Abstract
Plants face many stresses in their natural environment. Different types of phytohormones help the plant to adapt to stressful conditions. Melatonin is a newly known substance in the plant kingdom, that is effective in relieving stress for plants. In this study, the effect of melatonin on Phaseolus vulgaris L. cv. Pak that was exposed to salinity stress at concentrations of 100 and 200 mM NaCl was investigated. Treatment of stressed plants and control group (0 mM NaCl) with concentrations of 100, 200 μM melatonin improved effects of salinity on the dry weight of shoot, the root and the photosynthetic pigments, net photosynthesis rate, leaf stomatal conductance, transpiration rate, K+, Na+, and Ca2+ content, and the ratio of K+/Na+. Melatonin application increased proline and sugar content while decreasing malondialdehyde and H2O2 content that were increased by salinity stress. Also, melatonin increased the activity of antioxidant enzymes of catalase, peroxidase, ascorbate peroxidase, polyphenol oxidase, and superoxide dismutase. Overall, lower concentrations of melatonin had significant ameliorating effects on salinity stress-induced damage to the bean plant. While, the use of 400 μM melatonin could somewhat exacerbate the effects of salinity in P. vulgaris L. cv. Pak.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a soil condition characterized by a high concentration of soluble salts such as NaCl, which is the most abundant and soluble salt in the soil (Munns and Tester 2008). Salinity stress has an unfavorable impact on agricultural products worldwide, with a greater impact in the arid and semi-arid areas (PasandiPour et al. 2013). According to the Food and Agriculture Organization of the United Nations (FAO) above 6%, more than 800 million hectares of land throughout the world are affected by salinity problems (Munns and Tester 2008). Salinity causes ionic imbalance both outside and inside the plant. First, soil salinity reduces soil water potential leading to a decrease in water level in plants and consequently resulting in a lower rate of cell division and plant growth. Then, the high density of the Cl− and Na+ ions inside the cells and decreased K+/Na+ ratio impair plant enzymes and membranes function, induce cell dehydration, stomata closure, and minimize CO2 level inside the photosynthetic cell. As well, the decrease in Mg2+ and toxicity caused by the increase of Cl− in leaf cells decreases the quantity of chlorophylls, resulting in reduced photosynthesis (Parihar et al. 2015; Rady and Mohamed 2015; Sytar et al. 2017; Liu et al. 2018). For plants to grow in saline soil, they need to adjust osmotically to maintain a positive turgor pressure (Flowers et al. 2015). The plant strives to adapt to osmotic stress and ion toxicity due to salinity by producing osmolytes such as proline and soluble sugars and intracellular compartmentation of ions (Li et al. 2017).
Another consequence of salinity is oxidative stress. As a result of oxidative stress, reactive oxygen species (ROSs) damage proteins and nucleic acids, oxidize carbohydrates, negatively affect the K+ and Ca2+ cation channels, and eventually damage pigments and membranes. Lipid peroxidation disrupts membrane integrity and metabolism balance. The most sustained ROS produced in saline stress is H2O2, which plants must balance its production and decomposition to avoid its effects. The use of enzymatic and non-enzymatic antioxidant systems to neutralize ROS is one of the strategies that plants take in the stress conditions (Zhang et al. 2014b; Demidchik 2015; Rady and Mohamed 2015).
Plants differ greatly in their tolerance of salinity, as reflected in their different growth responses (Munns and Tester 2008). Previously, researchers have applied a variety of chemical compounds to raise the extent of resistance of plants to salinity stress conditions (Khadri et al. 2007). In recent years, Melatonin (MEL) has been at the core of attention. MEL is a tryptophan-derived indoleamine-type hormone that is first found in the bovine pineal gland and then in many vertebrate and invertebrate animals (Arnao and Hernández-Ruiz 2014). In vertebrates, several physiological roles like regulation of circadian rhythms, photoperiodism, acting as an antioxidant compound and impacting on sleep, reproduction, and the immune system as an immune buffer or as an anti-inflammatory compound in the presence of exacerbated immune responses (Carrillo-Vico et al. 2013), have been attributed to MEL (Li et al. 2017; Afreen et al. 2006). Its discovery in higher plants dates as back as 1995. Plants’ chloroplasts are where the biosynthesis of MEL occurs. The MEL content of plant cells is much higher than that of animal cells (Afreen et al. 2006; Zheng et al. 2017). What makes the study of MEL important in plants is the multiple roles it plays in plant growth and defense against environmental factors. There have been reports of the role of MEL in improving plant responses to stresses such as drought (Ye et al. 2016; Li et al. 2019a, b), salinity (Huang et al. 2017; Jiang et al. 2016a, b; Liu et al. 2018; Zhang et al. 2014a), cold stress (Yang et al. 2018; Zhang et al. 2014a), attack of pathogens and protection against herbicide (Park et al. 2013), and ion toxicity (Posmyk et al. 2008; Yadu et al. 2018).
A report of increased levels of endogenous MEL in UV-B-treated Glycyrrhiza uralensis indicates its effective role in protecting against UV-induced oxidative damage (Afreen et al. 2006). The use of external MEL in Melon with the support of chlorophyll stability improved photosynthetic efficiency and light absorption by increasing the activity of superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT) enzymes mitigated the effects of cold stress (Zhang et al. 2017). MEL improved salinity in watermelon, apple, and cucumber by improving photosynthesis and ion homeostasis (Gao et al. 2019). Pre-treatment with MEL increases the photosynthetic capacity and development of the root system. Increased expression of one of the genes in the biosynthetic pathway of MEL (MzASMT9) in Arabidopsis increased its tolerance to salt than wild-type plants (Zheng et al. 2017). A similar result was observed for transgenic Panicum virgatum L. with more MEL biosynthetic ability in salt tolerance. Proline content in this plant was higher than in the control group (Huang et al. 2017). The application of MEL in the oats exposed to salinity increased chlorophyll content and leaf area and increased fresh and dry weight of the plant, while the activity of SOD, POX, CAT, and APX enzymes (Gao et al. 2019). Liang et al. (2015) showed that using external MEL will promote tolerance of rice to salinity by direct/indirect inhibition of H2O2 accumulation (Liang et al. 2015).
Common bean (Phaseolus vulgaris L.), with high protein and other nutrients such as fiber, vitamins, and nutrient ions, has high food intake in the developing countries and is considered as one of the most important crops in areas such as the Middle East, Latin America, and Africa (Talaat et al. 2015; Rady and Mohamed 2015; López-Barrios et al. 2016; Hnatuszko-Konka et al. 2014). This plant shows low tolerance to salinity and is known as a glycophyte plant. A decrease in growth and weight loss of plants due to salinity has been reported in P. vulgaris L. (Sytar et al. 2017; Brugnoli and Lauteri 1991; Rady and Mohamed 2015). In this study, we attempt to understand the effect of MEL treatment on P. vulgaris L. cv. Pak plant under salinity stress conditions. We hypothesize that the use of MEL improved beanʼs tolerance to salt stress by an increase in antioxidant enzyme activity and ion homeostasis.
Materials and methods
Plant material and growth conditions
A total of 0.058 g of MEL (Sigma-Aldrich) were dissolved absolutely with 25 mmol/L concentration and it was then stored at the temperature of − 20 °C. Twenty-five mmol/L of MEL was further diluted to 400, 200, and 100 µM. To soak the sterilized seeds of P. vulgaris distilled water or MEL solutions with a respective concentration of 100, 200, and 400 µM were used for 12 h. As the seedlings sprouted, they were transplanted into perlite-containing, 10 cm pots which were placed in a greenhouse and exposed to the light of 16/8 h interval along with other controlled conditions (heat 25 ± 5 °C, approximate humidity of 60 ± 10%, and 1200–1400 light flux). A half-strength (pH range of 6.8–7) solution of Hoagland was used to irrigate them. In the four-leaf stage, 21 days after planting, melatonin spray was used with a concentration of none, 100, 200, and 400 μM (40 mL). Then, the pots were divided into three groups as each group was exposed to either 0, 100, or 200 mM NaCl. Concentrations of 100 and 200 mM salt were supplied by adding NaCl by weight to the half-strength Hogland nutrient solution. Salinity stress was applied to the plants by irrigating them with a half-strength solution of Hogland containing NaCl, and 12 plants received each treatment 48 h after salinity stress, plants were harvested, and were either frozen at – 80 °C using liquid nitrogen or dried for measurements. A minimum of three biological replicates per condition were used.
Measurement of photosynthetic characteristics
Evaluations for net photosynthesis rate (PN), leaf stomatal conductance (C), and transpiration rate (E) were taken from not separated young, healthy, and well-grown leaves of the main stem. To comply with the standard conditions for all treatments, the conditions of the leaf chamber were adjusted according to the culture room conditions. Measurements were taken using a portable photosynthesis system (CI-340, CID Bio-Science). Measurement was carried out on the second and third leaves of bean plants under similar developmental stages and environmental conditions for all plants.
Photosynthetic pigments’ measurement
For measuring chlorophyll (Chl) and carotenoid (Car) contents, Arnon method was used: 0.5 g of leaf sample in 20 mL of 80% acetone was ground. Extracts were centrifuged (Model SIGMA 2-16KL) at 6000 rpm for 10 min, and the absorbance of the supernatant was measured at 663, 645, and 470 nm with a spectrophotometer (Model Epoh, BioTek company, England) for Chl a, Chl b, and Car, respectively. The total chlorophyll (Total Chl), Chl a and Chl b, and Car contents were calculated using the following equations (Arnon 1967):
Evaluation of proline and sugars
The Bates et al.’s method was used to measure proline content. The leaf sample (0.2 g) was homogenized with aqueous sulfosalicylic acid (3%). Ninhydrin, acetic acid finally, toluene were added to the supernatant. The colored product of the reaction was read at 520 nm using toluene as the blank (Bates et al. 1973). For Sugar measurement, approximately 0.2 g of the leaves was extracted with distilled water based on the Somogyi method (1952). The mixture of extract and CuSO4 was heated for 20 min. After cooling, the phosphomolybdic acid solution was added to the mixture and the absorbance of the product of the reaction was measured at 600 nm (Somogyi 1952).
Measurement of H2O2 content and evaluation of malondialdehyde
To determine the relationship between melatonin use and reduction of H2O2, its concentration in stressed plants and the control group was measured in the presence of melatonin test concentrations based on the Sergiev et al.’s method. A sample of leaf tissues (0.5 g) was homogenized with 0.1% (w/v) trichloroacetic acid (TCA). After centrifuging at 12,000×g for 15 min, the supernatant was mixed with 10 mM potassium phosphate buffer (pH 7) and 1 M potassium iodide (KI). The sample absorbance was read at 390 nm (Velikova et al. 2000). MDA content, as a product of unsaturated fatty acid peroxidation, was determined by the thiobarbituric acid (TBA) reaction as described by Heath and Packer. The leaf material (0.2 g) was homogenized in 0.1% (w/v) TCA. The absorbance of MDA was read at 532 nm (Heath and Packer 1968).
Antioxidant enzyme activity measurement
For total protein extraction, 0.1 g of leaf tissue was ground in a chilled mortar with 1% (w/v) polyvinylpyrrolidone. Extract homogenized with 10 mL of 50 mM potassium phosphate buffer (pH 7.8) containing 1 mM EDTA-Na2, and then centrifuged at 12,000×g for 20 min. The supernatant was used to measure total protein and antioxidant enzyme activity. Leaf soluble protein was measured by the Bradford method using bovine serum albumin as a standard (Bradford 1976). To measure the activity of CAT (CAT; EC 1.11.1.6) according to the method of Kato and Shimizu, the initial rate of disappearance of H2O2 was measured. The CAT reaction solution (1 mL) contained 20 µL of enzyme extract. The absorbance of the sample was measured every 5 s for 1 min at 240 nm (Kato and Shimizu 1987). Peroxidase (POX; EC 1.11.1.7) activity was measured using the Mac-Adam et al. method. The reaction solution consisted of 0.1 M potassium phosphate buffer, 3% H2O2 solution, and pure guaiacol. The activity of POX was evaluated as a result of guaiacol oxidation by increasing the adsorption at 436 nm at 30-s intervals (MacAdam et al. 1992). Ascorbate peroxidase (APX; EC 1.11.1.11) was determined according to Nakano and Asada method. The reaction solution (1 mL) consisted of 50 mM potassium phosphate buffer (pH 7), 0.5 mM ascorbate, 0.25 mM H2O2, and 100 µL enzyme extract. The absorption of the reaction solution was read at 290 nm (Nakano and Asada 1981). Polyphenol oxidase (PPO; EC 1.14.18.1) activity was examined using the method of Raymond et al. with slight modifications, and the reaction mixture (1 mL) contained 0.2 M phosphate buffer (pH 7.6), 20 mM pyrogallol, and 200 μL enzyme extract. The increase of absorbance was recorded at 430 nm and the temperature of the reaction mixture was 25 °C (Raymond et al. 1993). All of these enzyme activities were expressed as units per mg protein. To measure the activity of superoxide dismutase enzyme (SOD; EC 1.15.1.1) according to Giannopolittis and Ries method. The reaction mixture included 50 mM sodium phosphate buffer (pH 7.5), 13 mM l-methionine, 75 μM NBT, 0.1 mM EDTA, 2 μM riboflavin, and 10 μL enzyme extract used. The reaction solution was exposed to the white fluorescent light for 15 min. Then, the reduction in NBT was measured by reading the absorbance at 560 nm (Giannopolitis and Ries 1977).
Measurement of Na+, K+, and Ca2+
Na+, K+, and Ca2+ contents were assayed based on the Buendia et al.’s method. The samples of shoot and root of bean plants ash (0.1 g) were mixed with 50 mL of 6 M HCl, then the added acid was evaporated by the heater, and 30 mL of 0.1 M HNO3 was added and remained for 2 h. The residual solution volume (about 1 mL) was reached 100 mL by deionized water and was filtered. The ion’s content was measured using atomic absorption (Model AAS240FS, Agilent Company, USA) and was reported based on mg g−1 DW (Buendía-González et al. 2010).
Statistical analysis
Data are reported as mean ± standard error (SE) values of triplicate experiments and were analyzed using SPSS 20. General Linear Model analysis of variance (ANOVA) and Duncan’s tests were performed to determine the significance of the difference among samples, with a significance level of 0.05. All data generated or analyzed during this study are included in this published article.
Results
Photosynthesis, photosynthetic pigments, and plant growth
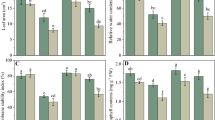
As salt levels rose, PN, C, and E decreased significantly in bean leaves. In unstressed plants, except for PN, 100 µM MEL had an improving effect on other factors. In salt stress conditions, compared to zero MEL, 100 µM MEL affected better at any salinity level. At 200 mM NaCl, application of 100 µM MEL, compared to zero MEL, increased PN, C, and E up to 334%, 118%, and 148%, respectively (Fig. 1). Different levels of salinity decreased leaf chlorophylls and carotenoid content significantly. Compared to other concentrations of MEL, its 400 μM concentration had a small effect on these parameters at different levels of salt. Concentrations of 100 and 200 μM MEL improved the effect of salinity on pigment content. The ratio of Chl a/b decreased as salinity increased. MEL application increased this ratio up to 58% (Fig. 2).
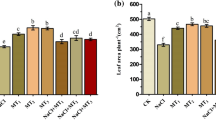
In this study, shoot dry weight (SDW) and root dry weight (RDW) were identified as a factor showing plant growth. Salinity induced a significant decrease in SDW and RDW, MEL at 100 and 200 μM concentrations increased these factors. The highest amount of SDW and RDW was observed in salt-free conditions which were increased up to 25% and 7%, respectively, compared to controls (Fig. 3a, b).
Sugar and proline contents
Salinity significantly increased the content of sugar and proline content in leaves of P. vulgaris cv. Pak. The highest levels were observed under the 200 mM NaCl condition and 100 μM MEL treatment. At this salinity level, sugar and proline contents were increased by 54% and 52%, respectively, with MEL (Fig. 3c, d).
The amount of hydrogen peroxide and malondialdehyde
Salinity significantly increased leaf H2O2 and MDA content. The highest values of H2O2 and MDA have been observed in 200 mM salt and 0 μM MEL. At different levels of NaCl, the effect of MEL at 100 µM concentration was better than others. In the best results, at 200 mM NaCl, 100 µM MEL significantly reduced H2O2 and MDA levels to 31% and 54%, respectively, and at 100 mM NaCl reduced MDA level to 60% compared to controls (Fig. 4).
Antioxidant enzymes’ activity
Increased activity of antioxidant enzymes is one of the plant’s responses to oxidative stress induced by salinity. In this study, MEL increased the activity of SOD, CAT, POX, PPO, and APX. MEL at 400 µM showed the least additive effect. At all levels of NaCl, SOD, and CAT activity significantly increased by 100 µM MEL, compared to the control plants treated by 0 µM MEL. The highest activity of APX was at 200 mM NaCl and 200 µM MEL, with a 102% increase compared to the controls. The 100 µM concentration of MEL significantly increased the activity of PPO at all salt levels. The positive effect of 200 µM MEL on PPO activity at 100 mM NaCl was also significant, compared to the controls. The highest POX activity was measured in 100 µM MEL and 200 mM NaCl, which was significantly different from the control with an increase of 65%. Table 1 shows the effect of MEL and salinity interaction on the activity of these enzymes.
Content of Na+, K+, and Ca2+ ions
Increasing salinity level decreased root and shoot K+ and Ca2+ and increased Na+ in these organs. In roots, at 100 mM NaCl, and K+ did not decrease in the absence of MEL. Moreover, the K+/Na+ ratio also decreased significantly in root and shoot. MEL decreased the salinity effect on Na+ root and shoot and increased K+ content of root and shoot. It also increased the K+/Na+ ratio in these two fractions. In roots at 100 and shoots at 200 mM NaCl, MEL application increased Ca2+ levels by 16% and 69%, respectively. The increasing effect of MEL on K+ and Ca2+ content was observed at concentrations of 100 and 200 μM, respectively, while 400 μM concentration of MEL hurt ions content (Table 2).
Discussion
Melatonin improved photosynthesis and plant biomass
Salinity stress affects the water status of the tissue, the metabolic processes, and plant growth by limiting water absorption and ion toxicity (Rady and Mohamed 2015). Drought and salinity stress significantly decrease growth through decreasing chlorophyll content and photosynthesis rate (Zhang et al. 2014b). A decrease in photosynthesis occurs in salinity due to stomatal or non-stomatal reasons (Zhang et al. 2017). In the present study, salinity decreased PN, C, and E significantly. Although the improving effect of melatonin 100 µM on these factors was significant, the highest amount was observed in control treatments of NaCl. This shows that melatonin cannot eliminate some of the damages that salinity has resulted in the plant’s photosynthetic system.
There is a positive relationship between photosynthesis, stomatal conductance, intrinsic CO2, and biomass production (Qu et al. 2017). When non-stomatal factors are involved, intracellular CO2 stays constant or decreases parallel to the decrease in stomatal conductance (Zhang et al. 2017). As the results of the present study show, MEL increased photosynthetic pigments levels in the control group and stressed plants. Chl a/b ratio analysis showed that 100 µM MEL had a positive and significant role in raising this ratio under salinity conditions. In plants, the amount of Chl a is higher than that of Chl b, but with increasing salinity, Chl b decreases more than Chl a (Parihar et al. 2015). MEL stabilizes photosynthetic processes by preventing the breakdown of chlorophyll and proteins and regulating glucose and nitrogen metabolism (Siddiqui et al. 2019). Other studies have also reported the beneficial effect of MEL on chlorophyll content, photosynthesis, stomatal conductance, and ion homeostasis under drought and salinity stress conditions (Jiang et al. 2016b; Ye et al. 2016; Gao et al. 2019; Liang et al. 2015).
According to the results of this study, SDW and RDW reduction caused by salinity stress was compensated by MEL treatment. By downregulating enzymes involved in lipid degradation via beta-oxidation and slowing the degradation of chlorophyll proteins by changing those proteases, MEL improves chloroplast and chlorophyll stability. Also, the effect of MEL on photosynthesis and the activity of enzymes such as Rubisco can have a positive effect on early net production, which eventually leads to increased plant growth (Wang et al. 2014). Pre-treatment with MEL increases photosynthetic capacity and develops the root system (Zhang et al. 2013). In a study by Zhang et al. (2017) on Cucumis melo L., the use of external MEL at a concentration of 200 μM as leaf spray significantly prevented the suppression of cold-induced growth and the plants treated by MEL showed higher growth and chlorophyll content than non-treated ones (Zhang et al. 2017).
Melatonin enhanced sugar and proline content
Osmotic stress affects the metabolism of carbohydrates and osmolytes such as amino acids, and has a role in osmotic regulation by affecting carbohydrate metabolism (most of all proline) (Qian et al. 2015; Siddiqui et al. 2019). Similar to other studies (Palma et al. 2009), salinity in P. vulgaris cv. Pak significantly increased the concentration of soluble sugars and proline in the present study, and the additive effect of MEL on these factors under stress and non-stress conditions was depending on concentration. Since there is a positive relationship between the accumulation of proline osmolyte and plant tolerance to stress (Nanjo et al. 1999), it is possible to emphasize the effective role of MEL in plant tolerance to salinity based on the present study results.
Proline has positive effects on the enzymes and integrity of the cell membrane and plays an adaptive role as an osmotic mediator in plants under stress (Ashraf and Foolad 2007). Qian et al. (2015) showed that the use of MEL in Arabidopsis plants, exposed to bacterial invasion, increased the levels of sugars such as fructose, glucose, and glycerol. Among the 16 amino acids they measured, proline levels were significantly increased after treatment. Results of their study demonstrated the effective role of MEL in pathogen tolerance in Arabidopsis by affecting the metabolism of sugars. Huang et al. (2017) showed that transgenic Panicum virgatum L. with more MEL biosynthetic ability compared to the control group has increased proline content and more resistance to salt stress.
Melatonin protected membranes by reducing H2O2 level
All stresses increase ROS levels and disrupt ROS homeostasis, causing damage to the membrane and enzymatic systems. Plant response to stress-induced damage usually begins in the early hours of the confronting to the stress by applying a strong protein and non-protein antioxidant system. Damage to membranes is associated with increased levels of MDA due to environmental stresses such as salinity stress (Posmyk et al. 2008). MEL treatments improved the potential of the plant to tolerate oxidative stress, induced by salinity through increasing transcript levels and growing antioxidant enzyme activity. MEL significantly suppresses H2O2 production and hydroxyl radicals (Zhang et al. 2013). In the present study in P. vulgaris L. cv. Pak, application of 100 and 200 µM MEL significantly decreased H2O2 and MDA in stress conditions, although higher concentrations of MEL increased these two factors which were observed in earlier studies (Posmyk et al. 2008; Zhang et al. 2013).
The results of Gao et al.’s (2019) research on oats showed that application of external MEL reduced the H2O2 and MDA content under saline stress conditions (Gao et al. 2019). Similarly, the application of MEL reduced lipid peroxidation and MDA production in corn seedlings under drought stress (Ye et al. 2016). Li et al. (2017) showed that external MEL can reduce salt stress damage in rice, decrease H2O2 levels, and increase the activity of SOD and CAT enzymes (Li et al. 2017). Similarly, the use of MEL in wheat and cucumber and melon seedlings increased plant resistance to cold stress (Zhao et al. 2017; Cao et al. 2018; Zhang et al. 2017), which was associated with a decrease in H2O2 level compared to the control group (Cao et al. 2018) and with an increase in net photosynthesis (Zhao et al. 2017).
Melatonin alleviated salt stress by increasing antioxidant enzyme activity
Oxidative stress is a complex chemical and physiological phenomenon that is caused by almost all biotic and abiotic stresses in plants. The produced ROS damages and deactivates all the important plant polymers, altering the activity of K+ and Ca2+ channels and catalyzing Ca2+-dependent signaling events, causing K+ leakage, and cell death (Demidchik 2015). Among the antioxidant defense systems, SOD is the first line of defense against oxidative stress that reduces O2− to H2O2. CAT can then convert H2O2 to H2O in plant cells. APX can also convert H2O2 to monodehydroascorbate (MDHA) using ascorbic acid (AsA) and neutralize its toxicity (Cao et al. 2018).
In addition to being directly ROS-scavenging (Park et al. 2013; Cao et al. 2018), MEL has an effective role in reducing oxidative damage by increasing the activity of antioxidant systems, including the SOD, POX, CAT, and APX enzymes. Increasing effects of MEL on alteration of antioxidant enzymes activity in plants exposed to salinity (Zhang et al. 2014a; Li et al. 2017), drought (Wang et al. 2013; Ye et al. 2016), oxidative (Afreen et al. 2006), and cold stress (Zhang et al. 2017) have been reported.
In the present study, MEL increased the activity of SOD, PPO, POX, CAT, and APX enzymes in P. vulgaris L. cv. Pak under salinity stress. The use of MEL at a concentration of 100 µm increased the activity of these enzymes at the highest salinity level compared to the control group, which confirms the results of previous studies (Liang et al. 2015; Zhang et al. 2017; Gao et al. 2019). In a previous research MEL at the same concentration, improved fenugreek tolerance to drought stress by increasing CAT, POX, and PPO activity (Zamani et al. 2019). Jiang et al. (2016a, b) also reported an increase in the activity of the POX and APX enzymes by applying MEL in corn leaves under salinity stress (Jiang et al. 2016b).
Melatonin alleviated salt stress by improving ion homeostasis
In the present study, MEL had an alleviative effect on increased Na+ and decreased amounts of root and shoot K+ and Ca2+ induced by salt stress. MEL also increased the K+/Na+ ratio in the shoot and root of bean plants. Li et al. (2017) studied rice, showing that pretreatment with 75 µM MEL reduced Na+ and Cl− content and Na+/K+ ratio in roots and leaves, but had no significant effect on K+ values (Li et al. 2017), whereas in the case of P. vulgaris L. cv. Pak, MEL increased K+ in the control group and the stressed plants. Research by Zhang et al. has shown that MEL increases the expression of genes for the K+ (AKT1) and sodium (NHX1) channels that are involved in the development of ion homeostasis (Zhang et al. 2014b). Increased K+ content in shoots after MEL application has a positive effect on stomatal conductance and increased CO2 uptake (Qu et al. 2017). The results of the present study showed that MEL (100 µM) improved stomatal conductance under NaCl 100 and 200 mM, which is consistent with increased amounts of potassium in the shoot.
The effects of different MEL concentrations on alleviating salt-induced damages
Melatonin is involved in plant development and abiotic stress responses. However, the effect of MEL, like hormones such as auxin, is significantly related to its concentration. In this study, treatment with 100 or 200 µM MEL alleviated the salt-induced inhibition of plant growth and biomass accumulation, and improved NaCl-induced damages by increasing antioxidant activity. Whereas in most cases, a concentration of 400 μM MEL intensified salinity stress damages, which agrees with the results in the previous studies.
There are a very limited number of reports indicating the beneficial impact of high MEL concentrations on plant tolerance to stress conditions. Our results at 400 μM concentration level demonstrate the inhibitory effects of MEL concentration. The inhibitory effects of higher levels of MEL, such as auxin, have been reported in cases like root growth. In mustard, low MEL concentration (0.1 mM) stimulated root growth, whereas higher concentration (100 mM) had an inhibitory effect on growth. Low concentrations of MEL led to rooting and high concentration inhibited growth in the case of cherry under tissue culture (Zhang et al. 2014b). MEL in the high and low concentration ranges in different species and even close to one another have had varying effects (Arnao and Hernández-Ruiz 2009; Zhang et al. 2014b, 2017). For instance, in Brassica oleracea L., concentrations of 1 and 10 μM MEL subsided the inhibitory effects of copper and 100 μM MEL concentration exacerbated these effects (Posmyk et al. 2008), whereas MEL with the concentration levels of 200 μM have had an ameliorative effect on cucumber seedlings, being subjected to the chilling stress (Zhao et al. 2017). MEL with a concentration level of 0.1 μM improved corn growth under salinity stress conditions, while increasing net photosynthesis and antioxidant activity of its enzymes and improving the ion homeostasis (Jiang et al. 2016b).
Conclusions
In the present study, NaCl stress inhibited plant growth and biomass accumulation in bean plants, which was associated with a decrease in photosynthesis and the content of photosynthetic pigments and a decrease in K+ in stressed plants. The results of atomic absorption and change in K+ values in shoots, as well as comparison of the results of changes in stomatal conductance and pigments content, indicate that possibly photosynthetic degradation, in this case, is related to non-stomatal factors such as chlorophyll degradation and damage to the membranes, which are also linked to stomatal causes.
In general, the relationship between different physiological parameters in P. vulgaris L. cv. Pak under salinity stress revealed that an increase in salinity level from 100 to 200 mM NaCl, in most cases, promoted significant changes, and treatment with MEL for both levels of salinity had alleviating effects. The effects are testified to be concentration-dependent to the extent that MEL concentrations of 100 µM and, in some cases, 200 µM had ameliorating effects, and in many cases, 400 µM MEL concentration intensified the effects of salinity.
Author contribution statement
F. Azizi took part in performing the experiments and preparation of the manuscript, H. Amiri designed experiments and revised the final version of the manuscript. A. Ismaili evaluated the statistical sections of the research and revised the final version of the manuscript.
References
Afreen F, Zobayed SM, Kozai T (2006) Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV-B radiation. J Pineal Res 41(2):108–115. https://doi.org/10.1111/j.1600-079X.2006.00337.x
Arnao MB, Hernández-Ruiz J (2009) Chemical stress by different agents affects the melatonin content of barley roots. J Pineal Res 46(3):295–299. https://doi.org/10.1111/j.1600-079x.2008.00660.x
Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19(12):789–797. https://doi.org/10.1016/j.tplants.2014.07.006
Arnon AN (1967) Method of extraction of chlorophyll in the plants. Agron J 23:112–121
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Brugnoli E, Lauteri M (1991) Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol 95(2):628–635
Buendía-González L, Orozco-Villafuerte J, Cruz-Sosa F, Barrera-Díaz C, Vernon-Carter E (2010) Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour Technol 101(15):5862–5867. https://doi.org/10.1016/j.biortech.2010.03.027
Cao S, Shao J, Shi L, Xu L, Shen Z, Chen W, Yang Z (2018) Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci Rep 8(1):806. https://doi.org/10.1038/s41598-018-19363-5
Carrillo-Vico A, Lardone PJ, Álvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int J Mol Sci 14(4):8638–8683. https://doi.org/10.3390/ijms14048638
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228. https://doi.org/10.1016/j.envexpbot.2014.06.021
Flowers TJ, Munns R, Colmer TD (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot 115(3):419–431
Gao W, Feng Z, Bai Q, He J, Wang Y (2019) Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int J Mol Sci 20(5):1176. https://doi.org/10.3390/ijms20051176
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59(2):309–314. https://doi.org/10.1104/pp.59.2.309
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hnatuszko-Konka K, Kowalczyk T, Gerszberg A, Wiktorek-Smagur A, Kononowicz AK (2014) Phaseolus vulgaris—recalcitrant potential. Biotechnol Adv 32(7):1205–1215. https://doi.org/10.1016/j.biotechadv.2014.06.001
Huang Y-H, Liu S-J, Yuan S, Guan C, Tian D-Y, Cui X, Zhang Y-W, Yang F-Y (2017) Overexpression of ovine AANAT and HIOMT genes in switchgrass leads to improved growth performance and salt-tolerance. Sci Rep 7(1):12212. https://doi.org/10.1038/s41598-017-12566-2
Jiang C, Cui Q, Feng K, Xu D, Li C, Zheng Q (2016a) Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol Plant 38(4):82. https://doi.org/10.1007/s11738-016-2101-2
Jiang X, Li H, Song X (2016b) Seed priming with melatonin effects on seed germination and seedling growth in maize under salinity stress. Pak J Bot 48(4):1345–1352
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can J Bot 65:729–735. https://doi.org/10.1139/b87-097
Khadri M, Tejera N, Lluch C (2007) Sodium chloride–ABA interaction in two common bean (Phaseolus vulgaris) cultivars differing in salinity tolerance. Environ Exp Bot 60(2):211–218. https://doi.org/10.1016/j.envexpbot.2006.10.008
Li X, Yu B, Cui Y, Yin Y (2017) Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regul 83(3):441–454. https://doi.org/10.1007/s10725-017-0310-3
Li J, Yang Y, Sun K, Chen Y, Chen X, Li X (2019a) Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 24(9):1826. https://doi.org/10.3390/molecules24091826
Li J, Zhao C, Zhang M, Yuan F, Chen M (2019b) Exogenous melatonin improves seed germination in Limonium bicolor under salt stress. Plant Signal Behav 14:1–10
Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Wu H, Qian Y, Zhu XG, Tan DX (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res 59(1):91–101. https://doi.org/10.1111/jpi.12243
Liu Z, Cai J, Li J, Lu G, Li C, Fu G, Zhang X, Liu Q, Zou X, Cheng Y (2018) Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J Integr Agric 17(2):328–335. https://doi.org/10.1016/s2095-3119(17)61757-x
López-Barrios L, Antunes-Ricardo M, Gutiérrez-Uribe JA (2016) Changes in antioxidant and antiinflammatory activity of black bean (Phaseolus vulgaris L.) protein isolates due to germination and enzymatic digestion. Food Chem 203:417–424. https://doi.org/10.1016/j.foodchem.2016.02.048
MacAdam JW, Nelson CJ, Sharp RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue. Plant Physiol 99:872–878. https://doi.org/10.1104/pp.99.3.879
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (1999) Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett 461(3):205–210. https://doi.org/10.1016/S0014-5793%2899%2901451-9
Palma F, Lluch C, Iribarne C, García-Garrido JM, García NAT (2009) Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in Phaseolus vulgaris. Plant Growth Regul 58(3):307–316. https://doi.org/10.1007/s10725-009-9380-1
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res Int 22(6):4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K (2013) Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J Pineal Res 54(3):258–263. https://doi.org/10.1111/j.1600-079X.2012.01029.x
PasandiPour A, Farahbakhsh H, Saffari M, Keramat B (2013) Response of fenugreek plants to short-term salinity stress in relation to photosynthetic pigments and antioxidant activity. Int J Agric Res 3(1):80
Posmyk MM, Kuran H, Marciniak K, Janas KM (2008) Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 45(1):24–31. https://doi.org/10.1111/j.1600-079X.2007.00552.x
Qian Y, Tan DX, Reiter RJ, Shi H (2015) Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin mediated innate immunity against bacterial pathogen in Arabidopsis. Sci Rep 28:15815. https://doi.org/10.1038/srep15815
Qu M, Zheng G, Hamdani S, Essemine J, Song Q, Wang H, Chu C, Sirault X, Zhu X-G (2017) Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol 175(1):248–258. https://doi.org/10.1104/pp.17.00332
Rady MM, Mohamed GF (2015) Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci Hortic 193:105–113. https://doi.org/10.1016/j.scienta.2015.07.003
Raymond J, Rakariyatham N, Azanza J (1993) Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry 34(4):927–931. https://doi.org/10.1016/S0031-9422(00)90689-7
Siddiqui MH, Alamri S, Al-Khaishany MY, Khan MN, Al-Amri A, Ali HM, Alaraidh IA, Alsahli AA (2019) Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int J Mol Sci 20(2):353. https://doi.org/10.3390/ijms20020353
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Sytar O, Brestic M, Zivcak M, Olsovska K, Kovar M, Shao H, He X (2017) Applying hyperspectral imaging to explore natural plant diversity towards improving salt stress tolerance. Sci Total Environ 578:90–99. https://doi.org/10.1016/j.scitotenv.2016.08.014
Talaat NB, Ghoniem AE, Abdelhamid MT, Shawky BT (2015) Effective microorganisms improve growth performance, alter nutrients acquisition and induce compatible solutes accumulation in common bean (Phaseolus vulgaris L.) plants subjected to salinity stress. Plant Growth Regul 75(1):281–295. https://doi.org/10.1007/s10725-014-9952-6
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Wang P, Sun X, Li C, Wei Z, Liang D, Ma F (2013) Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res 54(3):292–302. https://doi.org/10.1111/jpi.12017
Wang P, Sun X, Xie Y, Li M, Chen W, Zhang S, Liang D, Ma F (2014) Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J Pineal Res 57(3):291–307. https://doi.org/10.1111/jpi.12169
Yadu B, Chandrakar V, Meena RK, Poddar A, Keshavkant S (2018) Spermidine and melatonin attenuate fluoride toxicity by regulating gene expression of antioxidants in Cajanus cajan L. J Plant Growth Regul 37(4):1113–1126. https://doi.org/10.1007/s00344-018-9786-y
Yang X, Xu H, Li D, Gao X, Li T, Wang R (2018) Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 56(3):884–892. https://doi.org/10.1007/s11099-017-0748-6
Ye J, Wang S, Deng X, Yin L, Xiong B, Wang X (2016) Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol Plant 38(2):48. https://doi.org/10.1007/s11738-015-2045-y
Zamani Z, Amiri H, Ismaili A (2019) Improving drought stress tolerance in fenugreek (Trigonella foenum-graecum) by exogenous melatonin. Plant Biosyst Int J Deal All Asp Plant Biol 154:643–655
Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD (2013) Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 54(1):15–23. https://doi.org/10.1111/j.1600-079X.2012.01015.x
Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, Li DB, Cao YY, Weeda S, Zhao B, Ren S (2014a) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 57(3):269–279. https://doi.org/10.1111/jpi.12167
Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo Y-D (2014b) Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 66(3):647–656. https://doi.org/10.1093/jxb/eru336
Zhang Y, Yang S, Chen Y (2017) Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol Plant 61(3):571–578. https://doi.org/10.1007/s10535-017-0717-8
Zhao H, Zhang K, Zhou X, Xi L, Wang Y, Xu H, Pan T, Zou Z (2017) Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci Rep 7(1):4998. https://doi.org/10.1038/s41598-017-05267-3
Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Wang L, Wang Z, Guo Y, Zhou J (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci Rep 7:41236. https://doi.org/10.1038/srep41236
Acknowledgements
The authors are grateful for the support of Lorestan University in completing this research.
Funding
This research was funded by grants from Lorestan University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or other competing conflicts of interest.
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azizi, F., Amiri, H. & Ismaili, A. Melatonin improves salinity stress tolerance of Phaseolus vulgaris L. cv. Pak by changing antioxidant enzymes and photosynthetic parameters. Acta Physiol Plant 44, 40 (2022). https://doi.org/10.1007/s11738-022-03373-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03373-y