Abstract
The effects of drought on growth and seed quality of oilseed crops are of crucial importance in edible oil production due to its pivotal role in sustainable agriculture. Plant growth-promoting bacteria (PGPB) can improve crop yield by promoting plant growth under various environmental conditions. In the present study, the physiological responses, growth, and seed quality of three camelina doubled haploid lines (DH51, DH69, and DH104) were assessed upon their exposure to two irrigation regimes at the presence of Micrococcus yunnanensis during their reproductive phase. The results showed that the investigated parameters of camelina were affected by genotype, irrigation regimes, and PGPB. Drought decreased crop yield as measured by silique length, silique, and seed number and 1000-weight seed. PGPB significantly decreased the adverse effects of stress consistent by increasing the branches per plant and root length. Drought also caused a significant enhancement in the hydrogen peroxide and malondialdehyde contents, but the PGPB-inoculated plants showed lower contents of both compounds. Relative water content significantly reduced in plant grown under stress but inoculation enhanced the potential of water retaining in plants under stress and non-stress conditions. Drought stress and PGPB elevated proline and total soluble carbohydrate content in genotypes. Drought stress had no significant effect on photosynthetic pigments content of genotypes while inoculation apparently moderated negative impact of drought with enhancement of pigments content. The obtained results were responsible for metabolic changes occurring in response to stress. PGPB improved the plant drought-tolerance by enhancing its physiological traits. The fatty acid profile showed some variations among camelina genotypes under drought stress and PGPB inoculation. Upon symbiosis association, an increase was observed in major constituents of polyunsaturated acids, linoleic and linolenic acids, and a significant increase in oleic acid as a main monounsaturated acid. They also altered another major constituent, gadeolic acid, under water deficit stress and/ or with PGPB. Both drought stress and PGPB decreased the poly unsaturated fatty acids/mono unsaturated fatty acids ratio. In general, there was a significant difference among camelina lines in terms of seed yield and quality in response to drought. Also, it strongly suggested that PGPB application can be a positive strategy to mitigate drought stress and increase crop yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Drought stress, the most major impending environmental factor, is the major cause of poor food production throughout the world (You et al. 2019; Falaknaz et al. 2019). Water deficient can markedly damage crop growth and yield. It is well documented that the severity of stress, the period of drought treatment, and timing of stress application can affect the qualitative and quantitative traits of most plants. The flowering and seed filling stages are the most drought-susceptible stages. Drought stress can substantially affect the photosynthesis process, assimilate production and their transport to reproductive organs altering seed size, number, weight, and composition (Sohrabi et al. 2012; Akbarabadi et al. 2015; Sehgal et al. 2018). Drought stress invariably induces oxidative stress due to the accumulation of reactive oxygen species (ROS) such as H2O2, OH−, and O2− which can directly destroy the biological systems including lipid peroxidation, damage the nucleic acids, degrade the photosynthetic pigments, and disturb the protein structure and function (Huang et al. 2017; Li et al. 2019). Acclimation mechanisms to long-term stress-induced physiology, biochemical and morphology responses (such as activation of the antioxidant defense systems, osmotic potential modulation and increase root- shoot growth) are rather complex in plants (Zhou et al. 2015; Okunlola et al. 2017; Laxa et al. 2019). Given the global expansion of drought in the last decades, it is very critical to find a suitable approach to decrease the stress symptoms. The role of plant growth-promoting bacteria (PGPB) in growth, development, and management of biotic and abiotic stress has been very well documented (Vurukonda et al. 2016). PGPB-mediated mechanisms for drought stress tolerance in plants include; (1) improvement of nutrient uptake; (2) modulation of phytohormones level such as enhancement of gibberellins, auxins, and cytokinins, and the decline of ethylene; (3) promoting mineral accessibility; (4) induction of resistance to stresses and (5) increase of plant metabolites (Vurukonda et al. 2016; Oliveira et al. 2018). Reports of Sukweenadhi et al. (2015) have shown that M. yunnanensis increased the tolerance of the plant to salinity and drought through IAA production with and/or without precursor, siderophore production and solubilization of phosphate. Camelina (Camelina sativa L.Cranz) plant is an annual oilseed crop from the Brassicaceae family. It is originated from southeast Europe and southwest Asia but nowadays is sporadically cultivated in various climates (Berti et al. 2016). Genetically, the highly conserved and similarity of Arabidopsis and Camelina genomes make C. sativa a suitable genetic model for researchers (Hutcheon et al. 2010; Kahrizi et al. 2015).
This oil crop has great agronomical potential due to its relatively short life period, low nutrient demand, and high resistance to biotic and abiotic stresses (Soorni et al. 2017; Yuan et al. 2017). Additionally, camelina could be used as a rotational crop, improving soil quality (Anderson et al. 2019). Some studies showed that camelina seed contains at least 30% total oil. Its oil contains 90% unsaturated fatty acid (Yuan and Li 2020). The high level of unsaturated fatty acid has highlighted the commercial value of camelina in different industries such as feedstock, biofuel production, medicine, and oleochemical (Malik et al. 2018; Załuski et al. 2020). Thus, it is important to find the genotype and environmental effects on the fatty acid compositions of camelina. PGPBs can alleviate drought stress but there is limited information on PGPBs symbiosis with camelina and its influence on improving crop yields under water deficit stress.
The aim of the present study is to evaluate the effect of drought stress during the reproductive phase on growth, physiological responses, seed quality, and fatty acid composition for various genotypes of camelina upon symbiotic association with PGPB.
2 Materials and methods
2.1 Plant material, soil and bacterial preparation
The study was carried out at the Research Greenhouse of Biology Department, College of Sciences, Shiraz University, Shiraz, Iran from December to February 2018. The plant material included three doubled haploid (DH) lines of Camelina sativa (L.) Crantz including (DH51, DH69, and DH104) were supplied from Bisetoon Shafa Co., Kermanshah, Iran. These DH lines have been derived from anther culture of different F1 hybrids (Table 1). The soil was selected at depth of 0 to 30 cm from the Bajgah Agricultural Station of Shiraz University. The soil samples were sieved to 2 mm, mixed thoroughly, and sterilized by streaming (100°C for an hour for 3 day) and used for pot experiment. Physical and chemical properties of the soil were as follows, sand of 25%, silt of 63.40% and clay of 11% (Gee and Bauder 1986, pH of 7.9 (Thomas 1996), electrical conductively (EC) of 0.64 dS m−1(Rhoades 1996), total N of 0.04% (Bremner 1996), organic matter (OM) of 0.71% (Nelson and Sommers 1996), and Olson or available –phosphorous (P) of 10.49 mg kg−1(Olsen 1954), other elements such as ammonium acetate extractable of potassium (K) of 345 mg kg-1 K, diethylenetriaminepentaacetic acid (DTPA) extractable of iron (Fe), manganese (Mn), copper (Cu) and zinc (Zn) 1.40 mg kg−1, 3.20 mg kg−1, 0.72 mg kg−1 and 3 mg kg−1(Lindsay and Norvell 1978). Essential nutrient elements based on soil testing were added to all pots uniformly at the rate of 75 mg N kg −1 soil as urea, at the rate of 10 mg P kg−1 soil Ca (HPO2)2), Fe, Zn and Mn at the rate of 5 mg kg−1 soil (as Fe-EDDHA, ZnSO4.7H2O, MnSO4. 4H2O2, respectively) and Cu at the rate of 5 mg kg−1 soil (as CuSO4.5H2O) before sowing. Besides, urea was again applied at the rate of 75 mg N kg −1 20 days after sowing as a top dressing (Pereira et al. 2007).
The pure bacterium of Micrococcus yunnanensis was obtained from the Department of Soil Sciences, Shiraz University, and was cultured on nutrient broth (NB) medium for 24 h at 28 °C. Upon sowing, the seeds were inoculated by 1.0 ml M. yunnanensis suspension (9 × 107 c colony-forming unit (CFU) ml−1) in the treated group.
2.2 Growth, treatment conditions and data collection
The experiment was organized in a randomized experimental design with three treatment groups. The groups were defined as follows: T0: control group (well-irrigated), T1: drought-treated plants, T2: PGPB inoculated plants, and T3: PGPB inoculated drought-treated plants. Three pots were set up for each treatment which were daily monitored and irrigated based on field capacity (FC 100%) before the flowering stage. The drought treatment was conducted from the beginning of the flowering stage at levels of 100% and 50% FC until full maturity stage of the seed.
The leaves were collected 21 days after flowering and used for the physiological analysis. At maturity, plants were harvested from each treatment and the following parameters were determined: length of root and shoot, number of the lateral branches per plant, number of silique in the plant, length of silique, number of seed in silique and plant, and 1000 seed weight. The biochemical analysis also, was conducted on mature seeds.
2.3 Determination of photosynthetic pigments
The value of photosynthetic pigments was determined according to the Arone method (1949). Chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Total Chl), and carotenoids (Car) contents were extracted from 100 mg of fresh leaves in 10 ml 80% acetone. Homogenates were maintained at 4 °C for 24 h in the dark tubes. The absorbance of pigments was measured at 646, 652, 663, and 470 nm using Shimadzu UV- Vis spectrophotometer, Japan. The concentration of pigments was determined according to Lichtenthaler and Wellburn method (1983).
2.4 Determination of relative water content, proline content and total soluble carbohydrate
For relative water content (RWC) determination, the weight of the leaves was measured immediately after collection (fresh weight = FW) and placed into distilled water at 4 °C (darkness) and then weighted after 24 h (turgor weight = TW). Finally, dry weight (DW) of the samples was determined after 48 h at 70 °C. The RWC was measured according to Dhopte and Livera (2002) through the following equation:
Proline was estimated according to Bates et al. (1973). Fresh leaves (100 mg) were homogenized with 3 ml 3% aqueous sulfosalicylic acid. The homogenate was centrifuged at 15000 g for 10 min, at 4 °C. Ninhydrin acid and glacial acetic acid (2:2) were added to 2 ml extraction and maintained at 100 °C for 1 h. Then, 4 ml toluene was added to cooled extraction and its absorbance was determined at 520 nm. Proline content was reported as mmol g1fresh weight.
Total soluble carbohydrate (TSC) was extracted from 100 mg of fresh leaves and extraction solution in glacial acetic acid, methanol, water 1:4:5 (v: v: v) was centrifuged for 10 min at 3000 rpm. Extraction was done thrice and its volume was finally increased to 50 mL using water. TSC was determined according to Dubois et al. (1956). The mixture of 0.5 mL extraction with 0.5 mL of 5% (v: v) phenol solution and 2.5 mL purred sulfuric acid were maintained at 90 °C for 30 min. The absorbance was read at 490 nm. TSC content was recorded as mg−1 g dry weight.
2.5 Determination of malondialdehyde and H2O2 content
To evaluate lipid peroxidation, malondialdehyde (MDA) content was measured using thiobarbituric acid assay (Velikova et al. 2000). Fresh leaves (500 mg) were homogenized in 5 ml 0.1% trichloroacetic acid (TCA) and centrifuged at 10000 rpm for 20 min. The supernatant (0.5 ml) was added to 1 ml of thiobarbituric acid (TBA) in 20% TCA. The mixture was heated at 95 °C for 30 min, then placed in an ice-water bath. The extract was centrifuged at 10000 rpm for 5 min. The absorbance of the supernatant was recorded at 532 nm and subtracted at 600 nm.
H2O2 contents were measured as described by Velikova et al., (2000). Fresh leaves (500 mg) were homogenized in 5 ml 1%TCA. Homogenates were centrifuged at 10000 g for 20 min and then to 0.05 ml extract, 0.5 ml of 10 mM phosphate buffer (pH 7.0), and 1 ml of 1 M iodide potassium were added. The absorbance value of the mixture was read at 390 nm and H2O2 content was calculated by a standard curve.
2.6 Fatty acid analysis
The fatty acid composition was determined using the modified method of Xue et al. 2013. Briefly, 300 mg dried seeds were mixed with 500 μl methanol and sulfuric acid (49:1 v,v) to produce fatty acid methyl ester (FAME). The mixture was vortexed for 30s and then heated at 80 °C for 2 h. When cooled down to room temperature, 300 μl %0.9 NaCl and 150 μl Hexan were added to the mixture followed by 30 s of vortexing and finally centrifugation at 3000 rpm for 5 min. 0.2 μl FAME at a split ratio of 1:20 was quantified by gas chromatography (GC), using a DB-225 column (30 m × 250 μm × 0.25 μm) on Agilent 7890B. The FAME profile for fatty acids was identified based on comparing the retention time with standard library (NIST05a.L and wiley7n.l).
2.7 Statistical analysis
This experiment was conducted in a completely randomized design with a factorial arrangement and three replicates. All data were statistically analyzed by analysis of variance using the SAS software 9.4. The least significant differences method (LSD) was used for the interaction effect of three genotypes upon two levels of irrigation and PGPB. Results were recorded as means ± SE and significant differences were determined at p ≤ 0.05.
3 Results
3.1 Growth
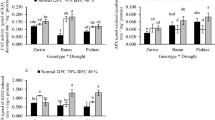
Drought stress reduced the number of branches, shoot, and root length compared to the controls (Fig. 1). The significant reduction in the number of branches was recorded by 70.35, 68.90, and 32% in DH69, DH104, and DH51, respectively, compared to control. The decrease of root length associated with a decrease of shoot length observed in drought- exposed plants. In DH51 genotype, we found the lower alternation in number branches, shoot and root length under drought stress than DH104 and DH69. It is suggested better growth performance of DH51 genotype under limited water condition. PGPB inoculation of camelina exhibited the highest increase in the number of branches (86.42% for DH51), shoot length (25.97% for DH104), and root length (27.33% for DH104) compared to the control (Fig. 1). PGPB also, had the strongest effect the number of branches in DH51 grown under drought stress compared to control.
Effect of drought stress and PGPR on (a) shoot length, (b) root length and (c) number of branches. T0: control, T1: drought stress, T2: PGPB, T3: drought stress with PGPB. Vertical bars indicate the standard error of the mean. The value with same letters have no significant difference (p < 0.05) at LSD test
3.2 Photosynthetic pigments
The effect of drought stress on photosynthetic pigments content in genotypes are presented in Table 2. The highest content of photosynthetic pigments (chlorophyll a, b and carotenoid), respectively, related to DH104 > DH69 > DH51 genotype. In general, Drought stress had no significant impact on genotypes pigments. PGPB application increased chlorophylls and carotenoid content and non- significantly mitigated the adverse effects of drought stress. In DH104, PGPB had the strongest of effect on carotenoid (19.04%) and total chlorophyll (48.17%) content as compared to controls.
3.3 Relative water content, proline and total soluble carbohydrate contents
Significant differences in relative water content (RWC) were no detected between genotypes at normal condition. RWC significantly decreased under drought stress (Fig. 2). These changes were ranged from 88.27 to 76.83 for DH51, 88.18 to 66.75 for DH69 and 92.42 to 79.06 for DH104 genotypes. In contrast, in DH51 genotype, the potential of water retaining under drought stress was higher than DH69 and DH104. Inoculation improved RWC in genotypes as compared to the non-inoculated plants. The strongest effect of PGPB on RWC was found in the non-stressed and stressed DH51genotype by 17 and 10%, respectively, improvement while it partially affected in DH69 and DH104 as compared to control.
Effect of drought stress and PGPB on (a) relative water content (RWC), (b) proline content, (c) total soluble carbohydrate (TSC) in three genotypes of Camelina sativa. T0 control, T1 drought stress, T3 PGPB, T4. Vertical bars indicate the standard error of the mean. The value with same letters have no significant difference (p < 0.05) at LSD test
There was a significant increase in proline content with both of treatment of drought stress and PGPB. The strongest proline increase under drought stress (up to 50%) was found in DH51genotype. PGPB markedly increased proline content under stress and non-stress conditions. Compared to control, proline content of PGPB-treated plants increased by 126.21, 211.2, and 73.06% in DH51, DH69, and DH104, respectively, under stress condition (Fig. 2).
The total soluble carbohydrate (TSC) content no significantly differed between genotypes (3.70, 3.62, and 2.96 mmolg−1 FW for DH104, DH51, and DH69, respectively) (Fig. 2). Drought stress significantly increased TSC content in both non-inoculated (1.4, 2.4 and 2.5 fold) DH104, and inoculated (3.1, 2.3, and 2.5) for DH51, DH69, and DH104, respectively. The highest TSC content was found in PGPB-treated DH51 genotype under drought stress while no significant difference was observed between DH69 and DH104 in terms of TSC content under same condition (Fig. 2).
3.4 H2O2 and MDA contents
The MDA and H2O2 content significantly increased in genotypes grown under drought stress as compared to genotypes grown under normal condition. For each of the three genotypes, H2O2 content elevated 2.5 fold under drought stress as compared to controls while no difference was observed in H2O2 content between genotypes under stress and non- stress condition. Lipid peroxidation rate and MDA content increased by 78, 55, and 64% in DH69, DH51and DH104, respectively, as compared with the control plants. The level of MDA was markedly modified with PGPB treatment under stress and non-stress conditions while PGPB had no impact on H2O2 content (Fig. 3).
3.5 Seed and silique
The long-term drought stress (70 days) strongly affected the seed and silique production. Silique length and number significantly decreased in the three genotypes under drought stress. The highest decrease of silique length (40.6%) was found in DH69 and DH51, respectively. Inoculation with PGPB caused an increase in silique length under stress and non-stress conditions. The number of silique per plant also declined under drought stress (Table 3) and the highest decrease was observed in DH51 (33.02%). The number of seeds in silique was significantly decreased in DH69 and DH51 under drought stress (25.15% and 28.14%, respectively) compared to the controls. PGPB had no significant effect on the number of seeds in DH69 and DH51 as compared with the control samples, however, it significantly decreased in PGPB- treated DH51 (11.28%) under drought stress. In general, a reduction was detected by 12.41, 24.54, and 50.9% in the number of seeds per plant of DH104, DH51, and DH69 under drought stress, respectively. The results also showed that the 1000-seed weight of all genotypes decreased under drought stress by 2% to 32% and inoculation with PGPB changed that by +0.43% to +26.92% under the same condition (Table 3). The application of PGPB significantly reduced the damage percentage in DH51 under drought stress as the number of silique, number of plant seeds and seed weight increased in inoculated plants.
3.6 Fatty acid compositions
Several different fatty acid compositions were detected in the oil of the maturated seeds (Table 4). There was a high variability between genotypes for fatty acids content under control and drought stress conditions. Water stress non-significantly increased palmitic acid (saturated fatty acid) composition of DH104 and DH69 (4.4 and 4.07%, respectively) and caused a non-significant reduction in DH51 (0.44%). PGPB caused a significant decrease in palmitic acid (C16:0) content by 5.68, 9.13, and 12.94% in DH51, DH69, and DH104, respectively. Oleic acid (C18:1) and linoleic acid (C18:2) levels were almost increased in all treatments. The oleic acid content ranged from 16.38 (DH69) to 18.5% (DH51). PGPB increased oleic acid content by 8.09, 14.57and 24.7% in DH51, DH104, and DH69, respectively, under non-stress conditions. Compared to the controls, the highest oleic acid content was recorded in PGPB-treated plants under stress conditions by 6.6, 25.1, and 27% for DH51, DH69, and DH104, respectively. Linoleic acid content was 23.14, 21.52, and 19.40% in DH51, DH69, and DH104, respectively. Linoleic acid content non- significantly decreased under drought stress by 7.51 and 8.1% in DH51 and DH104, respectively. A higher linoleic acid contents were also observed in PGPB-treated plants under stress and non-stress conditions as compared to control samples. The pattern of linolenic acid and linoleic acid was difference between genotypes in the response to drought stress. Linolenic acid (C18:3) was the most abundant polyunsaturated fatty acids as it ranged from 32.92 to 32.20%. The drought alone and/or with PGPP decreased the linolenic acid composition in DH69 and DH104 but not in DH51 which showed a significant increase upon drought treatment. However, the highest decrease of linolenic acid (31.24%) content was observed in PGPB-treated DH69 under stress conditions as compared to the control plants. It confirmed previous results that Linolenic acid was the most susceptible fatty acid against drought stress. Generally, higher content of unsaturated fatty acids belonged DH51 genotype under normal condition. Drought stress also positively affected unsaturated fatty acid content in particular linolenic acid and oleic acid suggesting that DH51 is a drought stress tolerant genotype compared to DH69 and DH104. There were three long-chain unsaturated fatty acids including gadeolic acid (C20:1), eicosadienoic acid (C20:2), and eicosatrienoic acid (C20:3). Gadeolic acid content for DH51, DH104, and DH69 under stress conditions was 12.97, 13.37, and 14.27, respectively. It increased in drought-exposed DH51 and DH104 compared to the control plants. Inoculation with PGPB decreased gadeolic acid content under non-stress (1.46, 4.34, and 1.72%) and stress (15.34, 33.84, and 14.21%) conditions for DH51, DH69, and DH104, respectively. Average of content total mono and polyunsaturated fatty acids content ranged from 30.65 to 31.5 and 55.21 to 57.77, respectively, in seeds oil. In genotypes, the monounsaturated fatty acids (MUFA) content increased under drought stress while poly unsaturated fatty acids (PUFA) content decreased under similar condition except DH51. The ratio of PUFAs/MUFAs was 1.83, 1.47, and 1.80% for DH51, DH69, and DH104, respectively, under normal condition. Both of drought stress and PGPB treatments altered PUFAs/MUFAs ratio. In DH51 and DH104, this ratio decreased with treatment of drought alone and / or whit symbiosis association but not in DH69 which showed an increase of PUFAs/ MUFAs ratio upon drought and symbiosis.
4 Discussion
The present study shows that camelina growth and yield are significantly affected by genotype and environment. It is in agreement with the results of Obour et al. (2017) reporting that genetic background and drought stress differently affect the camelina seed yield. It has been well been documented that drought stress can influence the physiological, biochemical, and morphological processes in plants, resulting in a considerable growth reduction (Hussain et al. 2018). Water deficit at the reproductive stage can severely impact fertilization and flowering, as water-deficient stress in the onset of the duration is conduced to inaccurate fertility, abortion of flower, and reduced seed number (Rad and Zandi 2012; Sehgal et al. 2018). It has been revealed that drought-induced reduction of seed yield is associated with cell membrane damage, physiological disorders in photosynthetic organs and mineral uptake, photoassimilates imbalance between the source (leave), and sink (development seed) during the seed filling stage (Sehgal et al. 2017). Furthermore, drought stress reduced the seed-developing duration and ultimately influenced the size and 1000-seed weight (Sehgal et al. 2018). Our findings are in line with the report of Yang et al. 2019 on rice, Mi et al. 2018 on maize, Dreccer et al. 2018 and Hatzig et al. 2018 on canola, and Singh et al. 2016 on safflower. It has been well documented that symbiosis with PGPB improved the plant growth, yield, and soil health by increasing the nutrients and water uptake under stress and non-stress condition (Kumar and Verma 2018; Pagnani et al. 2018). Our results are consistent with those reporting an increase of seed number in PGPB-inoculated Phaseolus vulgaris (Rezaei-Chiyaneh et al. 2019) and Vigna radiate (Mogal et al. 2019). PGPB-induced root elongation is in line with the PGPB effect on canola (Patten and Glick 2002) and wheat (Khan et al. 2017). It was suggested that PGPB-mediated increase of seeds weight can be related to prolonged seed filling duration and mobilization of nutrients to develop the seeds. Drought stress-induced ROS overproduction conduced to activation of an integrated defense system that controlled ROS accumulation and reduced oxidative damages in plants. Proline accumulation and stress-associated proline reproduction in the plants are important strategies to cope with the drought stress-induced oxidative damage. As an important osmolyte, TSC improves plant tolerance against stresses. Drought stress during the reproductive stage affected the TSC content of the leaves. Although drought leads to lower production of photoassimilates, TSC accumulation of leaves was consistent with the improvement of drought tolerance in the studied genotypes. The results are in agreement with Du et al. (2020) addressing the effect of drought stress on the distribution of TSC content of soybean leaves in early seed filling.
In the present research, we found that the proline level had an increasing trend under stress conditions. Genotypes showed various physiological responses under different conditions. It was consistent with a previous study showing that the genotypes have different mechanisms in response to stress (Kabbadj et al. 2017). The enhancement of proline and total soluble carbohydrate content could be due to the genotypes but more increase was observed in the PGPB inoculated-plants which can be assigned to their increased resistance against water-deficient stress. The role of PGPB in ameliorating abiotic stresses with an increase in osmolytes can be related to the upregulation of their synthesis, activity of enzymatic antioxidants as well as, water stress-induced enhancement of responsive gene expression (Bharti et al. 2016; Sarma and Saikia 2014). Similar results were reported for PGPB effect on the increase of proline and carbohydrate in Ocimum basilicum (Agami et al. 2016), Triticum aestivum (Khalilzadeh et al. 2016; Bharti et al. 2016) and Vigna radiata (Sarma and Saikia 2014) under stress condition.
The relative water content (RWC) is a suitable indicator of water stress, as the highest RWC belonged to water stress-tolerant plants. Our results demonstrated that drought stress caused a significant decrease in RWC of all genotypes. It has been suggested that PGPB can considerably increase the water-retaining and uptake ability by osmolytes accumulation, promoting root growth and development (Talbi et al. 2015; Khan et al. 2019). It is in agreement with our study and obtained results from Kariuki et al. 2016; Vardharajula et al. 2011; and Yasmin et al. 2017.
In the plant cells, accumulation of ROS is related to the reduction of O2 during photosynthesis and respiratory processes resulting in membrane lipid peroxidation-mediated production of MDA (Islam et al. 2014; Raza et al. 2017). Our findings showed a significant increase in the level of H2O2 and MDA under water stress. On the other hand, PGPB modulated ROS levels and lipid peroxidation with inducing enzymatic and non-enzymatic defense systems. In this study, a decreased level of MDA and H2O2 following PGPB inoculation is parallel with those published by Ghanbarzadeh et al. (2019) for Dracocephalum moldavica and Armada et al. (2015) for Zea mays. Similarly, PGPB treatment has decreased ROS level and lipid peroxidation in Cicer arietinum (Khan et al. 2019).
It is well documented that photosynthesis pigments will decrease under drought stress. It is suggested that water limitation–induced ROS production causes oxidation of photosynthesis system, pigments degradation and inhibition of chl production (Moghadam et al. 2011; Khalilzadeh et al. 2016; Wang et al. 2019). In PGPR-inoculated plants, the content of the pigment increased in control condition as well as under drought stress which might be related to higher availability of particular elements such as Mg and Fe. The results are consistent with reports on Phaseolus coccineus (Stefan et al. 2013), Vigna radiata (Sarma and Saikia 2014), Triticum aestivum (Khalilzadeh et al. 2016), Vicia faba (Li et al. 2016Cicer arietinum L. (Khan et al. 2019).
Water supply during the reproductive phase had a pronounced effect on oil content. The remarkable reduction in oil content is associated with the alternation of the fatty acids profile (Sehgal et al. 2018). Drought stress-induced reduction of requisite carbon for fatty acids biosynthesis is a possible explanation for the changes in fatty acids profile. Elferjani and Soolanayakanahally (2018) have reported that a major part of the seed oil skeleton was accreted from carbon assimilation of photosynthetic tissue and green silique. In developing seed, interplant competition between oil and protein biosynthesis for the achievement of essential nutrients is another possible reason for the alternations in fatty acids profile (Si et al. 2003; Bellalovi et al., 2013; Sehgal et al. 2018). Additionally, drought stress varied the enzymatic activity in fatty acid biosynthesis related-pathway and increased the oxidation of fatty acids (Upchurch 2008; El Sabagh et al. 2019) could change oil content and fatty acid profile. Genetic variations as well as environmental stresses could affect the fatty acids profile (Cosge et al. 2007; Moghadam et al. 2011; Hatzig et al. 2018). It is similar to that reported in our study. The present study found a shift in saturated fatty acid (palmitic acid) patterns of genotypes while observing a positive correlation between drought and unsaturated fatty acid content. It is similar to those reported for Helianthus annuus (Petcu et al. 2001), Brassica napus (Hatzig et al. 2018), Brassica campestris (Aslam et al. 2009) and Carthamus tinctorius (Fernández-Cuesta et al. 2014). It has been suggested that drought-induced decrease in activation of oleate desaturase could result in high accumulation of oleic acid during the seed filling stage (Wang and Frei 2011). Monounsaturated fatty acids (MUFA), particularly, oleic acid are more prone to oxidation as compared with polyunsaturated fatty acids (PUFA) (Ali et al. 2010; El Sabagh et al. 2019). A decrease was reported in both linoleic acid and linolenic acid content (polyunsaturated acid) in DH51 and DH104 genotypes which is in agreement with the findings on soybean (Bellaloui et al. 2013) and Canola (Dawood and Sadak 2014). Such a decline can be due to high sensitivity to oxidation during water stress and altering activation of ∆9 desaturase and ∆15 desaturase (Upchurch 2008; Yuan and Li 2020). Moreover, the increase of linoleic acid upon a decline in linolenic acid in the DH69 genotype is similar to the results observed in the case of Camelina sativa (Pavlista et al. 2016). Additionally, the linolenic acid content reduction could be due to the drought stress-induced decline in the time of seed filling. Gadeolic acid content also varied among Camelina genotypes. In some cases, the increase of gadeolic acid was associated with the increment of linoleic acid content. Such a trend may be due to coincide activation of fatty acid-desaturase and elongase during the seed development stage (Abdullah et al. 2016).
The fatty acid content altered after PGPB inoculation. Although the exact reasons are unclear, it can be due to the impact of PGPB on augmenting the grain filling time (Sharifi et al. 2017). In most cases, PGPB decreased saturated fatty acid (palmitic acid) and increased oleic acid and linoleic acid. Silva et al. (2013) reported that PGPB inoculation enhanced the oleic acid due to PGPB possessing all the enzymes involved in its synthesis. These results could be confirmed upon comparison with the studies published by Sharifi et al. (2017) on safflower and Silva et al. (2013) on soybean seeds. In the present study, inoculated seeds were richer in linolenic acid as compared to the controls which could be probably due to the prolonged seed development period upon the use of PGPB.
Camelina oil is a rich source of unsaturated fatty acids whose oxidation stability could be maintained by a balance between polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA). In general, the low PUFAs/MUFAs ratio is more preferable for vegetable oils, thus, it has been tried to reduce this ratio (Abdullah et al. 2016). In our study, PGPB decreased PUFAs content while increasing MUFAs giving rise to lower PUFAs/MUFAs ratio which can be assigned to enhanced monounsaturated acid accumulation upon the application of PGPB.
5 Conclusion
Physiological and biochemical analysis of C. sativa genotypes indicated that drought during the reproductive phase can affect their growth and seed yield. Furthermore, PGPB inoculation improved the growth and yield by decreasing the ROS while enhancing proline, carbohydrate, and photosynthetic pigments. In general, the variations in seed quality and fatty acid contents corresponded well with genetic variation and different responses to various environmental conditions.
References
Abdullah HM, Akbari P, Paulose B, Schnell D, Qi W, Park Y, Dhankher OP (2016) Transcriptome profiling of Camelina sativa to identify genes involved in triacylglycerol biosynthesis and accumulation in the developing seeds. Biotechnol Biofuels 9:136–155
Agami RA, Medani RA, Abd El-Mola IA, Taha RS (2016) Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress Int. J Agric Res 2:78–94
Akbarabadi A, Kahrizi D, Rezaizad A, Ahmadi GH, Ghobadi M, Molsaghi M (2015) Study of variability of bread wheat lines based on drought resistance indices. Biharean Biol 9:88–92
Ali Q, Ashraf M, Anwar F (2010) Seed composition and seed oil antioxidant activity of maize under water stress. J Am Oil Chem Soc 87:1179–1187
Anderson JV, Wittenberg A, Li H, Berti MT (2019) High throughput phenotyping of Camelina sativa seeds for crude protein, total oil, and fatty acids profile by near infrared spectroscopy. Ind Crop Prod 137:501–507
Armada E, Azcón R, López-Castillo OM, Calvo-Polanco M, Ruiz-Lozano JM (2015) Autochthonous arbuscular mycorrhizal fungi and Bacillus thuringiensis from a degraded Mediterranean area can be used to improve physiological traits and performance of a plant of agronomic interest under drought conditions. Plant Phisiol Bioch 90:64–74
Arnon DI (1949) Copper enzymes in isolated chloroplast. Polyphenoloxidase in Beta vulgaris Plant Physiol 24:1–15
Aslam MN, Nelson MN, Kailis SG, Bayliss KL, Speijers J, Cowling WA (2009) Canola oil increases in polyunsaturated fatty acids and decreases in oleic acid in drought-stressed Mediterranean-type environments. Plant Breed 128:348–355
Bates LS, Walderen RD, Taere ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bellaloui N, Mengistu A, Kassem MA (2013) Effects of genetics and environment on fatty acid stability in soybean seed. Food Nutr Sci 4:165–176
Berti M, Gesch R, Eynck C, Anderson J, Cermak S (2016) Camelina uses, genetics, genomics, production, and management. Ind Crop Prod 94:690–710
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768
Bremner JM (1996) Nitrogen total. In: Sparks DL, Page AL, Helmke PA and Loeppert RH (eds) Methods of soil analysis, part 3. Chemical methods, 3rd ed. American Society of Agronomy, Inc, Madison WI, pp. 1085–122
Cosge B, Gurbuz B, Kiralan M (2007) Oil content and fatty acid composition of some safflower (Carthamus tinctorius L.) varieties sown in spring and winter. Int J Nat Eng Sci 1:11–15
Dawood MG, Sadak MS (2014) Physiological role of glycine betaine in alleviating the deleterious effects of drought stress on canola plants (Brassica napus L.). Middle East J. Agric Res 3:943–954
Dhopte AM, Livera-M M (2002) Principles and techniques for plant scientist [s]. Agrobios (India)
Dreccer MF, Fainges J, Whish J, Ogbonnaya FC, Sadras VO (2018) Comparison of sensitive stages of wheat, barley, canola, chickpea and field pea to temperature and water stress across Australia. Agric For Meteorol 248:275–294
Du Y, Zhao Q, Chen L, Yao X, Zhang H, Wu J, Xie F (2020) Effect of drought stress during soybean R2–R6 growth stages on sucrose metabolism in leaf and seed. Int J Mol Sci 21(2):618
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
El Sabagh A, Hossain A, Barutcular C, Gormus O, Ahmad Z, Hussain S, Akdeniz A (2019) Effects of drought stress on the quality of major oilseed crops: implication and possible mitigation strategies–a review. Appl Ecol Env Res 17:4019–4043
Elferjani R, Soolanayakanahally R (2018) Canola responses to drought, heat, and combined stress: shared and specific effects on carbon assimilation, seed yield, and oil composition. Front Plant Sci 9:1224–1241
Falaknaz M, Aalami A, Mehrabi A, Sabouri A, Kahrizi D, Karimi N (2019) Cellular and physiological responses to drought stress in Aegilops tauschii genotypes. Cell Mol Biol 65:84–94
Fernández-Cuesta Á, Velasco L, Ruiz-Méndez MV (2014) Novel safflower oil with high γ-tocopherol content has a high oxidative stability. Eur J Lipid Sci Technol 116:832–836
Gee GW, Bauder JW (1986) Particle-size analysis. In Methods of soil analysis: part 1. Physical and mineralogical methods, ed. G. S. Campbell, R. D. Jackson, M. M. Mortland, D. R. Nielsen, and A. K. Chair, 2nded., 383–409. Madison, WI: ASA, Inc.
Ghanbarzadeh Z, Mohsenzadeh S, Rowshan V, Moradshahi A (2019) Evaluation of the growth, essential oil composition and antioxidant activity of Dracocephalum moldavica under water deficit stress and symbiosis with Claroideoglomus etunicatum and Micrococcus yunnanensis. Sci Hortic 256:108652
Hatzig SV, Nuppenau JN, Snowdon RJ, Schießl SV (2018) Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol 18:297–310
Huang YM, Zou YN, Wu QS (2017) Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep.7. 42335–42344
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci 9:393–414
Hutcheon C, Ditt RF, Beilstein M, Comai L, Schroeder J, Goldstein E, Kiser J (2010) Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol 10:233–248
Islam F, Yasmeen T, Ali Q, Ali S, Arif MS, Hussain S, Rizvi H (2014) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol Environ Saf 104:285–293
Kabbadj A, Makoudi B, Mouradi M, Pauly N, Frendo P, Ghoulam C (2017) Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS One 12(12):e0190284
Kahrizi D, Rostami-Ahmadvandi H, Akbarabadi A (2015) Feasibility cultivation of Camelina (Camelina sativa) as medicinal-oil Plant in Rainfed Conditions in Kermanshah-Iran's first report. Journal of Medicinal Plants and By-Products 2:215–218
Kariuki LW, Masinde P, Githiri S, Onyango AN (2016) Effect of water stress on growth of three linseed (Linum usitatissimum L.) varieties. Springerplus 5:759–775
Khalilzadeh, R., Seyed Sharifi, R., Jalilian, J., 2016. Antioxidant status and physiological responses of wheat (Triticum aestivum L.) to cycocel application and bio fertilizers under water limitation condition. J. Plant Interact.11, 130-137
Khan N, Bano A, Babar MA (2017) The root growth of wheat plants, the water conservation and fertility status of sandy soils influenced by plant growth promoting rhizobacteria. Symbiosis 72:195–205
Khan N, Bano A, Rahman MA, Guo J, Kang Z, Babar MA (2019) Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci Rep 9:2097–2116
Kumar A, Verma JP (2018) Does plant-microbe interaction confer stress tolerance in plants: a review? Microbiol Res 207:41–52
Laxa M, Liebthal M, Telman W, Chibani K, Dietz KJ (2019) The role of the plant antioxidant system in drought tolerance. Antioxidants. 8:94–125
Li Y, Xu S, Gao J, Pan S, Wang G (2016) Bacillus subtilis-regulation of stomatal movement and instantaneous water use efficiency in Vicia faba. Plant Growth Regul 78:43–55
Li Y, Zhang T, Zhang Z, He K (2019) The physiological and biochemical photosynthetic properties of Lycium ruthenicum Murr in response to salinity and drought. Sci Hortic.256
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 603:591–592
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Malik MR, Tang J, Sharma N, Burkitt C, Ji Y, Mykytyshyn M, Snell KD (2018) Camelina sativa, an oilseed at the nexus between model system and commercial crop. Plant Cell Rep 37:1367–1381
Mi N, Cai F, Zhang Y, Ji R, Zhang S, Wang Y (2018) Differential responses of maize yield to drought at vegetative and reproductive stages. Plant Soil Environ 64:260–267
Mogal CS, Jha S, Raj Kumar BK, Parekh VK, Chauhan DA, Karmakar N (2019) Quantification of plant hormones and synergistic effect of PGPR on yield attributing characters of mungbean (Vigna radiata (L.) Wilczek). Int. J. Chem. Stud.7, 2246-2250
Moghadam HRT, Zahedi H, Ghooshchi F (2011) Oil quality of canola cultivars in response to water stress and super absorbent polymer application. Pesqui Agropecu Trop 41:579–586
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In Methods of soil analysis, part 3. Chemical methods, ed. D. L. Sparks, A. L. Page, P. A. Helmke, and R. H. Loeppert, 3rd ed., 961–1010. Madison, WI: ASA, Inc.
Obour AK, Obeng E, Mohammed YA, Ciampitti IA, Durrett TP, Aznar-Moreno JA, Chen C (2017) Camelina seed yield and fatty acids as influenced by genotype and environment. Agron J 1093:947–956
Okunlola GO, Olatunji OA, Akinwale RO, Tariq A, Adelusi AA (2017) Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci Hortic 224:198–205
Oliveira, DM, Lima ALA, Diniz NB, Santos CEDRES, da Silva SLF, Simões ADN (2018) Inoculation of plant-growth-promoting rhizobacteria in Myracrodruon urundeuva Allemão supports in tolerance to drought stress. J. Plant Interact 13, 91–99
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In: Circ no 939. States Department Of Agriculture, Washington, DC, United
Pagnani G, Pellegrini M, Galieni A, D’Egidio S, Matteucci F, Ricci A, Stagnari F, Sergi M, Sterzo C, Lo Pisante M (2018) Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’cultivation: an alternative fertilization strategy to improve plant growth and quality characteristics. Ind Crop Prod 123, 75–83
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Pavlista AD, Hergert GW, Margheim JM, Isbell TA (2016) Growth of spring camelina (Camelina sativa) under deficit irrigation in Western Nebraska. Ind Crop Prod 83:118–123
Pereira BFF, Abreu CA, Romeiro S, Lagôa AMMA, Paz-González A (2007) Pb phytoextraction by maize in a Pb-EDTA treated oxisol. Sci Agric 64:52–60
Petcu E, Arsintescu A, Stanciu D (2001) The effect of drought stress on fatty acid composition in some Romanian sunflower hybrids. Rom Agric Res 15:39–43
Rad AHS, Zandi P (2012) The effect of drought stress on qualitative and quantitative traits of spring rapeseed (Brassica napus L.) cultivars. Zemdirbyste. 99:47–54
Raza MAS, Shahid AM, Saleem MF, Khan IH, Ahmad S, Ali M, Iqbal R (2017) Effects and management strategies to mitigate drought stress in oilseed rape (Brassica napus L.): a review. Zemdirbyste. 104:85–94
Rezaei-Chiyaneh E, Amirnia R, Amani Machiani M, Javanmard A, Maggi F, Morshedloo MR (2019) Intercropping fennel (Foeniculum vulgare L.) with common bean (Phaseolus vulgaris L.) as affected by PGPR inoculation: a strategy for improving yield, essential oil and fatty acid composition. Sci Hortic
Rhoades, J. D. 1996. Salinity: electrical conductivity and total dissolved solids. In Methods of soil analysis, part 3. Chemical methods, ed. D. L. Sparks, A. L. Page, P. A. Helmke, and R. H. Loeppert, 3rd ed., 417–435. Madison, WI: ASA, Inc.
Sarma RK, Saikia R (2014) Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 377:111–126
Sehgal A, Sita K, Kumar J, Kumar S, Singh S, Siddique KH, Nayyar H (2017) Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front Plant Sci 8:1776–1798
Sehgal A, Sita K, Siddique KH, Kumar R, Bhogireddy S, Varshney RK, Nayyar H (2018) Drought or/and heat-stress effects on seed filling in food crops: impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 9
Sharifi RS, Namvar A, Sharifi RS (2017) Grain filling and fatty acid composition of safflower fertilized with integrated nitrogen fertilizer and biofertilizers. Pesqui Agropecu Bras 52:236–243
Si P, Mailer RJ, Galwey N, Turner DW (2003) Influence of genotype and environment on oil and protein concentrations of canola (Brassica napus L.) grown across southern Australia. Aust J Agric Res 54:397–407
Silva LR, Pereira MJ, Azevedo J, Mulas R, Velazquez E, González-Andrés F, Andrade PB (2013) Inoculation with Bradyrhizobium japonicum enhances the organic and fatty acids content of soybean (Glycine max (L.) Merrill) seeds. Food Chem 141:3636–3648
Singh S, Angadi SV, Grover K, Begna S, Auld D (2016) Drought response and yield formation of spring safflower under different water regimes in the semiarid southern High Plains. Agric Water Manag 163:354–362
Sohrabi Y, Heidari G, Weisany W, Golezani KG, Mohammadi K (2012) Changes of antioxidative enzymes, lipid peroxidation and chlorophyll content in chickpea types colonized by different Glomus species under drought stress. Symbiosis 56:5–18
Soorni J, Kazemitabar SK, Kahrizi D, Dehestani A, Bagheri N (2017) Screening of camelina (Camelina sativa l.) doubled haploid lines for freezing tolerance in the seedling stage. Genetika. 49:173–181
Stefan M, Munteanu N, Stoleru V, Mihasan M, Hritcu L (2013) Seed inoculation with plant growth promoting rhizobacteria enhances photosynthesis and yield of runner bean (Phaseolus coccineus L.). Sci Hortic 151:22–29
Sukweenadhi J, Kim YJ, Choi ES, Koh SC, Lee SW, Kim YJ, Yang DC (2015) Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol Res 172:7–15
Talbi S, Romero-Puertas MC, Hernández A, Terrón L, Ferchichi A, Sandalio LM (2015) Drought tolerance in a Saharian plant Oudneya africana: role of antioxidant defences. Environ Exp bot.111, 114-126
Thomas GW (1996) Soil pH and soil acidity. In Methods of soil analysis, part 3. Chemical methods, ed. D. L. Sparks, A. L. Page, P. A. Helmke, and R. H. Loeppert, 3rd ed., 475–490. Madison, WI: ASA, Inc.
Upchurch RG (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30:967–977
Vardharajula S, Zulfikar Ali S, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interac 61:1–14
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Vurukonda SSKP, Vardharajula S, Shivastava M, SKZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res.184, 13-24
Wang Y, Frei M (2011) Stressed food–the impact of abiotic environmental stresses on crop quality. Agr Ecosyst Environ 141:271–286
Wang Y, Jie W, Peng X, Hua X, Yan X, Zhou Z, Lin J (2019) Physiological adaptive strategies of oil seed crop Ricinus communis early seedlings (cotyledon vs. true leaf) under salt and alkali stresses: from the growth, photosynthesis and chlorophyll fluorescence. Front. Plant. Sci. 9, 1939
Xue JA, Mao X, Yang ZR, Wu YM, Jia XY, Zhang L, Yue AQ, Wang JP, Li RZ (2013) Expression of yeast acyl-CoA-9 desaturase leads to accumulation of unusual monounsaturated fatty acids in soybean seeds. Biotechnol Lett 35:951–959
Yang X, Wang B, Chen L, Li P, Cao C (2019) The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci Rep 9
Yasmin H, Nosheen A, Naz R, Bano A, Keyani R (2017) L-tryptophan-assisted PGPR-mediated induction of drought tolerance in maize (Zea mays L.). J Plant Interac. 12:567–578
You J, Zhang Y, Liu A, Li D, Wang X, Dossa K, Zhou R, Yu J, Zhang Y, Wang L, Zhang X (2019) Transcriptomic and metabolomics profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol 19:267–283
Yuan L, Li R (2020) Metabolic engineering a model oilseed Camelina sativa for the sustainable production of high-value designed oils. Front Plant Sci 11:11
Yuan L, Mao X, Zhao K, Ji X, Ji C, Xue J, Li R (2017) Characterization of phospholipid: diacylglycerol acyltransferases (PDATs) from Camelina sativa and their roles in stress responses. Biol Open 6:1024–1034
Załuski D, Tworkowski J, Krzyżaniak M, Stolarski MJ, Kwiatkowski J (2020) The characterization of 10 spring Camelina genotypes grown in environmental conditions in north-eastern Poland. Agron 10(1):64
Zhou SX, Medlyn BE, Prentice IC (2015) Long-term water stress leads to acclimation of drought sensitivity of photosynthetic capacity in xeric but not riparian Eucalyptus species. Ann Bot 117:133–144
Acknowledgments
The authors truly appreciate the Shiraz University Research Council for financially supporting (1952) this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borzoo, S., Mohsenzadeh, S., Moradshahi, A. et al. Characterization of physiological responses and fatty acid compositions of Camelina sativa genotypes under water deficit stress and symbiosis with Micrococcus yunnanensis. Symbiosis 83, 79–90 (2021). https://doi.org/10.1007/s13199-020-00733-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-020-00733-5