Abstract
Chitosan as natural product might have potential application in the production of Origanum majorana “Marjoram”, a known historic plant with economic importance in the agriculture and pharmaceutical industries. Under three irrigation periods (1, 3, and 5 days) in marjoram plants for 8 weeks, chitosan was applied as water solution at 50, 200 and 500 ppm. Chitosan increased selected morphological parameters and associated with elevated physiological and molecular performance. Enhanced metabolism of the treated plants was expressed as increased rates of stomatal conductance and photosynthetic rates. In addition, soluble sugars and proline levels were higher. Elevated expression of MnSOD Cu/ZnSOD, FeSOD, APX, DREB2 and ERF3 s were also detected. Further elevated expression of CYP71D179/182 and CYP71D178 PII, essential oil composition-related genes, was also found. The SOD and APX enzymes were more active and there were reductions in the levels of reactive oxygen species. Thymol and cis-Sabinene were higher in treated plants essential oils. Chitosan may alleviate water stress in marjoram by enhancing the metabolism and stress related genes in treated plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lack of irrigation water or drought is restricting agricultural development worldwide. Some aromatic crops are oversensitive to prolonged irrigation intervals (PII). Stress may influence the morphology and physiology and cause reduced growth and lower metabolic performance and these changes might be related proline and sugar osmotic (Toscano et al. 2014; Bhattacharjee and Saha 2014; Elansary et al. 2017). Furthermore, molecular changes in specific antioxidant genes (e.g., APX and SOD) may occur under stress conditions (Elansary et al. 2017). Other genes are also regulated by stress condition including DREB2 and erf (Liu et al. 1988; Kang et al. 2002; Zhang et al. 2009; Ali et al. 2017).
The alleviation of water stress had been achieved using seaweed extracts (Elansary et al. 2017), growth regulators (El-Esawi et al. 2017), nanoparticles (Saxena et al. 2016) and chitosan (Yin et al. 2012; Bistgani et al. 2017). Chitosan oligosaccharide is relatively new biostimulant, produced by heating chitin then removing calcium and proteins (Sharp 2013). Chitosan is a natural product that might be a potent metabolic elicitor (Yin et al. 2012; Ramírez et al. 2010). The chitosan is nontoxic, environmental friendly or biodegradable and could be blended with starch, gelatin and alginates in the food industry (Kong et al. 2010). However, the stress related uses of chitosan are not well studied.
Origanum majorana L. is a known culinary herb that belongs to the family Lamiaceae with origins to southern Europe, Asia and North Africa (Makri 2002). The plant traditional uses roots in the history in Middle East and Arabian countries for long time (Mansfeld 1986; Small 2006). Tender stems and leaves are used fresh or dry for seasoning and spicing food in the Arabian kitchen. Leaves and flowers of the plant contain delicate fragrant essential oil that is widely used in traditional medicinal uses including lotions, perfume, cream and soaps (Khan and Abourashed 2010). The use of the plant is further documented in history for headaches, asthma, ear problems and others (Morton 1981; Simon et al. 1984).
In this investigation, several approaches were followed to explore the possible use of chitosan to overcome PII stress in marjoram plants. The essential oil is sensitive to stress conditions and chitosan sprays by means of ratio and composition. The morphological responses of the plants were also deeply investigated. The genetic background which is related to the metabolic performance is illustrated by several ways. The study may have applications as novel protocol for the essential oil production in this plant. It is important to associate these changes as conducted in this study.
Materials and methods
Materials and conditions
Origanum majorana L. was obtained from commercial nurseries in the years 2016 and 2017 (January). These plants were identified by Dr Abdullah A. Al-Ghamdi then routinely vouchered at the Botany and Microbiology Department, College of Science, King Saud University. The plants were grown a 14 cm diameter plastic pots that have a mixture of peat and sand (1:1) as well as chemical fertilizer (Crystalon® 2 g L− 1,NPK 20:20:20) in a in growth chamber of the Department of Botany and Microbiology. Substrate field capacity quantification followed the gravimetric method. In this method, the soil was saturated with water and weighed, then the water was drained for 40 min and the difference was determined.
Treatments
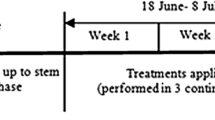
The plants were watered every 1, 3, and 5 days during an 8 week experiment. Sprays of chitosan (powder, Ghala Co., Riyadh) at 0, 50, 200 or 500 ppm dissolved in water was used weekly and starting 2 weeks before stress condition. Irrigation periods were the main effect (main plot) and chitosan was the subplots. Plants receiving 0 ppm of chitosan were considered control. Three blocks (RCBD) with five replicates for each treatment design was used. The total number of plants was 60 (three irrigation intervals × 4 chitosan conc. × 5 replicates) for each block in each year.
Measurements
Morphological measurements including leaf numbers, plant height, leaf area, root dry weight and overall all plant dry weight were determined after 8 weeks of treatments. Root dry weight as well as overall plant dry weight were quantified by drying fresh plants in the oven at relatively low temperature (35 °C for 72 h) to maintain the essential oil and until reaching constant weight. Metabolic performance of the plant was measured by determining the photosynthetic rate (Pn). The transpirations rate (E) and related measurements including the stomatal conductance (gs) were also determined using CO2/H2O IRGA (ADC, LCi, Bioscientific Ltd, Hoddesdon, UK). These metabolic measurements were taken in sunny conditions and on leaves located in middle of the canopy. Other metabolic related measurement including proline and sugars were also determined following Bates et al. (1973) and Dubois et al. (1956).
Essential oil determination and analyses
A Clevenger apparatus was used to obtain the oil from dried leaves by hydrodistillation. The GC/MS analyses followed the oil extraction and the experiment conditions and compounds identification followed previous studies (Adams 2007; Elansary 2015).
Enzymes and genes
The activities of Superoxide dismutase (SOD) is an important measurement in determining stress conditions. In addition, other enzymes such as ascorbate peroxidase (APX) play an important role under stress. The activities of theses enzymes were quantified in fresh frozen leaves following Elansary et al. (2017). Reactive oxygen species (ROS) accumulation is usually reduced under normal conditions and may increase under stress conditions. This accumulation of ROS was expressed as H2O2 using the Beyotime Colorimetric Kit. Total RNA isolation, cDNA preparation and qRT-PCR followed El-Esawi et al. (2017a, b) to quantify the possible responses of Cu/ZnSOD, APX, FeSOD, and MnSOD to stress and chitosan. Additional genes expression including DREB2 (Liu et al. 1988), AREB1 (Kang et al. 2002), and ERF3 (Zhang et al. 2009) were also investigated. PCR conditions followed El-Esawi et al. (2017). Additional cytochrome P450 genes (CYP71D179/182 and CYP71D178) responsible for carvacrol and thymol biosynthesis in O. vulgare were also used following Crocoll et al. (2010), Crocoll (2010) and Morshedloo et al. (2017). Actin was used as house keeping gene and for comparison reasons by employing the 2−∆∆Ct method (Radyukina et al. 2011). Experiments were also repeated to ensure the fidelity of the data.
Statistical analyses
To simplify the presentation of the data of the 2 years of 2016 and 2017, the data were subjected to the analyses of variance and no significant differences were found between 2 years. The data were pooled and differences among means were expressed by least significant differences (LSD) at probability level of 0.05 using the SPSS software.
Results
Morphological parameters and essential oils
The morphological effects of PII and chitosan sprays are presented in Table 1. Under PII of 3 and 5 days, chitosan caused noticeable peak in leaf number and area in marjoram plants compared to untreated plants. Dry weights of roots only as well as dry weights of the whole plant increased in stressed plants treated with chitosan compared to untreated ones. It was noticed that the plants were shorter under stress conditions and that chitosan treatments reduced this shortness. The PII treatment of 3 days showed high ratio of essential oil (2.9%), which is more than controls 1 and 2 (2.6%) (Table 1). Under PII of 3 and 5 days, chitosan increased the essential oils. For example, PII of 3 days showed increasing oil from 2.9 to 3.7% in chitosan treatment at 200 ppm. The 200–500 ppm chitosan spray elevated leaf number and area and increased dry weights as well as plant heights.
Metabolic plant performance
The effects of PII on the photosynthetic performance as well as the stomatal conductance and transpiration are presented in Table 2. There were increases in the three parameters following chitosan sprays compared to untreated plants. The highest increase in the three parameters of gas exchange was found in treatments of 200 and 500. These high chitosan treatments showed no differences regarding their effects on gas exchange in general. Under PII treatment in control plants, the proline and sugars were high. However, chitosan increased the accumulation of proline.
Enzymes gene expression
SOD and APX activities peaked following chitosan applications and PII of 3 and 5 days, as shown in Fig. 1. Normal irrigation intervals accompanied by chitosan had no effects on these enzymes. H2O2 levels were greatly reduced following chitosan applications (Fig. 2). The expression of Cu/ZnSOD, MnSOD, APX FeSOD, DREB2, AREB1 and ERF3 is shown in Figs. 3 and 4. It was found that APX, FeSOD, Cu/ZnSOD, MnSOD are stimulated in chitosan treated plants under PII. The concentrations of 200 and 500 ppm showed the highest levels regarding genes expression. However, other genes including DREB2, AREB1 and ERF3 varied in their expressions (Fig. 4). 1 day irrigation intervals showed no variation compared to control. PII conditions showed increases in the expression of DREB2 and ERF3, but AREB1 had no changes. Under PII, chitosan high doses of 200 and 500 ppm stimulated DREB2 and ERF3. Furthermore, the increases in the expression levels associated with increasing stress conditions. CYP71D179/182 and CYP71D178 showed significant fold changes in their expression following chitosan treatments (Fig. 5).
Essential oil composition
The essential oil composed of thymol, cis-sabinene hydrate, 4-terpineol, terpinolene, α-Terpinene and sabinene, as shown in Table 3. PII and chitosan influences the chemical constitutes of different treatments, as shown in Table 3. PII modulated the oil constitutes in control. The thymol, cis-sabinene hydrate and sabinene increased from 30.40, 15.41 and 7.30% to 31.61, 16.52 and 7.6%, respectively, following 3 day irrigation intervals prolongation. 4-Terpineol, terpinolene and α-Terpinene were reduced in control plants watered every 3 and 5 days compared to normal conditions (1 days).
PII and chitosan affected oil constitutes as shown in increased thymol, cis-sabinene hydrate and sabinene.
Discussion
In other plants such as Brachiaria brizantha (Fialho et al. 2009), Mentha sp. (Elansary 2017), turfgrasses (Elansary et al. 2017) and Medicago sativa (Zhang et al. 2018), the diminished vegetative growth is a mechanism for reducing water loss. Ninou et al. (2017) reported that stress in Origanum vulgare has effects on dry matter percentage in grown plants. Furthermore, Radácsi et al. (2010) found reductions in the dry mass of stressed Ocimum basilicum. The reduced dry weights found here is drought avoidance method (Nilsen and Orcutt 1996; Elansary et al. 2017). The plant shortness is caused by slow growth rate under PII and similar patternhad been described in marjoram (Hekmat et al. 2010), Ocimum basilicum (Elansay et al. 2015) and Mentha sp. (Elansary 2017).
Essential oil ratio peak after stress might be related to reduced water content (Ninou et al. 2017). However, continuous water stress conditions may reduce the essential oil ratios in marjoram plants (Hekmat et al. 2010).
Enhanced growth following chitosan treatment may increases essential oils (Bistgani et al. 2017). They found increased essential oil yields in water stressed Thymus daenensis Celak plants treated with 400 ppm chitosan sprays.
Stress may cause temporal reduction in metabolic performance including gas exchange measurements (Toscano et al. 2014). Chitosan may increase the proline content and lipid peroxidation (Bistgani et al. 2017) or cause increased polyphenolic composition in treated plants (Yin et al. 2012). Chitosan may alleviate stress by influencing gas exchange as well. Indeed, this is related to increased chlorophyll content and synthesis efficiency as well as proline and sugars peak in treated plants.
Chitosan relatives including chitin increase the chlorophyll accumulation and may affect the size of the chloroplast (Dzung and Thang 2004) and this activity may influence the aeration through enhancing stomatal conductance and the transpiration rates. The accumulation of proline is related to improved stress tolerance (Bandurska 2001; Zhang et al. 2018). The increase in the photosynthetic rates in plants treated with chitosan may result from increased accumulation of chlorophylls which enhance consequently the photosynthetic rates and such conclusion is strongly supported by previous work on chitosan (Farouk et al. 2008, 2011). However, other reports revealed opposite results (Bittelli et al. 2001; Song et al. 2006) which indicate that the effect might be species dependent.
Previous study indicate the antioxidant stress tolerance is supported by the oxidative enzymes (Ma et al. 2008; Sheikh-Mohamadi et al. 2017). Modulation of stress genes (e.g., FeSOD) might be related to stress tolerance (El-Esawi et al. 2017). Chen et al. (2016) related the photosynthetic apparatus with stress tolerance as found here. Chitosan sprays did not affect AREB1 but affected other genes such as CYP71D179/182 and CYP71D178, which are related to carvacrol and thymol and explaining essential oil shifts. Patricelli et al. (2015) reported parallel results as stress responses of these genes in O. vulgare and Morshedloo et al. (2017) also.
The current study support previous investigations (Soliman et al. 2009; Elansary 2015). Baatour et al. (2013) found cis-Sabinene hydrate and 4-terpineol (terpinen-4-ol) in the oil as found here.
Stress affects major oil constitutes in basil (Elansary 2015), mint (Elansary et al. 2016) and rosemary (Elansary et al. 2017). The morphological and physiological changes affect oil glands population and manipulate the essential oil composition (El-Keltawi and Croteau 1987).
Chitosan relation to essential oil ratio and constitutes is clear now and the highest quality of the oil were found in conditions, where chitosan is at high doses. These conditions enhance the metabolic and morphological status of the plants. The high quality oil is related to high cis-Sabinene hydrate in marjoram (Franz and Novak 2002; Elansary 2015). Other reports indicated high phenolics in the leaves of Ocimum basilicum L. plants treated with chitosan but they did not study the essential oil (Ghasemi Pirbalouti et al. 2017).
Conclusion
Chitosan is important in stress alleviation in some plants including marjoram. It increases the vegetative and metabolic levels of the plants subjected to unfavorable conditions. Increased expression of specific antioxidant and specific stress genes in marjoram is expected following chitosan application. The essential oils quality of marjoram are largely improved by chitosan as well as oil ratio. The chitosan is highly recommended under stress conditions in marjoram.
Author contribution statement
The author responsible for the experimental design, performing the experiment, analyzing the data and writing the manuscript.
References
Adams RP (2007) Identification of essential oil compounds by gas chromatography/mass spectrometry, 4th ed. Allured pub 1 Corp., Carol Stream, IL, pp 35–48
Ali F, Bano A, Fazal A (2017) Recent methods of drought stress tolerance in plants. Plant Growth Regul 82:363–375
Baatour O, Kaddour R, Tarchoun I et al (2013) Modification of fatty acid, essential oil and phenolic contents of salt-treated sweet marjoram (origanum majorana L.) according to developmental stage. J Food Sci 77:1047–1054
Bandurska H (2001) Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injuries? II. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiol Plant 23:483
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bhattacharjee S, Saha AK (2014) Plant Water-Stress Response Mechanisms. In: Gaur R, Sharma P (eds) Approaches to plant stress and their management. Springer, New Delhi
Bistgani ZE, Siadat SA, Bakhshandeh A, Pirbalouti AG, Hashemi M (2017) Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. The Crop J. 2017:407–415
Bittelli M, Flury M, Campbell GS, Nichols EJ (2001) Reduction of transpiration through foliar application of chitosan. Agric For Meteorol 107:167–175
Chen Y, Chen C, Tan Z, Liu J, Zhuang L, Yang Z, Huang B (2016) Functional identification and characterization of genes cloned from halophyte seashore paspalum conferring salinity and cadmium tolerance. Front Pant Sci 7:102
Crocoll C (2010) Biosynthesis of the phenolic monoterpenes, thymol and carvacrol, by terpene synthases and cytochrome P450s in oregano and thyme. PhD dissertation, Friedrich-Schiller-Universität, Max-Planck-Institut für chemische Ökologie, Jena
Crocoll C, Asbach J, Novak J, Gershenzon J, Degenhardt J (2010) Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol Biol 73:587–603
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dzung NA, Thang NT (2004) Effect of oligoglucosamine on the growth anddevelopment of peanut (Arachis hypogea L.). In: Khor E, Hutmacher D, Yong LL (eds.), Proceedings of the 6th Asia-Pacific on Chitin, Chitosan Symposium Singapore
Elansary HO (2015) Chemical diversity and antioxidant capacity of essential oils of marjoram in Northwest Egypt. Essent Oil Bear Plants 18:917–924
Elansary HO (2017) Green roof Petunia, Ageratum, and Mentha responses to water stress, seaweeds, and trinexapac-ethyl treatments. Acta Physiol Plant 39:145
Elansary HO, Yessoufou K, Abdel-Hamid AME, El-Esawi MA, Ali HM, Elshikh MS (2017) Seaweed extracts enhance Salam turfgrass performance during PII and saline shock. Front Plant Sci 8:830
El-Esawi MA, Elansary HO, Elshanhory N, Abdel-Hamid AME, Ali HM, Elshikh MS (2017) Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front Physiol 8:716
El-keltawi NE, Croteau R (1987) Influence of foliar applied cytokinins on growth and essential oil content of several members of the lamiaceae. Phytochemittry 26:891–895
Farouk S, Ghoneem KM, Ali A (2008) Induction and expression of systematic resistance to downy mildew disease in cucumber plant by elicitors. Egypt J Phytopathol 2:95–111
Farouk S, Mosa AA, Taha AA, Heba IM, EL-Gahmery AM (2011) Protective effect of humic acid and chitosan on radish (Raphanus sativus L. var. sativus) plants subjected to cadmium stress. J Stress Physiol Biochem 7:99–116
Fialho CMT, Ferreira EA, Meira RAS et al (2009) Anatomical characters of Brachiaria brizantha submitted to trinexapac-ethyl application. Planta Daninha 27:533–539
Franz C, Novak J (2002) Breeding of oegano. In: Kintzios SE (ed) Oregano: the genera Origanum and Lippia. Taylor and Francis Inc., New York
Ghasemi Pirbalouti A, Malekpoor F, Salimi A, Golparvar A (2017) Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) underreduced irrigation. Sci Hort 217:114–122
Hekmat MY, Abdalah MYA, Mosa AAA, Nour Eldeen EAE (2010) Effect of water stress and foliar spray of humic acid on growth and essential oil quality of marjoram (Majorana hortensis Moench) Plant. J Plant Prod Mansoura Univ 1:1113–1123
Kang JY, Choi HI, Im MY, Kim SY (2002) Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14:343–357
Khan IA, Abourashed EA (2010) Leung’s encyclopedia of common natural ingredients: Used in food, drugs and cosmetics, 3rd edn. Wily, New Jersey, pp 437–438
Kong M, Chen XG, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 133:51–63
Liu Q, Kasuga M, Sakuma Y et al (1988) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Ma YH, Ma FW, Zhang JK et al (2008) Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Sci 175:761–766
Makri O (2002) Cultivation of Oregano. In: Kintzios SE (ed) Oregano: the genera Origanum and Lippia. Taylor and Francis Inc., New York
Mansfeld R (1986) Verzeichnis landwirschftlicher and gartnerischer Kulturpflanzen (ohne Zierpflanzen) 2nd edn. 4 Vol. In: Schultze-Motel et al Springer, New York, USA, pp 224–225
Morshedloo MR, Craker LE, Salami A, Nazeri V, Sang H, Maggi F (2017) Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono- and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol Biochem 111:119–128
Morton JF (1981) Atlas of medicinal plants of Middle America. Charles C. Thomas, Springfield, pp 1420
Nilsen ET, Orcutt DM (1996) Physiology of plants under stress: Abiotic factors. Wiley, New York
Ninou E, Paschalidis K, Mylonas I (2017) Essential oil responses to water stress in Greek oregano populations. Essent Oil Bear Plants. https://doi.org/10.1080/0972060X.2016.1264278
Patricelli D, Barbero F, Occhipinti A, Bertea CM, Bonelli S, Casacci LP, Zebelo SA, Crocoll C, Gershenzon J, Maffei ME (2015) Plant defences against ants provide a pathway to social parasitism in butterflies. Proc R Soc B R Soc 282:20151111
Radácsi P, Inotai K, Sarosi SZ, Czovek P, Bernath J, Nemeth E (2010) Effect of water supply on the physiological characteristic and production of basil (Ocimum basilicum L.). Eur J Hortic Sci 75:193–197
Radyukina NL, Shashukova AV, Makarova SS, Kuznetsov VV (2011) Exogenous proline modifies differential expression of superoxide dismutase genes in UV-B-irradiated Salvia officinalis Plants. Rus J Plant Physiol 58:51–59
Ramírez M, Rodriguez AT, Alfonso L, Peniche C (2010) Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnol Apl 27:270–276
Saxena R, Tomar RS, Kumar M (2016) Exploring nanobiotechnology to mitigate abiotic stress in crop plants. J Pharm Sci Res 8:974–980
Sharp RG (2013) A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agron 3:757–793
Sheikh-Mohamadi MH, Etemadi N, Nikbakht A, Arab M, Majidi MM, Pessarakli M (2017) Antioxidant defence system and physiological responses of Iranian crested wheatgrass (Agropyron cristatum L.) to drought and salinity stress. Acta Physiol Plant 39:245
Simon JE, Chadwick AF, Craker LE (1984) Herbs: an annotated bibliography, 1971–1980. The Shoe String Press, Inc., Hamden, pp 770
Small E (2006) Culinary herbs, 2nd edn. NRC Research Press, Ottawa, pp 611–613
Soliman FM, Yousif MF, Zaghloul SS, Okba MM (2009) Seasonal variation in the essential oil composition of Origanum majorana L. cultivated in Egypt. Z Naturforsch C 64:611–614
Song SQ, Sang QM, Guo SR (2006) Physiological synergisms of chitosan on salt resistance of cucumber seedlings. Acta Bot Boreali-Occidentalia Sin 26:435–441
Toscano S, Scuderi D, Giuffrida F, Romano D (2014) Responses of Mediterranean ornamental shrubs to drought stress and recovery. HortScience 178:145–153
Yin H, Frette XC, Christensen LP, Grevsen K (2012) Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J Agric Food Chem 60:136–143
Zhang G, Chen M, Li L et al (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERT type transcription factor for increased tolerance to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60:3781–3796
Zhang C, Shi S, Wang B, Zhao J (2018) Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol Plant (2018) 40:25
Funding
This project was supported by the King Saud University. Deanship of Scientific Research. College of Science Research Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Apostol.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Ghamdi, A.A. Marjoram physiological and molecular performance under water stress and chitosan treatment. Acta Physiol Plant 41, 44 (2019). https://doi.org/10.1007/s11738-019-2830-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2830-0