accordingly, it can be clearly said that p-NMA with a higher cloud point can be used alone in these reactions as the emulsion polymerization temperature is about 80 °C.
References
Chen L, Yan L, Li Q, Wang C, Chen S (2010) Controllable synthesis of new polymerizable macrosurfactants via CCTP and RAFT techniques and investigation of their performance in emulsion polymerization. Langmuir 26:1724–1733
Matahwa H, Mcleary JB, Sanderson RD (2006) Comparative study of classical surfactants and polymerizable surfactants (surfmers) in the reversible addition–fragmentation chain transfer mediated miniemulsion polymerization of styrene and methyl methacrylate. J Polym Sci A Polym Chem 44:427–442
Prasath RA, Ramakrishnan S (2005) Synthesis, characterization, and utilization of itaconate-based polymerizable surfactants for the preparation of surface-carboxylated polystyrene latexes. J Polym Sci A Polym Chem 43:3257–3267
Asua JM, Schoonbrood HAS (1998) Reactive surfactants in heterophase polymerization. Acta Polym 49:671–686
Morizur JF, Irvine DJ, Rawlins JJ, Mathias LJ (2007) Synthesis of new acrylate-based nonionic surfmers and their use in heterophase polymerization. Macromolecules 40:8938–8946
Aramendia E, Barandiaran MJ, Cal JC (2002) Improving latex performance by using polymerizable surfactants. In: Daniels ES, Sudol ED, El-Aasser MS (eds) Polymer colloids: science and technology of latex systems. ACS symposium series. American Chemical Society, Washington, DC, p 168
Joynes D, Sherrington DC (1997) Novel polymerizable mono-and divalent quaternary ammonium cationic surfactants: 2.Surface active properties and use in emulsion polymerization. Polymer 38:1427–1438
Gon AM, Sherrington DC (1999) Reactive surfactants in heterophase polymerization XXIII. Synthesis and characterisation of novel dialkyl maleate cationic surfmers. Polymer 40:1067–1079
Hellgren AC, Weissenborn P, Holmberg K (1999) Surfactants in water-borne paints. Prog Org Coat 35:79–87
Aramendia E, Mallegol J, Jeynes C, Barandiaran MJ, Keddie JL, Asua JK (2003) Distribution of surfactants near acrylic latex film surfaces: a comparison of conventional and reactive surfactants (surfmers). Langmuir 19:3212–3221
Klimenkovs I, Zhukovska I, Uzulina I, Zicmanis A, Guyot A (2003) Maleic diamide polymerizable surfactants: applications in emulsion polymerization. C R Chim 6:1295–1304
Samakande A, Hartmann PC, Sanderson RD (2006) Synthesis and characterization of new cationic quaternary ammonium polymerizable surfactants. J Colloid Interface Sci 296:316–323
Guyot A (2004) Advances in reactive surfactants. Adv Colloid Interface Sci 108:3–22
Freedman HH, Mason JP, Medalia AI (1958) Polysoaps-II: the preparation of vinyl soaps. J Am Chem Soc 23:76–82
Chen SA, Chang HS (1985) Kinetics and mechanism of emulsifier-free emulsion polymerization: styrene/surface active ionic comonomer system. J Polym Sci A Polym Chem 23:2615–2630
Amalvy JI, Unzue MJ, Schoonbrood HAS (2002) Reactive surfactants in heterophase polymerization: colloidal properties, film-water absorption, and surfactant exudation. J Polym Sci A Polym Chem 40:2994–3000
Rongqiang L, Fengmei Y, Junli Z, Xu C, Wang J (2014) The self-assembly properties of a series of polymerizable cationic gemini surfactants: effect of the acryloxyl group. Colloids Surf A Physicochem Eng Asp 444:276–282
Kinoshita K, Fukakusa K, Fukuzumi T, Takahata N (1994) Jpn Pat 94(49):108
Zhenga J, Luoa J, Zhoua DW (2010) Preparation and properties of non-ionic polyurethane surfactants. Colloids Surf A Physicochem Eng Asp 363:16–21
Berber H, Sarac A, Yıldırım H (2011) Synthesis and characterization of water-based poly(vinyl acetate-co-butyl acrylate) latexes containing oligomeric protective colloid. Polym Bull 66:881–892
Hu T, Fang Y, Yu H, Chen L, Chen S (2007) Synthesis of poly(N-methylolacrylamide)/polymethylacrylamide hybrids via frontal free-radical polymerization. Colloid Polym Sci 285:891–898
Brown NR, Frazier CE (2007) Cross-linking poly[(vinyl acetate)-co-nmethylolacrylamide latex adhesive performance. Part I. N-methylolacrylamide (NMA) distribution. Int J Adhes Adhes 27:547–553
Krishnan S, Klein A, El-Aasser MS, Sudol D (2003) Influence of chain transfer agent on the crosslinking of poly(N-butyl methacrylate-co-N-methylol acrylamide) latex particles and films. Macromolecules 36:3511–3518
Chen L, Hu T, Yu H, Chen S, Pojman JA (2007) First solvent-free synthesis of poly(N-methylolacrylamide) via frontal free-radical polymerization. J Polym Sci A Polym Chem 45:4322–4330
Cernakova L, Chrastova V, Volfova P, Zahoranova A (2002) Polystyrene/poly(butyl acrylate) dispersions having N-methylol groups a spectroscopic study. Macromol Symp 179:305–314
Berber H, Sarac A, Yıldırım H (2011) A comparative study on water-based coatings prepared in the presence of oligomeric and conventional protective colloids. Prog Org Coat 71:225–233
Tsubone K, Ghosh S (2004) Micellization of an anionic gemini surfactant having N,N-dialkylamide, carboxyl, and carboxylate groups in aqueous NaCl solutions. J Surf Deterg 7:47–52
Chiang WH, Hsu YH, Lou TW, Chern CS, Chiu HC (2009) Effects of mPEG grafts on morphology and cross-linking of thermally induced micellar assemblies from PAAc-based graft copolymers in aqueous phase. Macromolecules 42:3611–3619
Katritzky AR, Pacureanu LM, Slavov SH, Dobchev DA, Karelson M (2008) QSPR study of critical micelle concentrations of nonionic surfactants. Ind Eng Chem Res 47:9687–9695
Hait SK, Moulik SP (2001) Determination of critical micelle concentration (CMC) of nonionic surfactants by donor–acceptor interaction with iodine and correlation of cmc with hydrophile–lipophile balance and other parameters of the surfactants. J Surf Deterg 4:303–309
Borkovec M (2001) Handbook of applied surface and colloid chemistry, vol 18. Wiley, New York
Wilkinson TS, Boonstra A, Montoya-Goni A, van Es S, Monteiro MJ, German AL (2001) Synthesis and characterization of a novel addition–fragmentation reactive surfactant (TRANSURF) for use in free-radical emulsion polymerizations. J Colloid Interface Sci 237:21–27
Aguiar J, Carpena P, Bolívar JAM, Ruiz CC (2003) On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interface Sci 258:116–122
Ray GB, Chakraborty I, Moulik SP (2006) Pyrene absorption can be a convenient method for probing critical micellar concentration (CMC) and indexing micellar polarity. J Colloid Interface Sci 294:248–254
Thomas JK (1980) Radiation-induced reactions in organized assemblies. Chem Rev 80:283–299
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Maiti S, Chatterji PR (2000) Aggregation and polymerization of amphiphilic macromonomers with a double bond at the hydrophilic terminal. J Colloid Interface Sci 232:273–281
Mahmood ME, Al-Koofee DAF (2013) Effect of temperature changes on critical micelle concentration for tween series surfactant. Glob J Sci Front Res Chem 13(4):1–8
Hsu CH, Shau SM, Jeng RJ, Chiu HC, Dai SA, Conte ED, Suen SY (2013) Determination of critical micelle concentration of dendritic surfactant synthesized via a selective ring-opening addition reaction. Microchem J 110:48–53
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tamer, Y., Berber Yamak, H. & Yıldırım, H. Structural and Physicochemical Properties of a Polymerizable Surfactant Synthesized from N-Methylol Acrylamide. J Surfact Deterg 19, 405–412 (2016). https://doi.org/10.1007/s11743-015-1785-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1785-4