Abstract
Salinity stress is a major limitation to global crop production. Sugar beet, one of the world’s leading sugar crops, has stronger salt tolerant characteristics than other crops. To investigate the response to different levels of salt stress, sugar beet was grown hydroponically under 3 (control), 70, 140, 210 and 280 mM NaCl conditions. We found no differences in dry weight of the aerial part and leaf area between 70 mM NaCl and control conditions, although dry weight of the root and whole plant treated with 70 mM NaCl was lower than control seedlings. As salt concentrations increased, degree of growth arrest became obvious In addition, under salt stress, the highest concentrations of Na+ and Cl− were detected in the tissue of petioles and old leaves. N and K contents in the tissue of leave, petiole and root decreased rapidly with the increase of NaCl concentrations. P content showed an increasing pattern in these tissues. The activities of antioxidant enzymes such as superoxide dismutase, catalase, ascorbate peroxidase and glutathione peroxidase showed increasing patterns with increase in salt concentrations. Moreover, osmoprotectants such as free amino acids and betaine increased in concentration as the external salinity increased. Two organic acids (malate and citrate) involved in tricarboxylic acid (TCA)-cycle exhibited increasing contents under salt stress. Lastly, we found that Rubisco activity was inhibited under salt stress. The activity of NADP-malic enzyme, NADP-malate dehydrogenase and phosphoenolpyruvate carboxylase showed a trend that first increased and then decreased. Their activities were highest with salinity at 140 mM NaCl. Our study has contributed to the understanding of the sugar beet physiological and metabolic response mechanisms under different degrees of salt stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinization is a global environmental problem that can influence the productivity and quality of agricultural crops (Duarte et al. 2013). To date, over 800 million hectares of cultivated land in the world are affected by salinity (Munns and Tester 2008). In China, the area of saline soil occupies about 34 million hectares. It accounts for about 3.6% of the land area in China and equals one-third of its cultivated land (Wang et al. 2012). Usually, a large amount of salt accumulating in soil will inhibit seed imbibition and cause nutrient imbalance, enzymatic inhibition and metabolic dysfunction (Xu et al. 2016). For example, a high concentration of salt ions in the soil results in physiological water shortage and impedes nutrient absorption through the increasing osmotic potential in the soil. A high level of sodium ions in the shoot will also decrease photosynthesis rate (Julkowska and Testerink 2015). The ultimate aim of salinity tolerance research is therefore to enhance the ability of plants to maintain growth and productivity in saline soils, which is of urgent need for engineering or breeding salinity tolerant crops.
In the long term evolutionary process, plants form physiological adaptation mechanism in response to salt stress. Many adaptation strategies occurred at the same time or separately, depending on the stress duration and intensity. Several physiological and biochemical strategies to combat salt stress have been found in plants, including selective absorption and transport of salt ions, salt ions compartmentalization, accumulating of osmotic adjustment solutes and increasing of antioxidant enzyme activities (Munns and Tester 2008; Rahman et al. 2016). For example, under salt stress, large amounts of inorganic ions accumulating in plant cells are transported and stored in the vacuole, enabling water uptake and avoiding ion poisoning (Roy et al. 2014). In addition, superoxide anion radical, hydrogen peroxide and hydroxyl radical will be generated under salt stress conditions (Mishra et al. 2013). These reactive oxide species (ROS) affect the normal process of metabolism through lipid peroxidation or oxidative damage of biological macromolecules (Bose et al. 2014). However, plants make use of antioxidases such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione peroxidase (GPX) combined with low molecular weight antioxidants such as ascorbic acid (AsA) and glutathione (GSH), to remove different types of ROS (Kaur et al. 2016; Noctor et al. 2007). Another common change identified in plants exposed to salinity is that they synthesize and accumulate organic metabolites of low molecular weight, which are collectively known as compatible solutes. They act as osmolytes by maintaining osmotic equilibrium in different compartments (Yang et al. 2013). The combined action of these strategies plays an important role in improving the salt tolerance of plants.
Sugar beet is one of the main sugar yielding crops, and shows good adaptability under salt stress. It has been reported that sugar beet was able to grow in soil containing 85–140 mM salt (Li et al. 2007). Our previous report showed that sugar beet exhibited better growth status under 3 mM NaCl than 0 mM NaCl (Peng et al. 2014). Sugar beet thus shows a great potential to grow in saline soil. Therefore, the study of sugar beet response mechanism under salt stress is useful for sugar beet plantation in saline soil and cultivating salt tolerance varieties. The aim of this work was to understand the physiological and metabolic response mechanisms of sugar beet under different degrees of salt stress. Our study will add new knowledge to our understanding of sugar beet response mechanisms under salt stress.
Materials and methods
Plant materials and growth conditions
This experiment was performed with sugar beet (Beta vulgaris L.) variety ST13092. Plants were grown in a quantum flux density of 450 µmol m−2 s−1 and incubated at 25 °C with a 14 h/10 h photoperiod in a greenhouse at Heilongjiang University. Relative humidity was controlled at 60 ± 5%. The seeds were germinated in vermiculite with daily watering. Selected uniform seedlings were transferred to 5 L dark colored glass containers filled with half-strength Hoagland solution. Ten days after planting, seedlings were treated with 3 (control), 70, 140, 210 and 280 mM NaCl for 20 days. As our previous report showed sugar beet exhibited the best growth status under 3 mM NaCl compared with 0, 0.75, 1.5, 6 and 9 mM NaCl conditions (Peng et al. 2014). We thus selected the 3 mM NaCl as control condition. Each treatment contained four seedlings and has six biological repeats. The nutrient solution with different concentrations of NaCl was aerated continuously and regularly added to replace water lost by transpiration.
Measurement growth status
Plants were harvested after 20 days of treatment, rinsed three times in ddH2O and dried with filter paper. The fresh weight (FW) was calculated after harvest and dry weight (DW) was measured after drying for 3 days at 80 °C. Relative water content (RWC) was measured using the following formula as follows: RWC (%) = [(fresh weight − dry weight)/(turgid weight − dry weight)] × 100. Osmotic potential ψs was determined by the method of Bendaly et al. (2016). Leaf area was measured by LI-3000C portable area meter (LI-COR Biosciences). The testing of stomatal conductance, transpiration rate, intercellular carbon dioxide intensity and net photosynthetic rate of blade was done using an LC4 photosynthesis tester (ADC Bio Scientific).
The measurement of ions and nutrients
The plants were dried at 80 °C to constant weight. The content of Na+ and K+ were determined using the flame spectrophotometer (FP640, Shanghai Precision & Scientific Instrument) (Pi et al. 2016). Cl− determination using the ultraviolet spectrophotometer referred to the method by Lu (2000). Total N and P measurement was conducted using Kjeldahl method and ammonium molybdate, respectively (Lu 2000).
Estimation of lipid peroxidation and antioxidant enzyme activities
Sugar beet leaves were homogenized, and the homogenate was centrifuged at 30,000 g for 30 min. The supernatant obtained was used as the enzyme extract. The ascorbate peroxidase (APX) activity was measured as the rate of oxidation of ascorbate by monitoring the absorbance decline at 290 nm at 25 °C as previously reported by Nakano and Asada (1981). The GPX activity was analyzed according to Sharma et al. (2013) by measuring change in absorbance at 436 nm. The reaction buffer contained 50 mM K-P buffer (pH 7.0), 10 mM guaiacol 10 mM H2O2 and 25 μL enzyme extract. For measurement of superoxide dismutase (SOD), 1 mL of reaction mixture was prepared in 50 mM K-P buffer (pH 7.8) containing 2 μM riboflavin, 75 μM nitrotetrazolium blue (NBT), 100 μM EDTA, 13 mM DL-methionine and 60 μl of enzyme extract and the absorbance was taken at 560 nm (Stewart and Bewley 1980). CAT enzymatic activity was calculated using the system reported by Aebi (1984). The decline of H2O2 absorbance was determined in 2 mL of reaction buffer containing 100 mM K-P buffer (pH 7.0), 20 mM H2O2 and 20 μL enzyme extract. Lipid peroxidation was determined by measuring the content of malondialdehyde (MDA) in leaves (Yang et al. 2015).
Measurement of indole acetic acid (IAA), abscisic acid (ABA) and gibberellic acid (GA)
The ABA and IAA contents were measured by enzyme-linked immunosorbent (ELISA) kits provided by China Agricultural University. The leaves (1 g) were homogenized in liquid nitrogen and hormones were extracted by 2.5 mL methanol (80%) containing 1 mM butylated hydroxytoluence as an antioxidant. The extract was incubated for 4 h at 4 °C and centrifuged at 5,000 rpm for 15 min at 4 °C. The resulting supernatant was vacuum evaporated to one-tenth of the initial. The evaporated residue was dissolved in a 1% acetic acid and filtered with 0.22 mm filters. The samples were extracted with pre-concentrated C18 SPE columns. The analyses were eluted from the column by 2.5 mL 100% (v/v) methanol from the column. ELISA was performed on a 96-well microtitration plate. Each well on the plate was coated with 100 μL coating buffer (1.5 g L−1 Na2CO3, 2.93 g L−1 NaHCO3 and 0.02 g L−1 NaN3, pH 9.6) containing 0.25 g mL−1 antigens against the hormones. The coated plates were incubated for 4 h at 37 °C for ABA, and overnight at 4 °C for IAA. After washing four times with PBS buffer (pH 7.4) containing Tween 20 (0.1% [v/v]), each well was filled with 50 μL of extracts or standards, and 50 μL of 20 g mL−1 antibodies against IAA and ABA, respectively. The plate was incubated for 3 h at 28 °C for ABA, and overnight at 4 °C for IAA, and then washed as above. 100 μL of 1.25 g mL−1 IgG-horseradish peroxidase substrate was added to each well and incubated for 1 h at 30 °C. The plate was rinsed five times with the above PBS + Tween 20 buffer, and 100 μL color-appearing solution containing 1.5 mg mL−1 O-phenylenediamine and 0.008% (v/v) H2O2 was added to each well. After the reaction progress was stopped, color development in each well was detected using an ELISA Reader at optical density A490. The ELISA method was also used for the extraction and measurement of GA1+3 (Zhang et al. 2016).
The activity of photosynthetic enzymes
For determination of NADP-malic enzyme (NADP-ME) activity, leaf tissue (2 g) was homogenized on ice in 5 mL extraction buffer 0.1 M HEPES-KOH (pH 8.0) containing 2 mM DTT and 2 mM MnCl2. The mixture was centrifuged at 15,000 g for 5 min at 4 °C. The NADP-ME activity was measured according to Gawronska et al. (2013). For analysis of NADP-malate dehydrogenase (NADP-MDH), leaf tissue (2 g) was homogenized on ice in 5 mL extraction buffer (100 mM HEPES-NaOH, 6 mM DTT, 0.5% BSA and 0.5% polyvinylpyrrolidone (pvp). The mixture was centrifuged at 15,000 g for 2 min at 4 °C and, the supernatant was collected and put on ice. The NADP-MDH activity was measured in the buffer containing 30 mM Bicine, 30 mM HEPES-NaOH (pH 8.0) and 0.2 mM NADPH and 1 mM EDTA. Finally, the reaction was measured at 340 nm for 7 min (Gawronska et al. 2013).
Rubisco activity measurement was performed spectrophotometrically according to Ma et al. (2010). 500 mg leaves were homogenized in 3mL extraction buffer (100 mM tris–HCl, 1%PVP, 5% glycerol, 1 mM EDTA, and 10 mM mercaptoethanol, pH 8.2). The mixture was centrifuged at 15,000 g for 10 min at 4 °C and the supernatant was collected. Rubisco activity was analyzed in 3 mL reaction buffer (1 mM Tris–HCl pH 8.0, 100 mM MgCl2, 1 mM EDTA, 50 mM ATP, 50 mM DTT, 15U 3-phosphoglycerate kinase, 200 mM NaHCO3, 2 mM NADH and 1 mL, 9 mM RuBP) and 100 μL enzyme extract. The conversion rate of NADH to NAD+ was measured at 340 nm. For Phosphoenolpyruvate carboxylase (PEPC) activity assays, 200 mg leaves were homogenized in 2 mL of extraction buffer (100 mM Tris–HCl pH 7.5, 1 mM EDTA, 10 mM MgCl2, 10 mM KF and 14 mM b-mercaptoethanol). The homogenate was centrifuged at 12,000 g for 3 min and the supernatant was collected and desalted. The PEPC activity was determined spectrophotometrically using the method of NAD-MDH-coupled measurement at 2.5 mM PEP (Feria et al. 2016). Chlorophyll was extracted from 50 mg leaves in 2.5 mL of 80% acetone and measured at 645 and 663 nm according to a previous report (Bendaly et al. 2016).
Organic solutes analysis
Determination of five kinds of organic acid was done according to Nisperos-Carriedo et al. (1992) with minor modifications. Briefly, 1 g leaves were ground in methyl alcohol on ice and then placed in a centrifuge tube. The homogenate was centrifuged at 10,000 g for 10 min. Supernatant was collected and rotary evaporated at 39 °C, −0.095 MPa. Concentrate was transferred to the centrifugal tube and extracted 4 times using cyclohexane. The oil phase in the mixture was removed and aqueous phase prepared for testing after filtering through the 0.45 μm filter. Chromatography (HPLC) mobile phase contained 0.1 M K2HPO4 phosphate buffer solution (pH 2.55). HPLC conditions were C18-column (5 μm, 4.6 mm × 250 mm), flow velocity 0.5 mL min−1, injection volume 20 μL; detection wave length 214 nm; column temperature 30 °C. Betaine and proline were extracted and measured as previously reported (Yang et al. 2015). The choline content was analyzed using the methods of Pearce et al. (1976). Determination of free amino acids was done using the ninhydrin colorimetric method (Yang and Hong 2012). For analysis of soluble sugar, 100 mg leaves (dry weight) were infiltrated in 10 mL of 80% ethanol at 80 °C for 2 h. Ethanol was removed by boiled water bath. The extracts were added to reaction buffer containing 1 mL ddH2O and 5 mL 0.15% anthrone at 95 °C for 20 min. Absorbance of the mixture solution was then measured at 620 nm (Wang et al. 2014a, b).
Statistical analysis
All data were subjected to analysis of variance according to the model for completely randomized design using an SPSS program (SPSS Inc, Chicago, IL). Differences among means ± SD (n = 6) of treatments were evaluated by the Duncan test at 0.05 probability level.
Results
Growth status
From observation of the plant aerial part, there was no obvious difference between seedlings growing at 70 mM NaCl and control condition (3 mM NaCl) (Fig. 1a). These results were confirmed by the measurement of leaf area and dry weight (Fig. 1b, c). However, the dry weight of root and whole plant treated with 70 mM NaCl was lower than control seedlings. Indeed, growth arrest of the plant aerial part began to be exhibited in seedlings treated with 140 mM NaCl. As the salt concentrations increased, dry weight of the aerial part, roots and whole plant decreased and reached a minimum at 280 mM NaCl (Fig. 1a, d, e). At 280 mM NaCl, dry weight of the whole plant was only 38.9% that of the control group (Fig. 1e).
Effect of NaCl on phenotype (a), total leaf area (b), aerial part dry weight (c), root dry weight (d) and whole plant dry weight (e) in sugar beet treated with different concentrations of NaCl for 20 days. Different letters indicate significantly different at P < 0.05. Six biological replicates were performed
The distribution of Na+ and Cl− under salt stress
We then examined alterations in Na+ content in various tissues. Under control conditions, the maximum Na+ concentration was detected in the petiole tissue (Fig. 2a). Under salt stress, the Na+ content had a remarkable increase in all tissues (Fig. 2a). At 280 mM NaCl, Na+ content in leaf, petiole and root increased 7.9 times, 6.5 times and 17.8 times compared with control group, respectively. The highest concentration of Na+ was also detected in the petiole under salt stress. The distribution of Cl− in different tissues was similar to that of Na+. The Cl− content in the petiole was also higher than that of other tissues under control and salt stress conditions, and Cl− content in all tissues exhibited an increasing trend with increasing salt concentration (Fig. 2b).
The distribution of Na+ and Cl− in different tissues (a, b) and in different leaf positions (c, d) of sugar beet treated with different concentrations of NaCl for 20 days. Osmotic potential and relative water content in different leaf positions of sugar beet treated with different concentrations of NaCl for 20 days are indicated in e and f. Different letters indicate significantly different at P < 0.05. Six biological replicates were performed
The distribution of Na+ and Cl− was also detected in different leaf positions. The contents of Na+ and Cl− exhibited high levels in old leaves (2nd pair leaves) under control condition (Fig. 2c, d). Under salt stress, leaves in different positions all showed obvious increasing Na+ and Cl− concentrations. The highest value of Na+ was detected in 2nd pair leaves under different levels of NaCl (Fig. 2c). In addition, the distribution of Cl− in different leaf positions was the same as that of Na+ under salt stress (Fig. 2d). Our results indicated that salt ions tended to accumulate in old leaves under salt stress. Furthermore, osmotic potential and relative water content in different leaf positions were also detected under salt stress. It can be seen in Fig. 2 e, f that osmotic potential and relative water content in young leaves are significantly higher than old leaves under 70 mM NaCl concentration. However, no difference was found between old leaves and young leaves under control, 140, 210 and 280 mM NaCl conditions. As salt concentrations increased, the relative water content and osmotic potential gradually decreased and reached the lowest in 280 mM NaCl treatment.
Analysis of mineral nutrients (N, P and K) and hormones
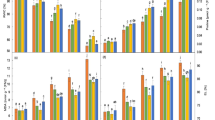
Salt stress effect on different nutrients in the tissues of leaf, petiole and root is shown in Fig. 3. Levels of N and K in these tissues changed in the opposite direction to NaCl treatment concentrations reflecting restriction in these nutrients. Conversely, a significant increase in P concentration was found (Fig. 3c).
N (a), K (b), P (c), Indole acetic acid (IAA) (d), gibberellic acid (GA) (e) and abscisic acid (ABA) (f) contents in leaves of sugar beet treated with different concentrations of NaCl for 20 days. Different letters indicate significantly different at P < 0.05. Six biological replicates were performed
To investigate the influence of saline stress on phytohormones, we examined the levels of GA, ABA and IAA under salt stress. The data indicated that there was no significant difference in the leaf content of IAA and GA1+3 between the low salt treatment (70 mM NaCl) and control group (Fig. 4). However, the production of GA and IAA was significantly lower under salt stress than to that under control conditions (Fig. 3d, e). The levels of IAA and GA1+3 reached their lowest value under 280 mM NaCl treatment, reducing by 57.4 and 51.1% compared to the control, respectively. However, the change trend of ABA was contrary to that of GA1+3 and IAA. ABA concentration increased under salt stress, and ABA accumulation reached its maximum under the 280 mM NaCl treatment (Fig. 3f).
Leaves malondialdehyde (MDA) content (a), relative electrical conductivity (b), superoxide dismutase (SOD) (c), catalase (CAT) (d), peroxidase (GPX) (e) and ascorbate peroxidase (APX) (f) of sugar beet treated with different concentrations of NaCl for 20 days. Different letters indicate significantly different at P < 0.05. Six biological replicates were performed
Lipid peroxidation and antioxidant enzyme activity
Oxidation-induced lipid membrane damage will usually appear in plants under salt stress. The damage is determined by lipid peroxidation. The MDA content reflects the level of lipid peroxidation. It is also one of the indexes for evaluating plasma membrane permeability. In our study, a slight increase in this parameter was found in sugar beet leaves, when plants were treated with low salt concentration (70 mM NaCl). The MDA level reached a maximum in the 280 mM NaCl treatment (Fig. 4a). Relative electrical conductivity is another index for measuring plasma membrane damage and permeability. In our study, the relative conductivity enhanced with the increasing of salinity (Fig. 4b). In the 280 mM NaCl treatment, relative electric conductivity in leaves was 77.5%, which was 3.4 times that of the control group. A change in antioxidant enzyme systems was also detected in our study. Under 70 mM NaCl, the activities of SOD and APX were not changed compared to control conditions (Fig. 4c, f), and the SOD activity presented an increasing pattern from 70 mM to 280 mM NaCl (Fig. 4c). APX activity showed the highest value in the 140 mM NaCl treatment, and remained stable with increasing NaCl concentrations in the culture medium (Fig. 4f). The activities of CAT and GPX showed an increasing pattern under salt stress and reached their highest at 280 and 210 mM NaCl, respectively (Fig. 4d, e).
Analysis of osmotic adjustment molecules
The contents of five organic acids under different salinity levels are listed in Table 1. The salt treatment did not affect the succinate and pyruvic acid contents. Instead, the malate content first increased and then decreased with increasing salinity. Citrate content began to be higher than the control group in the 210 mM NaCl treatment, but the fumarate content followed a decreasing pattern under salt stress. In addition, the betaine, choline, free amino acid, proline and soluble sugar contents were measured under different salinity levels (Table 2). At high salinity, betaine content in leaf increased remarkably. Choline content increased slowly and was higher than that of the control group in the 280 mM NaCl treatment. Furthermore, at 280 mM NaCl, the levels of soluble sugar and sucrose increased 0.9 times and 2.0 times compared to those at 3 mM NaCl, respectively. The free amino acids and proline accumulated progressively with increasing NaCl concentrations in the culture medium.
Photosynthesis related parameters
Chlorophyll plays a role of absorbing and transforming light energy in plant photosynthesis. We found an obvious decrease in chlorophyll content with the increase of NaCl concentrations (Fig. 5a). The content decreased by 38.4% at 280 mM NaCl. The net photosynthetic rate, stomatal conductance and intercellular carbon dioxide concentration also influence plant photosynthesis to varying degrees. It can be seen in Fig. 5 that the net photosynthetic rate, stomatal conductance and intercellular carbon dioxide concentration all followed a decreasing pattern and reached the lowest in the 280 mM NaCl treatment (Fig. 5b, c, d). Compared with the control group, the net photosynthetic rate, stomatal conductance and intercellular carbon dioxide concentration in the 280 mM NaCl treatment decreased by 74.5, 36.5 and 75.0%, respectively. Rubisco plays a major role in CO2 fixation, and its activity has a direct link to the accumulation of carbohydrate in plants. In our study, Rubisco activity was inhibited under salt stress (Fig. 6a). In addition, the activities of NADP-ME, PEPC, and NADP-MDH were determined under salt stress (Fig. 6b, c, d). These enzymes presented a trend that first increased and then decreased. Their activities were highest at 140 mM NaCl.
Analysis of chlorophyll content (a), intercellular carbon dioxide concentration (b), net photosynthetic rate (c) and stomatal conductance (d) in sugar beet leaves under different concentrations of NaCl treatment for 20 days. Different letters indicate significantly different at P < 0.05. Six biological replicates were performed
Activity of Rubisco (a), phosphoenolpyruvate carboxylase (PEPC) (b), NADP-malate dehydrogenase (NADP-MDH) (c), NADP-malic enzyme (NADP-ME) (d), in leaves of sugar beet treated with different concentrations of NaCl for 20 days. Different letters indicate significantly different at P < 0.05. Six biological replicates were performed
Discussion
Sugar beet is able to grow in saline soils (Yang and Hong 2012). In our study, no differences in total leaf area or dry weight of plant aerial part were detected between control seedlings and those treated with 70 mM NaCl (Fig. 1a, b, c), although the salt concentrations in leaves and petioles were significantly increased under 70 mM NaCl. It was reported that salt stress causes more damage to plant aerial part than plant roots (Eschie et al. 2002). Plant leaves may appear wilting and necrotic under salt stress. However, our results showed that sugar beet could maintain a good growth status in plant aerial part under low salt concentration (70 mM NaCl). This phenomenon reflects a strong salt-tolerance in the aerial part of sugar beet seedlings. However, root growth was slightly inhibited under 70 mM NaCl (Fig. 1d), and may be attributed to a high concentration of salt ions in the root environment.
Furthermore, the levels of Na+ and Cl− in the petiole tissue were significantly higher than those in roots or leaves under control or salt stress conditions (Fig. 2a, b). These results indicated that salt ions tended to accumulate in petioles of sugar beet. This may inhibit excessive accumulation of salt ions in the leaf tissues, which is essential to protect the photosynthetic machinery from ion toxicity (Eschie et al. 2002). In addition, our study found that salt ions tended to accumulate in old leaves under salt stress (Fig. 2c, d). Especially, at low salt concentration (70 mM NaCl), osmotic potential and relative water content in young leaves were significantly higher than in old ones (Fig. 2e, f). We therefore speculate that salt ions accumulating in old leaves may protect the physiological activity in young leaves from damage or ion toxicity.
Nutrients in plants can usually maintain a balanced state, but the increasing levels of Na+ and Cl− under salt stress affect the uptake of other nutrients. In our study, with the increasing concentrations of NaCl, the N and K contents in roots, stems and leaves of sugar beet decreased significantly (Fig. 3a, b). K and N are two indispensable nutrient elements in plants and play an important role in plant growth and development such as regulating permeability, maintaining the turgor pressure of cells or photosynthetic carbon metabolism and promoting cells growth. It has been reported that an increasing Na+ content induced K+ efflux and led to a lower K+/Na+ ratio under salt stress in plants (Percey et al. 2014). In addition, excessive Cl− can compete with NO3 − resulting in a decrease of NO3 − absorption efficiency (Chinnusamy et al. 2005). Therefore, high salt concentration may inhibit sugar beet growth by decreasing the absorption of K and N. However, the P content increased under salt stress (Fig. 3c). It is reported that salt stress can stimulate plants to absorb P and lead to an excessive accumulation (Alam et al. 2016). Several studies have shown that excessive P in plants will result in P toxicity and subsequently growth retardation (Ren et al. 2012; Zhou et al. 2008). Excessive accumulation of P in sugar beet leaves may thus be an important factor leading to a delay in plant growth under high salt concentrations.
Antioxidant enzymes, such as APX, POD, SOD and CAT, have been shown to significantly remove O2− and maintain reactive oxygen species (ROS) balance in plants for enhancing stress tolerance. SOD is a ubiquitous metalloenzyme in aerobionts, constituting the first line of defense against ROS to protect the cell membrane system from damage (Feng et al. 2016). SOD usually catalyzes the dismutation of the toxic superoxide anion to O2 and H2O2. But the byproduct, H2O2, can react with iron to produce hydroxyl radicals (Tepperman and Dunsmuir 1990). Timely elimination of excessive H2O2 is therefore an important step for antioxidation (Alscher et al. 2002). Other reports have shown that CAT, GPX and APX are essential enzymes for eliminating H2O2 and turning it into H2O and O2 (Mittler et al. 2004). Our study found that the activity of SOD, CAT, GPX and APX gradually increased under salt stress (Fig. 4c, d, e, f). The increasing activity of these antioxidant enzymes in sugar beet may play an important role in mitigating the damage from generated free radicals under salt stress. In addition, the change of APX activity under salt stress is different from that of SOD, CAT and GPX. It began to increase under 140 mM NaCl and no change was found with the increasing salt concentrations (Fig. 4f). These results indicated that different types of antioxidant enzymes may have adverse functions at different degrees of salt stress.
Osmotic adjustment is an important regulatory mechanism that plants adapt to saline stress (Volkmar et al. 1998). Plants usually accumulate soluble organic solutes such as betaine, free amino acids, proline, organic acid, soluble sugar or its derivative compound to maintain cell turgor and stabilize activity conformation of enzyme molecules (Flowers and Colmer 2008). It is reported that the role of proline is involved in protecting the cytomembrane system and maintaining the intracellular enzyme structure (Hong et al. 2000). Soluble sugar in plants is very sensitive to salt stress and has been reported to take part in maintaining the integrity of cell membranes in high salinity conditions (Tuteja 2007). Betaine has been reported to accumulate to osmotically significant levels in many salt-tolerant halophytes (Zeng et al. 2015). In our study, we found that the levels of most organic solutes were enhanced under salt stress. However, the betaine or free amino acids contents were much higher than those of proline or choline (Table 2). We thus speculate that betaine and free amino acids contributions to osmotic adjustment in the cytoplasm were significantly greater. Furthermore, other studies have shown that plants with high salt tolerance ability will accumulate a large amount of organic acid, which is an important intermediate product of material and energy metabolism in plants, and can participate various kinds of abiotic stress response (Wang et al. 2014a, b). In our research, the malate and citrate contents increased under high salt stress (Table 1). Similarly, it is reported that malate increased under salt stress in Atriplex halimus (Bendaly et al. 2016). Citrate, as first intermediate product in the tricarboxylic acid cycle, influences many metabolic pathways and takes part in plant response to high temperature (Hu et al. 2016). However, the exact role of citrate accumulation under salt stress conditions in sugar beet needs further exploration.
Chlorophyll plays an important role in the plant photosynthesis process. Chlorophyll content not only reflects the capacity of plant photosynthesis, but is also one of the important physiological indexes for evaluating plant salt tolerance. Studies have shown that salt stress may damage the chloroplast structure, decreasing chlorophyll content in plants, causing weakened photosynthetic ability (Liu et al. 2016). We detected a decline of chlorophyll content in sugar beet under salt stress (Fig. 5a). The net photosynthetic rate, stomatal conductance and intercellular carbon dioxide concentration also significantly decreased (Fig. 5b, c, d). Salt stress usually leads to the closing of stomata for relieving physiological drought and affects transpiration and respiration in plants (Robinson et al. 1997). In our study, stomatal closure and the decreasing of intercellular CO2 concentration may have caused weakened photosynthesis (Fig. 5c). Rubisco is a vital enzyme associated with carbon fixation that converts carbon dioxide into high-energy substances (Feller et al. 2008). In our study, the activity of Rubisco decreased under salt stress, indicating the decreasing of CO2 fixation (Fig. 6a). It has also been reported that Rubisco large subunit was cleaved by ROS under abiotic stress (Luo et al. 2002). NADP-ME and NADP-MDH are two key enzymes involved in the CAM pathway. A significant up-regulation of NADP-ME and NADP-MDH activities and transcript levels under salt stress was found in some halophytic plants (Cushman et al. 2008). However, the increasing activity of NADP-ME and NADP-MDH was detected in sugar beet under moderate salt stress. It is reported that a contribution of cytosolic NADP-ME to plant cell defense against oxidative stress is mainly related to providing NADPH for the biosynthesis of flavonoids and lignin (Cushman et al. 2008). Furthermore, NADP-MDH is involved in protection against oxidative stress by participating in the export of reduction equivalents from chloroplasts to the cytosol (Scheibe et al. 2005). Thus, the increasing activity of these two enzymes in sugar beet may engage in antioxidant functions under moderate salt stress (Fig. 6c, d).
Sugar beet is a moderate salt-tolerant glycophyte. Based on our results, a schematic diagram of potential salt stress tolerance mechanisms in sugar beet is proposed (Fig. 7). We found that salt tolerance of sugar beet was a complex trait involved in many physiological and metabolic pathways. Future studies focusing on characterizing the key physiological mechanisms will be highly valuable for designing molecular breeding or engineering programs to enhance crop salt tolerance and yield.
Conclusions
Based on the overall salt stress-mediated adaptive responses in our study, we concluded that the salt tolerance of sugar beet was integrated into many aspects, including storing salt ions in old leaves and petioles, increasing the levels of compatible solutes, enhancing the activity of antioxidants and increasing NADP-ME, NADP-MDHN and PEPC activities under moderate salt stress. Therefore, the salt tolerance of sugar beet is a complex trait determined by many physiological and metabolic pathways. These results have laid a foundation for understanding the physiological regulatory mechanisms of salt stress tolerance in Beta vulgaris.
References
Aebi H (1984) CAT in vitro. Meth Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Alam MA, Juraimi AS, Rafii MY, Hamid AA, Aslani F, Hakim MA (2016) Salinity-induced changes in the morphology and major mineral nutrient composition of purslane (Portulaca oleracea L.) accessions. Biol Res 49:24. doi:10.1186/s40659-016-0084-5
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341. doi:10.1093/jxb/53.372.1331
Bendaly A, Messedi D, Smaoui A, Ksouri R, Bouchereau A, Abdelly C (2016) Physiological and leaf metabolome changes in the xerohalophyte species Atriplex halimus induced by salinity. Plant Physiol Biochem 103:208–218. doi:10.1016/j.plaphy.2016.02.037
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257. doi:10.1093/jxb/ert430
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448. doi:10.2135/cropsci2005.0437
Cushman JC, Tillett RL, Wood JA, Branco JM, Schlauch KA (2008) Large-scale mRNA expression profiling in the common ice plant, Mesembryanthemum crystallinum performing C3 photosynthesis and crassulacean acid metabolism (CAM). J Exp Bot 59:1875–1894. doi:10.1093/jxb/ern008
Duarte B, Santos D, Marques JC, Caçador I (2013) Ecophysiological adaptations of two halophytes to salt stress: photosynthesis, PS II photochemistry and anti-oxidant feedback–implications for resilience in climate change. Plant Physiol Biochem 67:178–188. doi:10.1016/j.plaphy.2013.03.004
Eschie HA, Al-Barhi B, Al-Gheity S, Al-Khanjari S (2002) Root and shoot growth in salinity-stressed Alfaalfa in respone to nitrogen source. J Plant Nutr 25:2559–2569. doi:10.1081/PLN-120014713
Feller U, Anders I, Mae T (2008) Rubiscolytics: fate of rubisco after its enzymatic function in a cell is terminated. J Exp Bot 59:1615–1624. doi:10.1093/jxb/erm242
Feng K, Yu J, Cheng Y, Ruan M, Wang R, Ye Q, Zhou G, Li Z, Yao Z, Yang Y, Zheng Q, Wan H (2016) The SOD gene family in tomato: identification, phylogenetic relationships, and expression patterns. Front Plant Sci 7:1279. doi:10.3389/fpls.2016.01279
Feria AB, Bosch N, Sánchez A, Nieto-Ingelmo AI, de la Osa C, Echevarría C, García-Mauriño S, Monreal JA (2016) Phosphoenolpyruvate carboxylase (PEPC) and PEPC-kinase (PEPC-k) isoenzymes in Arabidopsis thaliana: role in control and abiotic stress conditions. Planta 244:901–913. doi:10.1007/s00425-016-2556-9
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963. doi:10.1111/j.1469-8137.2008.02531.x
Gawronska K, Romanowska E, Miszalski Z, Niewiadomska E (2013) Limitation of C3-CAM shift in the common ice plant under high irradiance. J Plant Physiol 170:129–135. doi:10.1016/j.jplph.2012.09.019
Hong Z, Lakkineni K, Zhang Z, Verma DP (2000) Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased prolineaccumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136. doi:10.1104/pp.122.4.1129
Hu L, Zhang Z, Xiang Z, Yang Z (2016) Exogenous application of citric acid ameliorates the adverse effect of heat stress in Tall Fescue (Lolium arundinaceum). Front Plant Sci 7:179. doi:10.3389/fpls.2016.00179
Julkowska MM, Testerink C (2015) Tuning plant signaling and growth to survive salt. Trends Plant Sci 20:586–594. doi:10.1016/j.tplants.2015.06.008
Kaur N, Dhawan M, Sharma I, Pati PK (2016) Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol 16:131. doi:10.1186/s12870-016-0824-2
Li W, Wang R, Wang W, Liu H, Liu J, Zhang S, Ai Y (2007) Effect of NaCl stress on sugar beet growth. Sugar Crops China 2:17–19. doi:10.3969/j.issn.1007-2624.2007.02.006
Liu A, Hu Z, Bi A, Fan J, Gitau MM, Amombo E, Chen L, Fu J (2016) Photosynthesis, antioxidant system and gene expression of bermudagrass in response to low temperature and salt stress. Ecotoxicology 25:1445–1457. doi:10.1007/s10646-016-1696-9
Lu R (2000) Soil agricultural chemical analysis method. Agricultural science and technology press, Bei Jing
Luo S, Ishida H, Makino A, Mae T (2002) Fe2+-catalyzed site-specific cleavage of the large subunit of ribulose 1,5-bisphosphate carboxylase close to the active site. J Biol Chem 277:12382–12387. doi:10.1074/jbc.M111072200
Ma D, Guo T, Song X, Wang C, Han Q, Yue Y, Cha F (2010) Effects of nitrogen fertilizer application on RuBP carboxylase activity and chlorophyll fluorescence parameters in flag leaves of winter wheat. Acta Bot Bor Occ Sin 30:2197–2202
Mishra P, Bhoomika K, Dubey RS (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250:3–19. doi:10.1007/s00709-011-0365-3
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. doi:10.1016/j.tplants.2004.08.009
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. doi:10.1146/annurev.arplant.59.032607.092911
NakanoY, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. doi:10.1093/oxfordjournals.pcp.a076232
Nisperos-Carriedo MO, Buslig BS, Shaw PE (1992) Simultaneous detection of dehydroascorbic, ascorbic and some organic acids in fruits and vegetables by HPLC. J Agric Food Chem 40:1127–1130. doi:10.1021/jf00019a007
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134. doi:10.1016/j.tplants.2007.01.005
Pearce RB, Strange RN, Smith H (1976) Glycinebetaine and choline in wheat: Distribution in relation to infection by Fusarium graminearum. Phytochemistry 15:953–954. doi:10.1016/S0031-9422(00)84377-0
Peng C, Geng G, Yu L, Yang Y, Pi Z, Sun F, Sun X, Zhao H (2014) Effect of different Na+ concentrations on growth and physiological traits of sugar beet. J Plant Nutr Fertil 20:459–465. doi:10.11674/zwyf.2014.0223
Percey WJ, Shabala L, Breadmore MC, Guijt RM, Bose J, Shabala S (2014) Ion transport in broad bean leaf mesophyll under saline conditions. Planta 240:729–743. doi:10.1007/s00425-014-2117-z
Pi Z, Stevanato P, Sun F, Yang Y, Sun X, Zhao H, Geng G, Yu L (2016) Proteomic changes induced by potassium deficiency and potassium substitution by sodium in sugar beet. J Plant Res 129:527–538. doi:10.1007/s10265-016-0800-9
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Calcium supplementation improves Na(+)/K(+) ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci 7:609. doi:10.3389/fpls.2016.00609
Ren F, Guo QQ, Chang LL, Chen L, Zhao CZ, Zhong H, Li XB (2012) Brassica napus PHR1 gene encoding a MYB-like protein functions in response to phosphate starvation. PLoS One 7:e44005. doi:10.1371/journal.pone.0044005
Robinson MF, Very A, Sanders D, Mansfield TA (1997) How can stomata contribute to salt tolerance? Ann Bot 80:387–393. doi:10.1006/anbo.1996.0435
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26:115–124. doi:10.1016/j.copbio
Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56:1481–1489. doi:10.1093/jxb/eri181
Sharma I, Ching E, Saini S, Bhardwaj R, Pati PK (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26. doi:10.1016/j.plaphy.2013.04.013
Stewart RR, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248. doi:10.1104/pp.65.2.245
Tepperman JM, Dunsmuir P (1990) Transformed plants with elevated levels of chloroplastic SOD are not more resistant to superoxide toxicity. Plant Mol Biol 14:501–511. doi:10.1007/BF00027496
Tuteja N (2007) Mechanisms of high salinity tolerance in plants. Meth Enzymol 428:419–438. doi:10.1016/S0076-6879(07)28024-3
Volkmar KM, Hu Y, Steppuhn H (1998) Physiological responses of plants to salinity: a review. Can J Plant Sci 78:19–27. doi:10.4141/P97-020
Wang Y, Zhan Y, Wu C, Gong S, Zhu N, Chen S, Li H (2012) Cloning of a cystatin gene from sugar beet M14 that can enhance plant salt tolerance. Plant Sci 191–192:93–99. doi:10.1016/j.plantsci.2012.05.001
Wang M, Li P, Li C, Pan Y, Jiang X, Zhu D, Zhao Q, Yu J (2014a) SiLEA14, a novel atypical LEA protein, confers abiotic stress resistance in foxtail millet. BMC Plant Biol 14:290. doi:10.1186/s12870-014-0290-7
Wang XY, Wang P, Qi YP, Zhou CP, Yang LT, Liao XY, Wang LQ, Zhu DH, Chen LS (2014b) Effects of granulation on organic acid metabolism and its relation to mineral elements in Citrus grandis juice sacs. Food Chem 145:984–990. doi:10.1016/j.foodchem.2013.09.021
Xu C, Tang X, Shao H, Wang H (2016) Salinity tolerance mechanism of economic halophytes from physiological to molecular hierarchy for improving food quality. Curr Genom 17:207–214. doi:10.2174/1389202917666160202215548
Yang S, Hong N (2012) Improvement on determination of free amino acids content in tea. Food Sci Techol 37:297–305.
Yang L, Zhang Y, Zhu N, Koh J, Ma C, Pan Y, Yu B, Chen S, Li H (2013) Proteomic analysis of salt tolerance in sugar beet monosomic addition line M14. J Prot Res 12:4931–4950. doi:10.1021/pr400177m
Yang C, Zhou Y, Fan J, Fu Y, Shen L, Yao Y, Li R, Fu S, Duan R, Hu X, Guo J (2015) SpBADH of the halophyte Sesuvium portulacastrum strongly confers drought tolerance through ROSscavenging in transgenic Arabidopsis. Plant Physiol Biochem 96:377–387. doi:10.1016/j.plaphy.2015.08.010
Zeng Y, Li L, Yang R, Yi X, Zhang B (2015) Contribution and distribution of inorganic ions and organic compounds to the osmotic adjustment in Halostachys caspica response to salt stress. Sci Rep 5:13639. doi:10.1038/srep13639
Zhang S, Zhang D, Fan S, Du L, Shen Y, Xing L, Li Y, Ma J, Han M (2016) Effect of exogenous GA3 andits inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple (Malus domestica Borkh.). Plant Physiol Biochem 107:178–186. doi:10.1016/j.plaphy.2016.06.005
Zhou J, Jiao F, Wu Z, Li Y, Wang X, He X, Zhong W, Wu P (2008) OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol 146:1673–1686. doi:10.1104/pp.107.111443
Acknowledgements
Lisa David from University of Florida is acknowledged for critical reading of the manuscript. This work was supported by the Project of Natural Science Foundation of Heilongjiang province (project no. C2016048: A preliminary study on the molecular drought tolerance mechanism of T510 strains of sugar beet); Project of international Cooperation (project no. 2010DFAN31530: Drought resistance, salt tolerance breeding, and cultivation for energy beet); Project of National Natural Science Foundation of China (project no. 31271779: Excavation of germplasm resources of higher salt tolerance and osmotic regulation mechanism in sugar beet); Youth Science Funds of Heilongjiang University (Project no QL201511: A preliminary study on salt tolerance mechanism of T510 strains of sugar beet).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Stevanato, P., Yu, L. et al. The physiological and metabolic changes in sugar beet seedlings under different levels of salt stress. J Plant Res 130, 1079–1093 (2017). https://doi.org/10.1007/s10265-017-0964-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0964-y