Abstract
Severe water shortage limits horticultural crop growth and development, thereby compromising plant quality. Novel tools to enhance stress tolerance in medicinal horticultural crops are crucial to cope with growing environmental challenges to world crop performance. In this study, water solutions of robinin (25, 100, and 200 ppm) and/or foliar sprays of chitosan (0, 50, and 200 ppm) were applied to Chrysanthemum morifolium Ramat subjected to a 2 (2DWI) or 6 day (6DWI) irrigation intervals for 6 weeks. Morphological, physiological, and genetic markers associated with plant-response mechanisms to water stress were explored. Robinin + chitosan-treated plants showed increased morphological performance associated with enhanced chlorophyll, carbohydrates, proline, K+, Ca+2, phenols, leaf water potential, antioxidants, and leaf water content. Superoxide dismutase (SOD), peroxidase (POD), and ascorbate peroxidase (APX) enzymes were more active in robinin + chitosan-treated plants, while H2O2 accumulation was diminished. Higher expression levels of the Chrysanthemum antioxidant gene of zinc-finger transcription factor gene (Cm-BBX24), Chrysanthemum roots fu (DREB1A-1), Chrysanthemum heat shock protein CgHSP70, pyrroline-5-carboxylate synthetases (P5CS), pyrroline-5-carboxylate reductase (P5CR), and proline dehydrogenase (ProDH) were found in robinin- and chitosan-treated plants. Robinin + chitosan treatment stimulated the accumulation of carbohydrates, K+, Ca+2, proline, and chlorophylls to achieve osmotic adjustment and maintain turgor pressure. Accumulation of reactive oxygen species was controlled by enzymatic and non-enzymatic means, as well as the overexpression of stress-related genes (Cm-BBX24, DREB1A-1, CgHSP70, P5CS, P5CR, and ProDH) in robinin + chitosan-treated plants. Plant-response mechanisms for enhanced drought resistance interacted under robinin + chitosan treatment to improve plant performance under stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing pressure on global irrigation water resources has forced prolonged irrigation intervals due to water shortage. This is undoubtedly one of the most challenging issues that agriculture, and particularly the horticulture industry, faces today (Mehran et al. 2017; Ahmadi et al. 2018). Water stress may have morpho-physiological, metabolic, and molecular responses on plants, ending to reducing growth and yield. These changes are associated with mechanisms of resistance to water stress that include tolerance, avoidance, and escape (Nilsen and Orcutt 1996; Li et al. 2018). Drought tolerance may be defined as the ability of the plant to maintain growth despite water stress. This can be accomplished by accumulating solutes (e.g., K), a condition termed osmotic adjustment (Huang et al. 2014). Drought avoidance targets increasing water uptake and/or minimizing water loss by reducing leaf area and/or increasing root growth (Nilsen and Orcutt 1996; Huang et al. 2014; Gonçalves et al. 2017). Drought escape is achieved by shortening the plant life cycle or by undergoing dormancy during periods of stress (Huang et al. 2014). Drought-tolerance and drought-avoidance mechanisms are common in plants and may interact or co-occur with metabolic and molecular regulation (Yu et al. 2012; Li et al. 2018). Plant metabolic performance under drought conditions includes increased carbohydrates (Pallas et al. 2013), synthesis of specific proteins (Chen et al. 2017), increased stress-related nutrient uptake (e.g., K), and accumulation of antioxidants that neutralize reactive oxygen species (ROS) (El-Esawi et al. 2017). Plant responses to water deficit also include proteomic regulation by activating specific antioxidant-related genes such as SOD, CAT, and APX (El-Esawi et al. 2017; Ju et al. 2018) and by activating stress-related genes such as those reported previously (Gupta and Huang 2014; Ali et al. 2017; Li et al. 2018).
Developing novel tools that may enable crops to cope with effects of water stress are growing worldwide, including the use of trinexapac-ethyl (Elansary 2017), seaweed extracts (Elansary et al. 2017), nanoparticles (Saxena et al. 2016), myo-inositol (Yildizli et al. 2018), chitosan (Yang et al. 2009), and robinin.
Chitosan is a biostimulant manufactured by treating chitin with high temperature, and then following by deacetylation process that removes proteins and calcium (Sharp 2013; Pichyangkura and Chadchawan 2015). Chitosan oligosaccharides may be prepared and sold as a liquid solution or as water-soluble powder. They are known as plant elicitors that increase secondary metabolites production (Karuppusamy 2009), particularly polyphenols (Yin et al. 2012). Chitosan also has antimicrobial effects and, in the same time, may enhance beneficial microbes’ growth (Ramírez et al. 2010). Additionally, increased crop yield had been reported following chitosan treatment (Sharp 2013). However, the mechanism whereby chitosan improves the tolerance in agricultural crops is not well understood. Furthermore, no additive effects of chitosan and robinin on plant growth have been previously described.
Robinin (syn. Kaempferol 3-O-robinoside-7-O-rhamnoside) is a flavone glycoside (Fig. 1) that was first isolated from Vinca erecta (Akhmedzhanova 1986) as well as other plants (Elansary et al. 2018) and is an important natural phenolic compound. Sergiev et al. (2004) reported that robinin has growth-stimulatory activities on wheat coleoptiles; this is the only report on robinin application in plants, to our knowledge.
Chrysanthemum (Dendranthema grandiflorum), which belongs to the family Asteraceae, is an important horticultural plant worldwide that is used as ornamental plant suitable to arid and semi-arid regions. Chrysanthemum genes related to abiotic stress are well characterized, including DREB1, which is strongly related to freezing and drought stresses (Tong et al. 2009); BBX24, which influences flowering and abiotic stresses (Yang et al. 2014); and ProDH, which encodes a mitochondrial protein representing the key enzyme in proline degradation (Xu et al. 2013). The response of this species to robinin and/or chitosan oligosaccharide elicitor under water stress has not yet been investigated.
In the present study, the objective was to study the different mechanisms involved in the effects of robinin and/or chitosan on Chrysanthemum grown under two different irrigation intervals using morphological, physiological, metabolic, and molecular markers. We hypothesized that stress tolerance, as well as stress avoidance mechanisms, co-occurs with the antioxidant pathway to control plant growth. In addition, enhanced molecular regulation of antioxidant enzymes and stress-related genes in plants subjected to robinin and/or chitosan do exist. We measured several physiological markers that reflect stress and stress alleviation in plants such as proline, carbohydrates, antioxidants, and leaf water potential. The information obtained from this study will contribute to our understanding of robinin and/or chitosan effects in plant subjected to water stress.
Material and methods
Plant material and treatments
Chrysanthemum morifolium Ramat plants that height is 10 cm (short-day flowering) were purchased from private commercial nurseries in January (for 2 successive years of 2017 and 2018). A polyethylene-covered greenhouse was used for growing the plants in Alexandria (Alexandria-Cairo Desert Road), Egypt. Identified plants by Hosam Elansary were registered at the Faculty of Agriculture-Elshatby, Alexandria. The plants were transplanted to 2.1 L pots that contained growing media (brown peat and perlite, 3:1 w/w). A compound fertilizer was used (Crystalon®; 20% N: 20% P: 20% K, 2 g/L media). The growing temperature ranged from 15.2 °C (night) to 27.6 °C (day); relative humidity between 58 and 68%; photosynthetically active radiation (PAR) around 1000 m−2 μmol m−2 s−1 at 12.00 p.m.; and manual daily watering of 38–50 mL/plant. Two groups of plants were used, one of which was watered at 2-day intervals (2 DWI) with 38–40 mL/plant to reach 100% of evapotranspiration (ET), while the other group was watered with 40–50 mL/plant at 6-day intervals (6 DWI) for 6 weeks to reach 100% ET. Day length control was applied to maintain vegetative growth by extending day length to 14 h using 1000 m−2 μmol m−2 s−1 incandescent light.
To investigate the optimal concentrations of robinin (Sigma-Aldrich, Germany) that boost plant growth (pilot study), 48 Chrysanthemum seedlings were watered separately for 3 weeks with a solution containing different concentrations of robinin. Then, in the final experiment, the plants were watered weekly with a solution containing robinin (Fig. 1) at 25, 100, and 200 ppm during extended irrigation interval conditions (every 6 days, 6DWI). Untreated plants with robinin/chitosan were considered as controls. Chitosan oligosaccharide (deacetylation > 95%, powder, Aldebeiky Group Co., Cairo, Egypt) water solution was applied using a sprayer at concentrations of 50 and 200 ppm until drop-off during the experiment and started 2 weeks prior to extending the watering interval from 2 days (2DWI) to 6DWI. The experiment was designed as split plot. Irrigation treatments were considered as the main plot and robinin and/or chitosan treatments as the subplot. Three blocks/repetitions (n = 3) were formed from plants containing five replicates for each treatment to reach a total of 360 plants in Randomized Complete Block Design (RCBD) experiment.
Morphology and physiology
After applying treatments for 6 weeks, the plant height and the leaf number were determined. Leaf area was determined by a scanner and AutoCAD program. Soil-free plants were dried in the oven at 40 °C, and then, the total constant dry weight was determined. Freeze-dried ground samples were also obtained and kept at 20°. The carbohydrates composition was quantified following Dubios et al. (1956) as percentage. The K+, Ca2+, and proline concentrations were measure in leaves following Elansary et al. (2017). Leaf midday water potential and relative water content were determined at 12 pm (solar time) following Elansary et al. (2016a).
Antioxidants, chlorophyll, phenols, and enzyme activities
The leaves were air dried and then ground into powder, and then, methanolic extracts were prepared and the antioxidant effects were measured by the 2,2′-diphenylpicrylhydrazyl (DPPH) and β-carotene-bleaching tests, which determine OH− radical effects (Elansary et al. 2017, 2019). Total phenolic and total chlorophyll contents were determined following Elansary et al. (2016b). Peroxidase (POD) activity was quantified in leaves following He et al. (2011). Superoxide dismutase (SOD), ascorbate peroxidase (APX), and H2O2 accumulation were quantified in leaf tissues following Elansary et al. (2017). One unit represented the enzyme amount inhibiting 50% of nitroblue tetrazolium (NBT).
RNA isolation and quantitative real-time PCR
The RNA was obtained using the RNeasy Plant Mini Kit (Qiagen, Germany) from fresh leaves as well as the roots 12 h after the last application of robinin and/or chitosan, and the cDNA was obtained using a Reverse Transcription Kit (Qiagen, Germany). Real-Time PCR (qRT-PCR) were performed in duplicates (SYBR Green, Qiagen, Germany) (Al-Ghamdi and Elansary 2018) to calculate the expression levels of genes. The expression of zinc-finger transcription factor gene (Cm-BBX24) (Yang et al. 2014), Chrysanthemum roots dehydration responsive element binding factor 1 (DREB1A-1) (Tong et al. 2009), Chrysanthemum heat shock protein CgHSP70 (Song et al. 2014), pyrroline-5-carboxylate synthetases (P5CS), pyrroline-5-carboxylate reductase (P5CR), and proline dehydrogenase (ProDH) (Xu et al. 2013) were examined. PCR conditions followed Al-Ghamdi and Elansary (2018). Amplification specificity was tested using melting curve analysis. A housekeeping gene (Actin) was used as a reference gene following Gu et al. (2011).
Statistical analyses
The data of 2017 and 2018 seasons showed no significant differences, and then, they were pooled and expressed as means, and least significant difference (LSD) was determined using SPSS (PASW Ver. 21) at P ≤ 0.05.
Results
Morphological performance
Under the 2 DWI, robinin and/or chitosan had no effect on chrysanthemum leaf numbers except at high doses of 100/200 ppm robinin + 200 ppm chitosan (Figs. 2 and 3). However, leaf area, dry weight, and height showed higher variation compared to leaf numbers in response to robinin and/or chitosan doses. The leaf area increased in response to the additive effects of robinin + chitosan. For example, the leaf area increased from 308.1 (control) to 360.4 cm2 plant−1, plant dry weight from 10.6 (control) to 13.9 g plant−1, and plant height from 27.2 (control) to 33.9 cm in response to 200 ppm robinin + 200 ppm chitosan application. In general, under 2 DWI, the application of high doses (robinin + chitosan) had effects on dry weight, leaf area and number, and height.
Under 6 DWI, there were increases in the morphological performance of plants subjected to robinin + chitosan at 200 ppm of each. For example, application at 200 ppm robinin + 200 ppm chitosan increased leaf numbers from 12.5 (control) to 19.5 leaf plant−1. In the same manner, the leaf area increased from 121.6 (control) to 150.9 to cm2 plant−1, the plant dry weight increased from 5.5 (control) to 6.4 g plant−1), and plant height increased from 17.2 (control) to 21.9 cm.
Physiological performance
Carbohydrates, K+, Ca2+, proline, and leaf water potential
Under 2 DWI, carbohydrates, K+, Ca2+, and proline compositions were the highest in chrysanthemum plants subjected to 200 ppm robinin + 200 ppm chitosan (Figs. 4 and 5). For example, total carbohydrates increased from 12.43% (control) to 14.35%, K+ increased from 2.4 (control) to 2.6 mg g−1 DW, Ca2+ increased from 1.81 (control) to 2.41 mg g−1 DW, and proline increased from 14.9 (control) to 19.7 mg g−1 DW.
Under 6 DWI, the carbohydrates increased in chrysanthemum subjected to 200 ppm robinin + 200 ppm chitosan as compared to other doses. In addition, lower doses of robinin such as 100 ppm robinin + 200 ppm chitosan showed comparable results to 200 ppm robinin. Lower doses of robinin and/or chitosan showed much lower carbohydrate compositions.
K+ and Ca2+ compositions showed increases in the robinin + chitosan treatments comparable to robinin/chitosan alone as well as control treatments. The proline content enhanced in robinin + chitosan-treated plants at 200 ppm of each compared to the control (Figs. 4 and 5).
Leaf water potential and relative content of water showed significant increases in plants treated with robinin + chitosan compared to other treatments as well as control (Fig. 6).
Antioxidants, phenolics, and chlorophylls
Under 2DWI, the DPPH (IC50) of chrysanthemum decreased from 11.5 (control) to 9.7 µg mL−1 following robinin (200 ppm) + chitosan (200 ppm) treatment, indicating an increase in scavenging activity (Fig. 7). An increase in the scavenging activity in 2 DWI was noted in the β-carotene–linoleic acid test following robinin + chitosan treatments.
Chrysanthemum leaves in plants growing under 6DWI showed enhanced scavenging activity following application of robinin (200 ppm + chitosan (200 ppm). For example, the application of 200 ppm robinin + 200 ppm chitosan increased the DPPH (IC50) from 9.7 (control) to 7.9 µg mL−1 and the β-carotene–linoleic acid activity from 10.8 (control) to 8.5 µg mL−1.
The total phenolic of chrysanthemum plants peaked following robinin + chitosan treatments under 2 and 6 DWI (Fig. 8). Robinin + chitosan treatments at 200 ppm each boosted the phenolic content from 12.1 (control) to 14.0 mg GAE g−1 and from 14.3 to 16.9 mg GAE g−1 in plants subjected to 2 DWI and 6 DWI, respectively.
Total chlorophyll content in chrysanthemum plants was reduced in control plants when the irrigation intervals were prolonged and this chlorophyll content increased from 2.2 (control 2 DWI) to 1.6 (control 6 DWI) mg g−1 DW (Fig. 7). Under 2 DWI, robinin + chitosan (at 200 ppm each) increased the chlorophyll compared to other treatments. Also, under 6 DWI, the robinin and chitosan increased also the chlorophyll of treated plants at different doses.
Antioxidant enzyme activities and gene expression
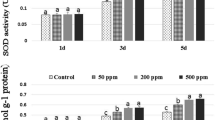
Major antioxidant POD, SOD, and APX enzymes increased in chrysanthemum subjected to robinin + chitosan (200 ppm each) compared to other treatments under 2 and 6DWI (Fig. 9). The H2O2 in chrysanthemum subjected to robinin + chitosan (200 ppm each) compared to other treatments in both seasons (Fig. 10).
Gene expression levels of Cm-BBX24, DREB1A-1, and CgHSP70 increased in chrysanthemum plants following application of robinin + chitosan at 200 ppm each as compared to other treatments (Fig. 11). There was an additive effect of robinin + chitosan on Cm-BBX24, DREB1A-1, and CgHSP70 under under 2 and 6DWI. Increased expression of P5CS and P5CR was detected under robinin + chitosan treatments (200 ppm each) compared to the other treatments (Fig. 12). ProDH expression was reduced following robinin + chitosan treatment at 100–200 ppm each (Fig. 12).
Discussion
Increasing pressure on global irrigation water resources has forced prolonged irrigation intervals due to water shortage. This is undoubtedly one of the most challenging issues that agriculture, and particularly the horticulture industry, faces today (Ali et al. 2017; Ahmad et al. 2018).
We recorded reductions in all the morphological parameters due to extension of the irrigation interval such as shortness of plants and reduction of leaf area and number as drought-avoidance mechanism (Elansary and Salem 2015; Ali et al. 2017; Gonçalves et al. 2017). The reduction in the morphological side was associated with stress-related physiological alterations including leaf water potential, carbohydrate, proline, chlorophylls, K, Ca, and antioxidant (Elansary 2017; Elansary et al. 2017, 2018).
Robinin + chitosan application improved chrysanthemum performance during different irrigation intervals. Previous study on chitosan indicated an increase in essential oil yield and dry matter in Thymus daenensis Celak (Bistgani et al. 2017). However, the mechanisms were not properly understood. Studies reporting robinin application to plants for vegetative stimulation purposes are limited. One study (Sergiev et al. 2004) reported that robinin might have stimulatory activities on the growth of wheat coleoptiles. However, this is the first comprehensive study investigating the physiological and molecular effects of robinin on an ornamental horticultural crop. Furthermore, the additive effects of robinin + chitosan on the vegetative growth of plants are novel and have not been reported previously.
Accumulation of proline, sugars, and ions is a mechanism of osmotic adjustment related to drought tolerance (Ali et al. 2017). The accumulation of proline assists in stabilizing cellular proteins under stress and control free radicals (Seki et al. 2007). Carbohydrate increase indicates stress conditions and helps in osmotic adjustment and free radical control (Yin et al. 2010; Gupta and Huang 2014). Additionally, the accumulation of proline in plant vegetative parts might be an important sign of stress tolerance, because such accumulation balances vacuolar ion osmotic pressure (Elansary and Salem 2015; Ali et al. 2017) and maintains water influx (Hoque et al. 2007). The decrease in leaf water potential and content are strong indicator of stress conditions that was alleviated by robinin and chitosan treatments. In addition, proline accumulation entailed a degree of water stress experienced by the plant tissues; furthermore, proline accumulation was induced in plants treated with robinin (100 and 200 ppm) + chitosan (200 ppm).
In this study, the osmotic adjustment mechanism was strongly present by means of accumulation of K+ and Ca+2 ions during water stress. Ion accumulation is related to carbohydrate, which may increase the growth and enhance cell turgor pressure (Ali et al. 2017; Elansary et al. 2017). Ion accumulation in chrysanthemum increased chlorophyll content (drought resistance mechanism). Low rate applications of robinin + chitosan increased the ion composition in chrysanthemum, and assisted and aided in the attainment of osmotic adjustment.
Excess ROS and related ions are usually present in plants subjected to stress due to electrons production and use imbalance which may end with damage and death of plant cells (Cruz 2008). The antioxidant defense mechanism is composed of enzymatic (POD, SOD, and APX) and non-enzymatic (e.g., phenols) pathways that crosstalk to minimize the intracellular redox (AbdElgawad et al. 2016; Elansary et al. 2017).
The phenolic composition was higher in robinin + chitosan-treated plants. These increases are associated with enhanced antioxidant performance of treated plants. Additionally, chitosan doses of 200 ppm increased the phenolic content of leaves compared to the control plants; these findings concord those reported by Yin et al. (2012). The novelty of this study could be in the additive effects of both substances used in enhancing the antioxidant bioactivities of treated plants. Phenols remove ROS in stressed plants by controlling OH− free radical and H2O2 accumulation.
Chrysanthemum plants subjected to robinin and chitosan showed increased expression of antioxidant-related genes. A strong indicator of the antioxidant mechanism of stress control is the increased expression of the chrysanthemum-specific Cm-BBX24 (Yang et al. 2014), as found in the current study. The increased expression of Chrysanthemum DREB1A-1 reported here is indicator of the molecular control of robinin + chitosan in treated plants.
These results are novel, and indicate a strong association among morphological, physiological, and genetic chrysanthemum-specific markers. There was robinin + chitosan additive effect on Cm-BBX24, DREB1A-1, and CgHSP70 expression in treated plants and caused increased expression under water stress. This effect was not reported before. Furthermore, P5CS and P5CR increased their expression as molecular control under stress in plants treated with robinin and/or chitosan. Plants treated with 200 ppm robinin + chitosan showed the highest expression of these five genes. Lower doses of robinin + chitosan had lower effects on these genes. In agreement with these results, several investigations have revealed higher expression of these genes under stress conditions in barley (Pérez-López et al. 2009), maize (AbdElgawad et al. 2016), and rosemary (El-Esawi et al. 2017). However, the application of robinin + chitosan is novel and has not been previously reported. Furthermore, drought and salinity stress-related genes such as DREB1 were confirmed to have enhanced expression under robinin + chitosan treatments. In addition, DREB gene overexpression has confirmed stress tolerance in several crops such rosemary (El-Esawi et al. 2017) and ERF3 in soybean (Zhang et al. 2009). The increase in P5CS expression is interesting, because it encodes pyrroline-5-carboxylate synthetase that controls the biosynthesis of proline (Xu et al. 2013). Also, the expression of ProDH reductions is important, because it encodes the main enzyme in the proline degradation pathway. The application of robinin + chitosan has main effect on proline in chrysanthemum plants which is related to water stress tolerance.
The longer irrigation interval of 6 days caused water stress conditions in chrysanthemum plants as found in reduced growth and relative water content of leaves. These conditions were alleviated at the morphological, physiological, and molecular levels by application of robinin + chitosan sprays. Several stress tolerance as well as avoidance mechanisms crosstalk with antioxidant responses at the morphological level as well as the physiological level. The findings of this investigation after water stress and the application of both chemicals were confirmed in several experiments in which we monitored different morphological measurements, all of which, taken together, are considered to be a drought-avoidance mechanism. These morphological changes co-occurred with physiological osmotic adjustment as revealed by carbohydrate, chlorophyll, proline, K+, and Ca+2 accumulation, which constitute the organic basis of a complex drought-tolerance mechanism. The antioxidant mechanism for stress tolerance was investigated by studying overall antioxidant activity, phenolic content, H2O2 content, and POD, SOD, and APX enzyme activities. Overall, the data obtained from these assays were confirmed at the molecular level by screening antioxidant enzyme-related genes as well as chrysanthemum-specific stress tolerance-associated genes. The application of robinin + chitosan during an extended irrigation interval might ameliorate drought stress effects on chrysanthemum. The current knowledge of stress resistance-related mechanisms might help in applying future novel tools that enable us to attenuate the unpleasant effects of earth temperature increase and lack of irrigation water around the world.
Conclusion
A novel finding emerged from this study exploring the mechanisms controlling morpho-physiological, and molecular performance in water-stressed Chrysanthemum treated with robinin + chitosan. The study revealed that several mechanisms interacted to enhance plant overall growth during water stress including drought tolerance, drought avoidance, and antioxidant pathway. Robinin + chitosan application successfully ameliorated the negative effects of water stress on plant growth, suggesting that it may be an excellent way in improving water stress tolerance in agricultural crops and in the future prospects of novel tools and strategies to further attenuate the unpleasant effects of global warming and lack of water resources.
Author contribution statement
HOE and AMEA-H designed and performed experiments. All the authors participated analyzing, writing, revising, and approving the final version of the manuscript.
References
AbdElgawad H, Zinta G, Hegab MM, Pandey R, Asard H, Abuelsoud W (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci 7:276
Ahmad Z, Waraich EA, Akhtar S et al (2018) Acta Physiol Plant 40:80
Ahmadi J, Pour-Aboughadareh A, Ourang SF, Mehrabi AA, Siddique KHM (2018) Acta Physiol Plant 40:90
Akhmedzhanova V (1986) Robinin and kaempfereol from Vinca erecta. Chem Nat Compd 22:601
Al-Ghamdi AA, Elansary HO (2018) Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol Biochem 129:273–284
Ali F, Bano A, Fazal A (2017) Recent methods of drought stress tolerance in plants. Plant Growth Regul 82:363–375
Bistgani ZE, Siadat SA, Bakhshandh A, Pirbalouti AG, Hashemi M (2017) Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J 5:407–415
Chen X-y, Yang Y, Ran L-p, Dong Z-d, Zhang E-j, Yu X-r, Xiong F (2017) Novel insights into miRNA regulation of storage protein biosynthesis during wheat caryopsis development under drought stress. Front Plant Sci 8:1707
Cruz CM (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
Dubios M, Gilles K, Hamlton J, Rebers P, Smith F (1956) Colourimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Elansary HO (2017) Green roof Petunia, Ageratum, and Mentha responses to water stress, seaweeds, and trinexapac-ethyl treatments. Acta Physiol Plant 39:145
Elansary HO, Mahmoud EA (2015) Basil cultivar chemotyping still favored over genotyping using core barcodes and possible resources of antioxidants. Essen Oil Res 27:82–87
Elansary HO, Salem MZM (2015) Morphological and physiological responses and drought resistance enhancement of ornamental shrubs by trinexapac-ethyl application. Sci Hortic 189:1–11
Elansary HO, Yessoufou K (2016) In vitro antioxidant, antifungal and antibacterial activities of five international Calibrachoa cultivars. Nat Prod Res 11:1339–1342
Elansary HO, Zin El-Abedin TK (2019) Omeprazole alleviates water stress in peppermint and modulates the expression of menthol biosynthesis genes. Plant Physiol Biochem 139:578–586
Elansary HO, Skalicka-Woźniak K, King IW (2016a) Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol Biochem 105:310–320
Elansary HO, Norrie J, Ali HM, Salem MZM, Mahmoud EA, Yessoufou K (2016b) Enhancement of Calibrachoa growth, secondary metabolites and bioactivity using seaweed extracts. BMC Complement Altern Med 16:341
Elansary HO, Yessoufou K, Abdel-Hamid AME, El-Esawi MA, Ali HM, Elshikh MS (2017) Seaweed extracts enhance Salam turfgrass performance during prolonged irrigation intervals and saline shock. Front Plant Sci 8:830
Elansary HO, Szopa A, Kubica P, Ekiert H, Ali HM, Elshikh MS, Abdel-Salam EM, El-Esawi M, El-Ansary DO (2018) Bioactivities of traditional medicinal plants in Alexandria. Evid Based Complement Altern Med 1463579:13
Elansary HO, Szopa A, Kubica P, Ekiert H, Mattar MA, Al-Yafrasi MA, El-Ansary DO, El-Abedin TKZ, Yessoufou K (2019) Polyphenol profile and pharmaceutical potential of Quercus spp. Bark Extr Plants 8:486
El-Esawi MA, Elansary HO, Elshanhory N, Abdel-Hamid AME, Ali HM, Elshikh MS (2017) Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front Physiol 8:716
Gu C, Chen S, Liu Z, Shan H, Luo H, Guan Z, Chen F (2011) Reference gene selection for quantitative real-time PCR in Chrysanthemum subjected to biotic and abiotic stress. Mol Biotechnol 49:192–197
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 2014:701596
He CF, Chen S, Lv G, Deng Y, Fang W, Liu Z, Guan Z, He C (2011) Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J Plant Physiol 168:687–693
Hoque MA, Banu MNA, Okuma E, Amako K, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J Plant Physiol 164:1457–1468
Huang B, DaCosta M, Jiang Y (2014) Research advances in mechanisms of turfgrass tolerance to abiotic stresses: from physiology to molecular biology. Crit Rev Plant Sci 33:141–189
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plant Res 3:1222–1239
Li Z, Li Y, Zhang Y, Cheng B, Peng Y, Zhang X, Ma X, Huang L, Yan Y (2018) Indole-3-acetic acid modulates phytohormones and polyamines metabolism associated with the tolerance to water stress in white clover. Plant Physiol Biochem 129:251–263
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1988) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Mehran A, Aghakouchak A, Nakhjiri N, Stewardson MJ, Peel MC, Phillips TJ, Wada Y, Ravalico JK (2017) Compounding impacts of human-induced water stress and climate change on water availability. Sci Rep 7:6282
Nilsen ET, Orcutt DM (1996) Physiology of plants under stress: abiotic factors. Wiley, New York
Pallas B, Clément-Vidal A, Rebolledo M-C, Soulié J-C, Luquet D (2013) Using plant growth modeling to analyze C source–sink relations under drought: inter- and intraspecific comparison. Front Plant Sci 4:437
Pérez-López U, Robredo A, Lacuesta M, Sgherri C, Muñoz-Rueda A, Navari-Izzo F et al (2009) The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol Plant 135:29–42
Pichyangkura R, Chadchawan S (2015) Biostimulant activity of chitosan in horticulture. Sci Hortic 196:49–65
Ramírez MÁ, Rodriguez AT, Alfonso L, Peniche C (2010) Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnol Appl 27:270–276
Saxena R, Tomar RS, Kumar M (2016) Exploring nanobiotechnology to mitigate abiotic stress in crop plants. J Pharm Sci Res 8:974–980
Seki M, Umezawa T, Urano K (2007) Shinozaki K (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10:296–302
Sergiev I, Alexieva V, Ivanov S, Bankova V, Mapelli S (2004) Plant growth regulating activity of some flavonoids. CR Acad Bulg Sci 57:63
Sharp RG (2013) A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agron 3:757–793
Song A, Zhu X, Chen F, Gao H, Jiang J, Chen S (2014) A chrysanthemum heat shock protein confers tolerance to abiotic stress. Int J Mol Sci 15:5063–5078
Tong Z, Hong B, Yang Y, Li Q, Ma N, Ma C, Gao J (2009) Overexpression of two chrysanthemum DgDREB1 group genes causing delayed flowering or dwarfism in Arabidopsis. Plant Mol Biol 71:115–129
Yang F, Hu J, Li J, Wu X, Qian Y (2009) Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul 58:131–136
Yang Y, Ma C, Xu Y, Wei Q, Imtiaz M, Lan H, Gao S, Cheng L, Wang M, Fei Z (2014) A zinc finger protein regulates flowering time and abiotic stress tolerance in chrysanthemum by modulating gibberellin biosynthesis. Plant Cell 23:2038–2054
Yildizli A, Çevik S, Ünyayar S (2018) Effects of exogenous myo-inositol on leaf water status and oxidative stress of Capsicum annuum under drought stress. Acta Physiol Plant 40:122
Yin YG, Kobayashi Y, Sanuki A, Kondo S, Fukuda N, Ezura H, Sugaya S, Matsukura C (2010) Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J Exp Bot 61:563–574
Yin H, Frette XC, Christensen LP, Grevsen K (2012) Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J Agric Food Chem 60:136–143
Yu S, Liao F, Wang F, Wen W, Li J, Mei H et al (2012) Identification of rice transcription factors associated with drought tolerance using the ecotilling method. PLoS ONE 7(2):e30765
Zhang G, Chen M, Li L, Xu Z, Chen X, Guo J, Ma Y (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERT type transcription factor for increased tolerance to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60:3781–3796
Acknowledgements
The work was supported by King Saud University, Researchers Supporting Project number (RSP-2019/118).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Gao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elansary, H.O., Abdel-Hamid, A.M.E., Yessoufou, K. et al. Physiological and molecular characterization of water-stressed Chrysanthemum under robinin and chitosan treatment. Acta Physiol Plant 42, 31 (2020). https://doi.org/10.1007/s11738-020-3021-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-3021-8