Abstract
Sugar beet is strongly resistant to salt-alkalinity. Understanding the physiological alkali stress resistance mechanism of sugar beet is important for fully utilizing saline–alkaline soil. Sugar beet seedlings from cultivars KWS0143 (alkali-tolerant) and Beta464 (alkali-sensitive) were treated with five concentrations of mixed alkaline solutions (NaHCO3: Na2CO3, 2:1), namely, 0 (control), 25, 50, 75, and 100 mM (mole concentration was calculated in Na+). A sharper decrease in dry weight per plant (87.1%) and total root length (91.7%) of Beta464 were observed compared to the 61.5% and 85.0% decrease in those of KWS0143 under 100 mM alkali treatment. With increasing alkaline stress, Na+ accumulation hindered K+ and Ca2+ absorption by roots. Free polyamines contents and phosphoenolpyruvate carboxylase (PEPC) activity in roots of both cultivars were all significantly enhanced by 50 and 75 mM alkali treatments. KWS0143 exhibited higher dissociated putrescine (Put), spermine (Spm), as well as spermidine (Spd) levels within the roots compared to Beta464 under alkali conditions. Root free Spd contents of KWS0143 and Beta464 increased by 154.2 and 64.5% treated with 50 mM alkali in comparison with the control. After treated with the dose of 25 mM, root succinic acid (SA) contents of KWS0143 and Beta464 increased by 90.4 and 14.3%, respectively, compared to the plants subjected to the control. Our results imply that polyamines and PEPC contribute to the tolerance of sugar beet to alkali stress. Those results could be useful for enriching the theory of plant stress response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinization has become the main global environment problem, and it becomes severe gradually (Jesus et al. 2015). Except for salinization, in the last several years, soil alkalinization is also identified, especially in the semi-arid and arid zones (Parihar et al. 2015; Paz et al. 2012). 8.31 × 108 hm2 of the soil in the world is affected by saline–alkaline, and in China, approximately 9.2 × 106 hm2 of the farmlands are in the saline–alkaline soil (Li et al. 2017). In Northeast China where soils have been extensively alkalized, Na2SO4, NaCl, NaHCO3, as well as Na2CO3 are the main salt components (Bai et al. 2016). Soil alkalization due to Na2CO3 and NaHCO3 is probably serious compared with the neutral salt-induced soil salinization, including Na2SO4 and NaCl (Liu and Shi 2010; Yang et al. 2011). Great salt contents within the growth medium of plant can decrease the K+ level and increase the accumulation and uptake of Na+, which results in K+ efflux and promotes the leakage of K+ ions through plant cells (Azooz et al. 2015; Latef and Tran 2016; Munns and Tester 2008). Similar to salt stress, alkaline stress impair plant development through inducing ion disequilibrium, oxidative damage, and hyperosmotic stress (Chen et al. 2012; Hazman et al. 2016; Radi et al. 2012). Nonetheless, alkaline challenge is distinctly different as a result of the great pH level. Notably, any slight increase in the pH value over the expected alkaline salt level can be poisonous, and has adverse effect on various physio-biochemical events, like mineral absorption, photosynthesis, plant yield, as well as membrane integrity (Chen et al. 2012; Radi et al. 2012).
Roots are an important crop organ, which participates in water and nutrients acquisition, plant hormone synthesis, amino acids, anchorage, organic acids, as well as environmental challenge adaptation (Liu et al. 2014). The root system in soils may be the directly damaged site resulting from salinity and alkalinity. Morphology of the plant root system is greatly different according to different salt stress treatments (Witzel et al. 2018).
Polyamines (PAs) exerts an important part in numerous physio-biochemical events related to plant development (Baron and Stasolla 2008; Bouchereau et al. 1999). In addition, PA also exerts a vital part in the preventing biotic as well as abiotic stress of plants (Bueno and Cordovilla 2019). Putrescine (Put), spermine (Spm), and spermidine (Spd) are several leading plant PAs (Zhou et al. 2019). According to Capell and colleagues (2004), transgenic plants that expressed gene Datura adc generated a greater Put amount in the case of stress, which promoted the synthesis of Spm and Spd, finally preventing droughts in plants.
Organic acids, the low-molecular-weight compound containing carboxyls, play a role of buffering. Diverse organic acids can be detected in plants, including malic acid, succinic acid, and citric acid (Ma et al. 2015). These compounds exert vital parts in adapting to different stresses of plants. Organic acids can adapt to and modulate diverse stresses of plants, such as metal ion, drought, and saline–alkali stresses (Chen et al. 2009; Fu et al. 2019; Li et al. 2016). Phosphoenolpyruvate carboxylase (PEPC), a critical enzyme involved in the tricarboxylic acid cycle, could catalyze phosphoenolpyruvate and CO2 synthesized into oxaloacetate, after which oxaloacetate was transformed into malic acid, and other organic acids were synthesized (Zhang and Fernie 2018). Therefore, PEPC exerts an important part in organic acids synthesis in plant adaption to salt–alkali stress. Ma and colleagues (2016) found that phosphoenolpyruvate carboxylation via PEPC might have little influence on the OxA synthesizing pathway and Kochia sieversiana PEPC activity was enhanced by alkali and salt stress.
Sugar beet (Beta vulgaris L.) has become a crucial industrial crop (Magaña et al. 2011). Special interested traits for improving sugar beet yield are acclimation to both abiotic and biotic challenges, like cold under the temperate climate, salinity, heat, and drought (Vastarelli et al. 2013). Exploring the physiological mechanism in tolerance of sugar beet to salt–alkali stress is important for fully utilizing saline–alkaline soil and promoting maintainable development of agriculture in our country. Many efforts have been made to examine saline stress response physiologically and molecularly, such as proteome, transcriptome, or antioxidant enzyme (Hossain et al. 2017; Li et al. 2015; Yang et al. 2012). Our previous studies have presented the alkali stress role in the dry matter accumulation, emergence rate, photosynthetic characteristics, membrane permeability, and chloroplast ultrastructure of sugar beet by pot and hydroponic experiments, and also reported the adaptation of sugar beet to alkali conditions through the osmotic substances as well as the antioxidative defense systems (Zou et al. 2018, 2019). Guo et al. (2016) showed changes in microbes and soil enzymes in rhizosphere environment of sugar beet seedlings under alkali conditions, and the correlation between those parameters and plant dry mass. Nonetheless, it remains largely unclear about its alkalinity role in root morphology, as well as physiological indexes, especially ion balance, free polyamines, and phosphoenolpyruvate carboxylase in roots of sugar beet.
This study aimed (1) to examine alkalinity role in dry matter accumulation as well as the morphology of root in sugar beet; (2) to study the ability of sugar beet maintain the balance of Na+, K+, and Ca2+ ions such as in roots, and (3) to explore the physiological responses, including osmotic substances, antioxidant enzymes, free polyamines, and phosphoenolpyruvate carboxylase, in sugar beet roots under alkaline challenge. Information generated from this study could shed light on sugar beet adaption to alkali conditions and add more knowledge on the plant tolerance theoretical system under experimental stress.
Materials and methods
Plant materials and culture environment
Two sugar beet cultivars that possessed distinctly different alkaline tolerability were screened, including the sensitive—Beta464 and the tolerant—KWS0143 (Chen et al. 2010; Guo et al. 2015).
The pelleted seeds from “KWS0143” (obtained from KWS, Germany) and “Beta464” (obtained from BETASEED, America) were subjected to germination within vermiculite that contained distilled water for one week, followed by 3-week irrigation using the Hoagland solution (first pair of real leaves fully expanded) as well as subsequent transplantation to the hydroponic device that contained Hoagland solution under 25℃/20℃ (day/night), 65% relative humidity, 450 μmol·m−2·s−1 light intensity, and the 16/8 h photoperiod. 1 week later, those resultant seedlings were treated with Hoagland solution containing Na2CO3:NaHCO3 mixed alkaline at the doses of 0 (control), 25, 50, 75, and 100 mM (1:2; mole concentration was calculated in Na+). The pH values of each treatment were 6.85, 9.16, 9.55, 9.67, and 9.75, respectively. Hoagland solution containing different levels of mixed alkaline was replaced per day to stabilize the pH values of each treatment. Root morphology and dry matter accumulation, together with root physiological parameters were determined at 7 days after treatments. The random complete block design was applied, and 3 duplicates were set for each treatment. Each block was a duplicate. Moreover, 100 seedlings existed in each block, and there was no significant variation between plants belonging to the same block. The distance between seedlings was 10 cm. Our blocking criterion was Hogland solution of the same formula.
Dry matter accumulation
For every duplicate, five plants would be screened randomly and washed using the deionized water; later, the dry weights of root and shoot were calculated. Shoots and roots were subjected to 15 min heating at the temperature of 105 ℃, followed by drying until reaching the constant weight under the temperature of 80 ℃, and thus, the dry weights were determined. Afterward, the dry weight and root/shoot ratio of each plant were calculated as equations below. The mean from the above five plants were determined as one replicate.
Root morphology
For every duplicate, five plants would be screened randomly and washed in deionized water, and the root volume, root-tip number in every plant, and total root length, root surface area, and root volume were measured by the root automatic scanning machine (Microtek Scan Maker i800) using the LA-S software. For five plants, the means were deemed as a duplicate.
Na+, K+, and Ca2+ ions
To estimate Na+, K+, and Ca2+ contents, root samples collected separately were sufficiently rinsed by deionized water for eliminating Na+, K+, and Ca2+ adhered onto surface. Later, 0.1 g oven-dried (48 h at 80 ℃) tissue sample was grinded, followed by digestion using the HNO3:HClO4 (5:1 v/v) mixture under 80 ℃ till the color disappeared. Contents of Na+, K+, and Ca2+ within leave and root samples were analyzed through flame atomic absorption spectrophotometry (Z-5000; Hitachi, Japan).
Moreover, 0.5 g shredded leaves were immersed within 50 ml deionized water; subsequently, Na+ content within the deionized water was measured through flame atomic absorption spectrophotometry (Z-5000; Hitachi, Japan). Finally, secreted Na+ from leaf surface (SNFLS) was calculated.
Free polyamines and amine oxidase
PAs were isolated according to Zhao et al. (2008). After washed in deionized water, root samples (0.5 g) were ground within the liquid nitrogen, followed by 1 h extraction within the 1.6 ml perchloric acid (PCA, 5%, v/v) under the temperature of 4 ℃, as well as 30 min centrifugation at 12,000 × g under the temperature of 4 ℃. Supernatants were utilized in determining free PAs contents. Supernatants were benzoylated in accordance with Aziz and Larher (1995), and the resulting benzoylated Put, Spd, and Spm were determined through HPLC by the use of the Waters 1525 chromatographic system (Waters, USA). The C18 column (250 × 4.6 mm, Kromasil, Sweden) was applied in separating polyamines. Then, 10 µl samples were loaded in every run. 0.8 ml·min−1 elution was conducted under the temperature of 25 ℃. 64% v/v methanol served as mobile phase. The Dionex UVD170U detector was used to detect the eluted polyamines at the wavelength of 254 nm through determining the respective retention time and that of standard solution (Sigma-Aldrich, Prague, Czech Republic).
The diamine oxidase (DAO) and polyamine oxidase (PAO) activities were measured through an improved method of Smith and Barker (1985). After washed in deionized water, 0.5 g fresh root sample was subjected to homogenization on ice within 1.6 ml of 100 mmol·L−1 phosphate buffer solution (PBS, pH 6.5); followed by 20 min centrifugal separation at 10,000×g. Later, those resultant supernatants were applied in determining the DAO and PAO activities. 3.1 ml reaction mixture contained 200 µl supernatant, 100 µl peroxidase (250 U·ml−1), 200 µl coloration liquid [25 µl N, N-dimethylaniline, and 10 mg 4-Aminoantipyrine in every 100 ml PBS (100 mmol·L−1)], 2.5 ml PBS (100 mmol·L−1, pH 6.5), and 100 µl of 20 mmol·L−1 Put or Spd. That reaction lasted for 30 min under the temperature of 25 ℃, and the optical density was determined at the wavelength of 555 nm. 0.01ΔA555·min−1 equaled one unit of enzymatic activity (1 U). For 3 separate samples, 3 values were measured independently.
Phosphoenolpyruvate carboxylase (PEPC) and organic acid
After washed in deionized water, 0.5 g fresh root tissues were sufficiently ground within the cooled mortar containing 2 ml extraction buffer that contained 100 mM Tris–HCl (pH 7.3), 10 mM MgCl2, 0.3% PVP, 25% (v/v) glycerol, and 1 M EDTA. Later, that homogenate was subjected to centrifugation for 8 min at 15,000 g and 4 °C. Those resultant supernatants were collected to carry out enzyme tests. We measured PEPC by Ma et al.'s method (2016) after mild modification. 2 ml reaction mixture supplemented with 1 mM NaHCO3, 100 mM Tris–HCl, 0.3 mg NADH, 5 mM MgCl2, and excess malate dehydrogenase. Following 15 min incubation under 27 °C, 0.05 ml enzymatic extracts were joined for reaction initiation. The NADH absence was monitored through PEPC activity for 3 min at the wavelength of 340 nm under ambient temperature.
Root organic acid contents were measured by the improved approach by Chen et al. (2009). The 0.1 g oven-dried root tissue samples were abraded, followed by 30 min boiling using the 15 ml deionized water, as well as 30 min centrifugation under 12,000 × g at 4 ℃. Supernatants were utilized for determining organic acid levels. Concentrations of succinic acid (SA), citric acid (CA), tartaric acid (TA), acetic acid (AA), malic acid (MA), and lactic acid (LA) were determined through HPLC by the use of the Waters 1525 chromatographic system (Waters, USA). The C18 column (250 × 4.6 mm, Kromasil, Sweden) was utilized to separate the organic acids. Then, 20 µl sample was added into every run, followed by elution under 30℃ at 0.6 ml·min−1. 50 mM dipotassium phosphate buffer (pH = 2.53) was used as the mobile phase. The Dionex UVD170U detector was employed for detecting eluted organic acids at 210 nm through measuring the respective retention time and that of standard solution (Sigma-Aldrich, Prague, Czech Republic).
Statistical analysis
Data are presented with the interaction of alkaline treatments and cultivars where these interactions were significant. Data were analyzed by ANOVA and least significant difference (LSD) multiple comparison test using SPSS 20.0. Microsoft Excel 2016 was utilized for generating diagrams and tables.
Results
Dry matter accumulation
Significant changes in morphology were detected following alkaline treatments (Fig. 1). Under control and 25 mM alkaline treatments, Beta464 showed high dry weight in each plant compared with KWS0143, while KWS0143 showed the high dry weight in each plant relative to Beta464 under 50, 75, and 100 mM treatments (Table 1). Dry weight per plant of Beta464 gradually reduced as the alkali dose increased, whereas that of KWS0143 increased to a maximum at 25 mM treatment and declined thereafter in response to alkaline stress. 50, 75, and 100 mM treatments markedly (P < 0.05) decreased dry weight in each plant for both cultivars. Such reduction in dry weight in each plant was sharper for Beta464 compared to KWS0143 under treatments at 50, 75, and 100 mM. In comparison with plants subjected to control, dry weight per plant of KWS0143 decreased by 13.7, 24.2, and 61.5% under 50, 75, and 100 mM treatments, respectively, whereas that of Beta464 decreased by 67.3, 73.0, and 87.1%, respectively.
Dry weights of root and shoot for Beta464 gradually declined with the increase in alkaline concentration, whereas those of KWS0143 showed the greatest value at 25 mM treatment and declined subsequently (Table 1). 50, 75, and 100 mM treatments remarkably (P < 0.05) reduced the dry weights of root and shoot in the two cultivars. Under 50, 75, and 100 mM treatments, KWS0143 exhibited higher root and shoot dry weights than Beta464. Sharper reductions of shoot (86.6%) as well as root (89.6%) dry weight were observed in Beta464 compared to the 60.3% and 67.3% decrease in KWS0143 under the 100 mM treatment.
The 25 mM treatment obviously (P < 0.05) elevated the ratio of root/shoot of both cultivars, whereas the 100 mM treatment resulted in markedly reduced (P < 0.05) root/shoot ratio in both cultivars (Table 1). Root/shoot ratio in KWS0143 was great compared with that in Beta464 under the 100 mM treatment.
Root morphology
Morphological factors of roots in sugar beet showed different responses to alkaline stresses (Table 2). According to the data in this work, most of root morphology parameters for both cultivars gradually decreased along with the increasing alkaline treatments. The total root length, root surface area, root volume, and root-tip number in KWS0143 elevated to a maximum at 25 mM and then decreased in response to alkaline conditions, whereas those of Beta464 gradually decreased. Under the control treatment, Beta464 showed higher total root length, root surface area, as well as root volume compared with KWS0143; however, those three parameters of KWS0143 were higher than Beta464 under 50, 75, and 100 mM treatments. The decrease of total root length, root surface area, as well as root volume were sharper in Beta464 compared with KWS0143 under the 100 mM treatment. Compared to the plants subjected to control, total root length, root surface area, as well as root volume of KWS0143 reduced by 77.9, 88.2, and 25.5% under the 100 mM treatment, respectively, whereas those of Beta464 decreased by 91.7, 96.7, and 89.6%, respectively. Besides, KWS0143 exhibited increased root-tip number compared with Beta464 of 100 mM treatment. The sharply decreased (by 62.0%) root-tip number was detected in Beta464 compared to the decrease (by 64.7%) in root-tip number in KWS0143 under 100 mM treatment.
Na+, K+, and Ca2+ ions
Na+ content in roots of both cultivars gradually increased with an increase in alkaline concentration, but K+ content, Ca2+ content, and K+/Na+ and Ca2+/Na+ decreased with alkaline concentration (Table 3), suggesting that Na+ accumulation hindered K+ and Ca2+ uptake of sugar beet roots. Under control and 25 mM treatments, root K+ content of both cultivars was obviously higher than Na+ content, but when treated with 50, 75, or 100 mM alkaline solutions, Na+ content was apparently higher compared to K+. Besides, among three sorts of ions, Ca2+ content was minimal under each treatment. Different concentrations of alkaline treatments all significantly (P < 0.05) increased within root Na+ content of two cultivars, but significantly (P < 0.05) reduced K+ content, Ca2+ content, and Ca2+/Na+ and K+/Na+. Besides, Na+ content in roots of Beta464 was higher, but Ca2+/Na+, K+/Na+ and K+ content were lower compared to those of KWS0143 under the same treatment. Under the control treatment, Beta464 exhibited higher Ca2+ content than that of KWS0143; however, KWS0143 had a higher Ca2+ content than Beta464 under each concentration of alkaline stress.
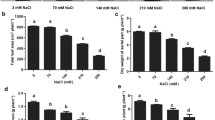
Under the control and 25 mM treatments, Beta464 showed a slightly higher SNFLS than that of KWS0143; however, KWS0143 had a higher SNFLS compared with Beta464 (Fig. 2). The SNFLS of KWS0143 and Beta464 gradually elevated as the alkaline dose increased. The increased SNFLS was sharper in KWS0143 compared to Beta464 under 50, 75, and 100 mM treatments. In comparison with plants subjected to the control treatment, the SNFLS of KWS0143 increased by 703.5%, 756.1%, and 1005.3% under 50, 75, and 100 mM treatments, respectively, whereas that of Beta464 increased by 140.5%, 292.4%, and 688.6%, respectively.
Effects of alkaline stress on secreted Na+ from leaf surface (SNFLS) of two sugar beet cultivars. Each histogram stands for mean ± SD from 3 duplicates. Those various capital letters followed mean values indicate differences of statistical significance between various cultivars at identical treatments (P < 0.05). Those diverse little letters stand for difference of statistical significance (P < 0.05) between different alkaline treatments for identical cultivars (P < 0.05). F value before ns letter indicates no significant difference upon LSD test, *P < 0.05; **P < 0.01; and ***P < 0.001
Free polyamines contents, DAO activity, and PAO activity
Of the three free polyamines, Spd showed the greatest level inn sugar beet root, while Put and Spm ranked the second and third places, respectively (Table 4). For three free polyamines, their contents markedly (P < 0.05) increased in the two cultivars under 25, 50, and 75 mM treatments. Under different concentrations of alkaline treatments, KWS0143 exhibited higher Spm, Spd, and Put levels within roots compared to Beta464. Contents of Spm, Spd, and Put within roots of both cultivars peaked at 50 mM treatment, followed by subsequent decline upon alkaline stress. Relative to control plants, contents of Spm, Spd, and Put within roots in KWS0143 increased by 56.3, 154.2, and 114.1% under the 50 mM treatment, respectively, whereas those of Beta464 increased by 60.2, 64.5, and 78.6%, respectively.
Root PAO activity of both cultivars peaked at 50 mM treatment, followed by subsequent decline in response to alkaline conditions (Table 4). In comparison with control plants, PAO activities of KWS0143 and Beta464 increased by 135.6% and 172.4%, respectively, under the 50 mM treatment. Peak value of DAO activity appeared later in comparison to that of Put content, Spd content, Spm content, and PAO activity. Beta464 exhibited higher root DAO activity than KWS0143 under each treatment. Root DAO activity of both cultivars showed the greatest value at 75 mM treatment and declined subsequently. A sharper increase in DAO activity (by 310.8%) was observed in Beta464 compared to the 156.4% increase in KWS0143 under the 75 mM treatment compared to the plants subjected to the control.
PEPC activity and organic acid contents
Various alkaline contents markedly affected the root PEPC activity in two sugar beet cultivars (Fig. 3). Root PEPC activity in the two cultivars showed a first increase and then decrease trend as the alkaline treatments increased. Under control, 25, 50, as well as 75 mM treatments, Beta464 had higher root PEPC activity than that of KWS0143; however, KWS0143 had a higher root PEPC activity than Beta464 under the 100 mM treatment. While treating with 50 mM alkaline solution, it could be found that root PEPC activity of KWS0143 and Beta464 increased by 39.9 and 66.8%, respectively. Under the 100 mM treatment, the KWS0143 root PEPC activity was slightly greater than that of control, but root PEPC activity of Beta464 was 24.2% lower compared with that of the control.
Alkaline stress effects on root PEPC activity in two sugar beet cultivars. Every histogram stands for mean ± SD from 3 duplicates. Those various capital letters followed mean values indicate differences of statistical significance between various cultivars at identical treatments (P < 0.05). Those various little letters stand for differences with statistical significance (P < 0.05) between different alkaline treatments for identical cultivars (P < 0.05). F values before the ns letter showed no statistical significance upon LSD test, *P < 0.05; **P < 0.01; as well as ***P < 0.001
TA and SA contents in roots of the two cultivars all showed a first increase and later decrease trend as the alkaline dose increased (Table 5). Root TA contents in Beta464 and KWS0143 rose to a maximum at 25 and 50 mM treatments, respectively, and declined thereafter in response to alkaline stress. In comparison with control plants, root TA content of Beta464 increased by 240.0% under the 25 mM treatment, whereas that of KWS0143 increased by 133.3% under the 50 mM treatment. The 75 and 100 mM treatments significantly reduced the root TA level in KWS0143, whereas those in Beta464 under 75 and 100 mM treatments were always significantly increased relative to control. The root SA contents of both cultivars increased to a maximum at the 25 mM treatment and then declined in response to alkaline stress. Under the 25 mM treatment, root SA contents of KWS0143 and Beta464 increased by 90.4 and 14.3%, respectively, relative to control plants. Decrease of SA was sharper in Beta464 (by 35.6%) compared to the 88.0% decrease in KWS0143 under the 100 mM treatment.
The contents of MA and CA in roots of both cultivars all gradually decreased with increasing alkaline concentration (Table 5). Under 75 and 100 mM treatments, KWS0143 exhibited higher contents of MA and CA than those of Beta464. The decreases of root MA and CA contents were sharper in Beta464 compared to KWS0143 under the 100 mM treatment. Relative to control plants, MA and CA contents in roots of KWS0143 decreased by 86.3 and 86.1% under the 100 mM treatment, respectively, whereas those of Beta464 decreased by 91.1 and 87.1%, respectively.
Under the control and 25 mM treatments, KWS0143 showed higher root LA content than that of Beta464; however, Beta464 exhibited a higher root LA content than KWS0143 of 50, 75, and 100 mM treatments. Root content of KWS0143 gradually reduced as the alkaline content increased, whereas that in Beta464 increased to a maximum at the 50 mM treatment and declined thereafter responding to alkali treatment. Root LA content decrease was sharper in KWS0143 (by 91.6%) compared to Beta464 (by 10.2%) under the 100 mM treatment.
Root AA content of Beta464 gradually decreased in response to alkaline concentrations, whereas that of KWS0143 showed the greatest value at the 25 mM treatment declined subsequently (Table 5). Under the control treatment, Beta464 exhibited a higher root AA content than that of KWS0143; however, KWS0143 showed higher root AA content that of Beta464. The decrease of root AA content was sharper in Beta464 (by 83.3%) compared to KWS0143 (by 30.2%) under the 100 mM treatment.
Discussion
At the seedling phase, plants show sensitivity to the unfavorable external parameters; as a result, the seedling stage has been recognized as the best timing for investigating the abiotic tolerance of plants (An et al. 2016). Dry matter accumulation reflects the plant developmental conditions. Decrease range in dry matter accumulation has been recognized to be the vital variable for determining stress degree of plants. Alkalinity or salinity usually suppresses the plant growth, or even lead to plant death (Guo et al. 2017; Yang et al. 2007). In the present work, shoot dry weight and root dry weight in two sugar beet cultivars all significantly (P < 0.05) decreased under 50, 75 as well as 100 mM alkaline stresses (Table 1), which demonstrated that alkalinity inhibited photo-assimilate accumulation of root and shoot of sugar beet. Moreover, compared to Beta464, KWS0143 showed higher dry weight per plant in exposure to 50, 75, and 100 mM alkaline conditions (Table 1). The sharper decrease in dry matter accumulation of Beta464 under alkaline conditions revealed that photo-assimilate accumulation of Beta464 was restrained more severely by alkali stress compared with that of KWS0143. Both Guo et al. (2016), together with Zou et al. (2018), suggested the increase in dry matter weight in each plant of Beta464 and KWS0143 plants due to low alkaline treatments. However, in the present study, the dry matter weight in each Beta464 plant remarkably reduced when exposed to a low level (25 mM) of alkalinity (Table 1). The differences between present work and previous ones may be due to the lack of buffering effects in hydroponic environment compared to those in soils. Root/shoot ratio was identified as an index to determine plant tolerance mode upon environmental stresses (Xu et al. 2018). In this work, root/shoot ratio and root morphological parameters in the two cultivars remarkably (P < 0.05) increased at 25 mM alkaline treatment (Table 1), which indicated that sugar beet may give priority to ensuring root growth when exposed to a low level of alkalinity.
The roots directly contact alkali-polluted soil; as a result, they are under the direct influence of excess Na+ and high pH. The morphological factors of roots, such as volume, root surface area, and length, can denote alkaline toxicity response in plants (Paz et al. 2012). Such significant inhibition of salt stress on root-tip number, root area, average root diameter, total root length, as well as total root volume has been also reported for wheat and blue panic grass (Eshghizadeh et al. 2012; Shafi et al. 2010). This work suggested that those morphological factors of roots were significantly inhibited under 50, 75, and 100 mM alkaline treatments (Table 2), which conformed to reports from Shafi et al. (2010), together with Eshghizadeh et al. (2012). Under the 100 mM alkaline treatment, root morphological parameters of KWS0143 were higher than Beta464, and decrease of those parameters in KWS0143 was slighter compared to Beta464 (Table 2), and this accounts for the cause of KWS0143 growth within certain alkali-contaminated areas. Under NaCl-derived salinity, a significant reduction in root morphological parameters is related to the mitotic or vacuolar abnormality and nuclear damage within the meristematic cells of roots (Radić et al. 2005). Information regarding pH influence on root growth is lacking. So far, it remains unclear about the role of pH in the development of lateral root has not been examined, which deserves more investigation.
Beta464 outperformed KWS0143 for root dry weight, shoot dry weight, as well as other parameters (Table 1) at control and 25 mM conditions, while, in general, both cultivars were similar at the remaining conditions. Thus, even when Beta464 is more inhibited than KWS0143 by alkali stress, the results suggest that it can still maintain an adequate performance under control and low alkali conditions. Besides, Beta464 was not significantly different from KWS0143 at 25 mM for the parameters included in Table 2. This is important for breeding purposes, since two alternative strategies are possible: (1) select for elite genotypes that outperform tolerant ones under control conditions while maintaining good performance under moderate stress levels; (2) select for tolerant genotypes under stress conditions with a yield penalty under optimal conditions.
Inorganic ions are accumulated in the vacuoles of plants subjected to saline stress for decreasing the water potential of cells, since the energy consumed after the absorption of inorganic ions is low compared with that in the synthesis of organic compounds. Excess ions passing through the plant increases the toxic level, decreases growth rate, and suppresses photosynthesis (Munns and Tester 2008). Na+ has been recognized as the major toxic ion within the salinized soils. The high cytoplasmic K+ and low Na+ exert an important part in maintaining numerous enzyme processes (Alzahrani et al. 2019). Controlling the uptake of Na+ represents an effective way in reducing Na+ content in a majority of crop plants (Zhang and Shi 2013). In the present study, K+ and Na+ were the predominant cations in cells for each case, while Ca2+ concentration was minimal; furthermore, Na+ content markedly increased relative to K+ in the case of alkali stress (Table 3). Those findings resembled the report from Yang et al. (2007). Moreover, root Na+ content gradually increased with alkaline concentration, but Ca2+/Na+, K+/Na+ as well as K+ level gradually declined (Table 3). These results suggested that increase in root Na+ concentration hindered K+ and Ca2+ uptake. Besides, K+/Na+ and Ca2+/Na+ in KWS0143 root were higher than Beta464 under different levels of alkaline stress (Table 3). This phenomenon indicated that KWS0143 has stronger capacity to control root Na+ uptake, maintain root K+ and Ca2+ assimilation compared with Beta464. Moreover, secreted Na+ from leaf surface significantly increased with increasing alkaline concentration (Fig. 2), which indicated that Na+ exclusion could be a way of sugar beet in adaption to alkali stress.
The dissociated polyamines exert a vital part within plant tolerance to stress. According to Liu et al. (2011), conspicuous accumulation of Put was found in the NaCl-treated Vitis vinifera seedlings. Besides, applying Spd in plants prevents the UV-C-induced damaging impacts to some extent (An et al. 2004). Our results suggested that, the dissociated polyamines levels in roots in KWS0143 as well as Beta464 increased significantly under alkaline stress (Table 4), which suggested that the dissociated polyamines participated in promoting alkaline stress resistance of sugar beet root. In this study, root polyamines might enhance critical enzyme activities participating within oxidative stress, including APX, GR, and SOD, in the meantime of decreasing lipid peroxidation of sugar beet (Tang and Newton 2005). The degradation of Put is catalyzed by a DAO, while a PAO is an enzyme involved in oxidative deamination of Spm and Spd (Moschou et al. 2008). According to our results, the increased polyamine level within the roots of sugar beet were associated with the elevated activities of PAO and DAO (Table 4), which suggested that accumulation of free polyamines stimulated the upregulation of DAO and PAO activity. On the other hand, in this experiment, the increase of DAO activity took place subsequently compared with that of PAO (Table 4). Such result might be due to the diverse locations within cells in 2 enzymes as well as 3 polyamines (Politycka and Kubiś 2000). DAO, PAO, and Spd can be detected within cell walls, whereas Put can be detected in vacuole and cytoplasm (Li et al. 2018). Therefore, Put was transported into apoplast prior to enzymatic oxidation (Politycka and Kubiś 2000).
With regard to the OAs metabolic modulation in the case of alkali stress, at least one critical enzyme is involved, including those known enzymes in the basal metabolic pathways, including glycolysis, glyoxylate cycle, and tricarboxylic acid cycle. In the presence of alkali condition, plants should keep the ion balance in cells, as well as the stability of pH value. The enhanced activity of PEPC in the presence of alkali or salt stress reflects the necessity for plants to boost carbon skeleton production for maintaining the pH neutrality in cells through synthesizing the organic acid (Ma et al. 2016). According to this study, PEPC activity in roots of two sugar beet cultivars was significantly enhanced at treatments of 25, 50, as well as 75 mM (Fig. 3). This may be because PEPC is one of key enzyme in process of organic acid synthesis (Jiang et al. 2019). Besides, similar to other physiological parameters in the present study, root PEPC activity of KWS0143 was higher compared with that of Beta464 under the 100 mM treatment (Fig. 2). This may be one of the reasons why KWS0143 can grow better than Beta464 under alkaline conditions. Based on our results, two major organic acids (tartaric acid and succinic acid) were accumulated in roots under alkali stress (Table 5). The modulation mechanism regarding the metabolism of organic acids within sugar beet was different compared with that for other halophytes, including Kochia sieversiana (Yang et al. 2007) as well as Suaeda glauca (Yang et al. 2008). The oxalate contents accumulated in those latter 2 species were about 90% of organic acids in the case of alkali stress. According to the above findings, modulating the metabolism of organic acids might exert a distinct role among various plant species. In conclusion, alterations of the metabolism of organic acids represent the vital in vivo response for halophytes to respond to alkaline condition, which can serve as the vital direction of research on plant physiology under alkali stress in the future.
Author contribution statement
C-LZ, Y-BW, and C-FL designed experiments. C-LZ, BW, DL, and LL carried out experiments. C-LZ analyzed the data and drafted the work. Y-BW provided instruments for experiments. C-FL critically revised the manuscript.
References
Alzahrani SM, Alaraidh IA, Migdadi H, Alghamdi S, Altaf Khan M, Ahmad P (2019) Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak J Bot. https://doi.org/10.30848/pjb2019-3(3)
An LZ et al (2004) Effect of enhanced UV-B radiation on polyamine content and membrane permeability in cucumber leaves Russian. J Plant Physiol 51:658–662. https://doi.org/10.1023/b:rupp.0000040753.29607.58
An YM, Song LL, Liu YR, Shu YJ, Guo CH (2016) De Novo Transcriptional Analysis of Alfalfa in Response to Saline-Alkaline Stress. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00931
Aziz A, Larher F (1995) Changes in polyamine titers associated with the proline response and osmotic adjustment of rape leaf discs submitted to osmotic stresses. Plant Sci 112:175–186
Azooz MM, Metwally A, Abou-Elhamd MF (2015) Jasmonate-induced tolerance of Hassawi okra seedlings to salinity in brackish water. Acta Physiol Plant 37:1–13
Bai L, Wang C, Zang S, Zhang Y, Hao Q, Wu Y (2016) Remote sensing of soil alkalinity and salinity in the Wuyu’er-Shuangyang river Basin. Northeast China Remote Sens 8:163
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44:384–395
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bueno M, Cordovilla M-P (2019) Polyamines in halophytes. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00439
Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101:9909
Chen SS, Xing JJ, Lan HY (2012) Comparative effects of neutral salt and alkaline salt stress on seed germination, early seedling growth and physiological response of a halophyte species Chenopodium glaucum. Afr J Biotechnol 11(40):9572–9581
Chen W et al (2009) Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.). Ind Crops Prod 30:351–358. https://doi.org/10.1016/j.indcrop.2009.06.007
Chen YT, Cai-Feng LI, Zhao LY, Peng Y, Wang YY, Teng XY (2010) Screening of Salinity Tolerance And Response Of Seedling To Salt Stress In Sugar Beet (Beta vulgaris L.). Plant Physiol Commun 46:1121–1128
Eshghiizadeh HR, Kafi M, Nezami A, Khoshgoftarmanesh A (2012) Studies on the role of root morphology attribution in salt tolerance of blue-panicgrass (Panicum antidotale Retz) using artificial neural networks (ANN). Res Crops 13:534–544
Fu HJ, Yu HY, Li TX, Wu Y (2019) Effect of cadmium stress on inorganic and organic components in xylem sap of high cadmium accumulating rice line (Oryza sativa L.). Ecotox Environ Safe 168:330–337
Guo J, Li CF, Chen M, Xu Y, Ma FM (2015) Effects of Na2CO3 stress on the growth and antioxidant enzymes of beta vulgaris seedlings. Plant Physiol J 51:840–846
Guo J, Li CF, Liu L, Sang LM, Wang YB (2016) Variation of rhizosphere environmental factors of sugarbeet seedlings under Na2CO3 stress and their correlation. Chin J Appl Ecol 27:904–910
Guo R et al (2017) Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol 17:41. https://doi.org/10.1186/s12870-017-0994-6
Hazman M, Hause B, Eiche E, Riemann M, Nick P (2016) Different forms of osmotic stress evoke qualitatively different responses in rice. J Plant Physiol 202:45–56
Hossain MS, ElSayed AI, Moore M, Dietz KJ (2017) Redox and reactive oxygen species network in acclimation for salinity tolerance in sugar beet. J Exp Bot 68:1283–1298. https://doi.org/10.1093/jxb/erx019
Jesus J, Castro F, Niemelä A, Borges MT, Danko AS (2015) Evaluation of the impact of different soil salinization processes on organic and mineral soils water. Air Soil Pollut 226:1–12
Jiang C-C, Fang Z-Z, Zhou D-R, Pan S-L, Ye X-F (2019) Changes in secondary metabolites, organic acids and soluble sugars during the development of plum fruit cv. ‘Furongli’ (Prunus salicina Lindl). J Sci Food Agric 99:1010–1019. https://doi.org/10.1002/jsfa.9265
Latef AAA, Tran LSP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00243
Li H et al (2015) Salt stress response of membrane proteome of sugar beet monosomic addition line M14. J Proteomics 127:18–33. https://doi.org/10.1016/j.jprot.2015.03.025
Li L et al (2018) Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem 129:35–55. https://doi.org/10.1016/j.plaphy.2018.05.017
Li Y, Huang L, Zhang H, Wang M, Liang Z (2017) Assessment of ammonia volatilization losses and ntrogen utilization during the rice growing season in alkaline salt-affected soils. Sustainability 9:132. https://doi.org/10.3390/su9010132
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by abscisic acid, salicylic acid, and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera ). Physiol Plant. https://doi.org/10.1111/ppl.12483
Liu D et al (2014) Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ Sci Pollut Res Int 21:13615–13624. https://doi.org/10.1007/s11356-014-3271-3
Liu J, Shi D-C (2010) Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 48:127–134. https://doi.org/10.1007/s11099-010-0017-4
Liu JH, Nakajima I, Moriguchi T (2011) Effects of salt and osmotic stresses on free polyamine content and expression of polyamine biosynthetic genes in Vitis vinifera. Biol Plant 55:340–344
Ma B et al (2015) Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem 172:86–91. https://doi.org/10.1016/j.foodchem.2014.09.032
Ma Y, Wang XP, Zhang SF, Shi DC, Sheng LX (2016) Effects of salt and alkali stress on growth, accumulation of oxalic acid, and activity of oxalic acid-metabolizing enzymes in Kochia sieversiana. Biol Plant 60:774–782. https://doi.org/10.1007/s10535-016-0650-2
Magaña C et al (2011) Direct prediction of bioethanol yield in sugar beet pulp using near infrared spectroscopy. Biores Technol 102:9542
Moschou P, Paschalidis K, Roubelakis-Angelakis K (2008) Plant polyamine catabolism: the state of the art. Plant Signal Behav 3:1061–1066. https://doi.org/10.4161/psb.3.12.7172
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Plant Biology 59:651–681
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Paz RC, Rocco RA, Reinoso H, Menéndez AB, Pieckenstain FL, Ruiz OA (2012) Comparative study of alkaline, saline, and mixed saline–alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of lotus tenuis. J Plant Growth Regul 31:448–459. https://doi.org/10.1007/s00344-011-9254-4
Politycka B, Kubiś J (2000) Changes in free polyamine level and di- and polyamine oxidase activity in cucumber roots under allelochemical stress conditions. Acta Physiol Plant 22:11–16
Radi AA, Abdelwahab DA, Hamada AM (2012) Evaluation of some bean lines tolerance to alkaline soil. J Biol Earth Sci 2
Radić S, Prolić M, Pavlica M, Pevalek-Kozlina B (2005) Cytogenetic effects of osmotic stress on the root meristem cells of Centaurea ragusina L. Environ Exp Bot 54:213–218
Shafi M, Guoping Z, Bakht J, AmanKhan M, Islam UE, Khan MD, Raziuddin GZ (2010) Effect of cadmium and salinity stresses on root morphology of wheat. Pak J Bot 42:2747–2754
Smith TA, Barker JH (1985) The DI- and polyamine oxidases of plants. Adv Exp Med Biol 13:319–322
Tang W, Newton RJ (2005) Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul 46:31–43
Vastarelli P, Moschella A, Pacifico D, Mandolino G (2013) Water Stress in Beta vulgaris: Osmotic Adjustment Response and Gene Expression Analysis in ssp. Vulgaris and maritime. Am J Plant Sci 04:11–16
Witzel K et al (2018) Plasma membrane proteome analysis identifies a role of barley membrane steroid binding protein in root architecture response to salinity Plant. Cell Environ 41:1311–1330. https://doi.org/10.1111/pce.13154
Xu Z-M et al (2018) Low root/shoot (R/S) biomass ratio can be an indicator of low cadmium accumulation in the shoot of Chinese flowering cabbage (Brassica campestris L. ssp. chinensis var. utilis Tsen et Lee) cultivars. Environ Sci Pollut Res 25:36328–36340. https://doi.org/10.1007/s11356-018-3566-x
Yang C, Chong J, Li C, Kim C, Shi D, Wang D (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294:263–276
Yang C, Shi D, Wang D (2008) Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regulat 56:179–190. https://doi.org/10.1007/s10725-008-9299-y
Yang JY, Zheng W, Tian Y, Wu Y, Zhou DW (2011) Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica 49:275–284. https://doi.org/10.1007/s11099-011-0037-8
Yang L, Ma C, Wang L, Chen S, Li H (2012) Salt stress induced proteome and transcriptome changes in sugar beet monosomic addition line M14. J Plant Physiol 169:839–850. https://doi.org/10.1016/j.jplph.2012.01.023
Zhang JL, Shi H (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosynth Res 115:1–22
Zhang Y, Fernie AR (2018) On the role of the tricarboxylic acid cycle in plant productivity. J Integr Plant Biol 60:1199–1216. https://doi.org/10.1111/jipb.12690
Zhao J, Shi G, Yuan Q (2008) Polyamines content and physiological and biochemical responses to ladder concentration of nickel stress in Hydrocharis dubia (Bl.) Backer leaves. Biometals 21:665
Zhou Y et al (2019) Application of exogenous glutathione confers salinity stress tolerance in tomato seedlings by modulating ions homeostasis and polyamine metabolism. Sci Hortic 250:45–58. https://doi.org/10.1016/j.scienta.2019.02.026
Zou C, Sang L, Gai Z, Wang Y, Li C (2018) Morphological and physiological responses of sugar beet to alkaline stress. Sugar Tech 20:202–211. https://doi.org/10.1007/s12355-017-0547-1
Zou CL et al (2019) Photosynthetic capacity, osmotic adjustment and antioxidant system in sugar beet (Beta vulgaris L.) in response to alkaline stress. Photosynthetica 57:350–360
Acknowledgements
This work was funded by the National Natural Science Foundation of China (31671622) and Modern Agricultural Industrial Technology System of China (CARS-170201).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. J. Reigosa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, CL., Wang, YB., Wang, B. et al. Effects of alkali stress on dry matter accumulation, root morphology, ion balance, free polyamines, and organic acids of sugar beet. Acta Physiol Plant 43, 13 (2021). https://doi.org/10.1007/s11738-020-03194-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03194-x