Abstract
Eclipta alba L. is a well known medicinal herb, found commonly on contaminated roadsides in Kerala, India. To assess its potential for copper tolerance and accumulation, pot culture experiment was carried out. Metal accumulation in the plant in relation to 50–800 mg kg−1 Cu in soils, administered as CuSO4·7H2O in solution, was examined. Biomass yield of shoot and root, pigment content, Cu accumulation in the plant, bio-concentration factor, and translocation factor were the parameters studied. At the highest level of treatment, Cu was found accumulated more in the roots than in shoots. A significant increase in lipid peroxidation, proline content, phenolics and flavanoids were observed in Cu treated plants, compared to the control. The activity of antioxidant enzymes like superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase was found significantly changed in all the treated plants than in the control. The Bradford assay revealed a significant increase in protein content of the plant at higher levels of Cu treatment. Transmission electron microscopy, images supported the uptake and sequestration of metal particles inside the plant cell. The overall data suggests Eclipta alba L. to be a plant with high potential to tolerate Cu toxicity in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination is an environmental problem common in the present day world. The sources of contamination includes industrial emission, improper waste management and chemical fertiliser applications (Cunningham et al. 1995; Raskin et al. 1997). Sustainable development depends on the availability of eco-friendly and economically affordable technologies to deal with heavy metal contamination. Phytoremediation is such an in situ ecotechnology for easy removal of metal contaminants from land, water and air (Chaney et al. 1997). The success of phytoremediation depends on the availability of metal tolerant and hyper accumulator species (Ent et al. 2012). Therefore, experimental trials with fast growing and high biomass yielding weeds are essential for the identification of suitable metal tolerant or hyper accumulator plants as promising species for phytoremediation. Eclipta alba L. is a fast growing weed of high biomass yield, found commonly on contaminated roadsides in Kerala (Ray and George 2009), with Ni accumulation in higher quantity than that in the contaminated roadside soils (Ray and George 2011). Hussain and Khan (2010) investigated the presence of Cu in different parts of Eclipta alba L., which suggested the hyper accumulation tendency of this plant. However, no comprehensive experimental data are available on either its metal accumulation potential or biochemical response to metal toxicity.
Cu—a redox-active metal—is an essential micronutrient required for growth and metabolism in plants. Many enzymes and proteins in plants and animals require Cu as a co-factor (Yruela 2005). An oversupply of Cu is often found toxic to plants (Khatun et al. 2008; Yang and Chu 2011). Cu ion (Cu 2+) is the real fungicidal component in the ‘Bordeaux mixture’. However, Cu contamination is quite common in many industrial and agricultural zones in the world (Reichman 2002).
Copper toxicity causes overproduction of reactive oxygen species, leading to oxidative stress in plants (Chai et al. 2014). Plants possess their own defence mechanism such as metal accumulation, metal exclusion, production of secondary metabolites and enzymatic antioxidant responses to overcome the stress (Sytar et al. 2013). The assessment of enzymatic antioxidants such as superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.11), catalase (CAT, EC 1.11.1.6), and glutathione reductase (GR, EC 1.6.4.2) in treated plant biomass is essential to explain the biochemical potential of plants to heavy metal stress. Therefore, experimentation on tolerance to a metal is the first step towards identification of potential species for phytoremediation.
The major objective of the present study was a comprehensive assessment of tolerance in Eclipta alba L. under different concentrations of Cu in soil. The metal accumulation potential and capacity of the plant to resist Cu toxicity in relation to different plant parameters are assessed. Various morphological, physiological and biochemical responses of the plant in terms of biomass, change in pigment content, lipid peroxidation, proline, flavanoid, phenolics, protein and enzymatic antioxidant activities were determined in response to the amount of Cu in soil. The study also aimed to Cu localisation and intracellular changes in the leaves of Eclipta alba L. by means of transmission electron microscopy (TEM). This is the first experimental work on Eclipta alba L. revealing its Cu accumulation characteristics, growth and biochemical response under different levels of Cu in soil.
Materials and methods
Preparation of soil and plant material
The experimental soil was collected from Mahatma Gandhi University premises at a depth of 0–20 cm, mixed with dried and powdered cow-dung and sand (3:1:1) for pot culture experiment. The physico-chemical analyses such as pH, electrical conductivity, salinity, available NPK, organic carbon, and Cu content were carried out (Jackson 1958) (Table 1). Each pot was filled uniformly with 1 kg of the ‘pot medium’ for experimental cultivation of the plant. The plant Eclipta alba L. was identified as per the type maintained in the Kerala State Regional Herbarium of the Botanical Survey of India at Department of Botany, St. Berchman’s College, Changanacherry and seeds were collected. After normal pre-treatments, the seeds were germinated under natural conditions using coir pith as a substrate to avoid the presence of any external Cu. The seedlings of uniform length (6–8 cm with 3–6 leaves) were collected for planting in the pots of 13 cm length and 15 cm diameter.
Experimental design and copper treatment
A completely randomized design was followed in the experiment. The five different concentrations of Cu selected for the study were 50, 100, 200, 400, 800 mg kg−1 dry weight (DW) of the soil. Six replicates of the control as well as each of the treatment groups were maintained. Each metal concentration was prepared by dissolving the respective concentrations of Cu (equivalent to CuSO4·7H2O) in 1000 mL distilled water and supplied to the plants as a daily dosage (50 mL) for 20 days. This treatment procedure was followed to prevent soil-mineral fixation, which may prevent the bioavailability of the metal as treatment dose and also to avoid metal leaching to an extent. The pots were kept on roof top in plastic trays (to collect any leak out fractions), covered by transparent polythene sheets under green net (mesh size to reduce 50% of light intensity), so as to avoid influence of rain and also to provide moderate and uniform culture conditions throughout the experimental period. The pot culture was maintained completely for a period of 30 days, followed by harvesting.
Chemical analysis of Cu in soil and plant samples
The soil samples (5 g) were taken and dried at 70 °C for 1 h in an oven. It was then powdered using mortar and pestle, sieved and stored in labelled sampling bags for quantitative analysis of Cu by Atomic Absorption Spectrophotometer (AAS, Thermo Scientific iCE 3000). For acid digestion, 500 mg of the soil samples were taken and digested in a microwave digester (Anton paar multiwave), by aqua regia method (Chen and Ma 2001), using 10 mL of concentrated HCl and 65% HNO3 in the ratio 3:1. The digest was filtered after cooling, and transferred into a 25 mL volumetric flask and made up to the mark with de-ionized water. The Cu accumulated in the respective soil samples (before and after treatment), was determined by AAS and used for further analysis of phytoremediation potential [Bio-concentration factor (BCF)] of the plant species.
The harvested plant material was carefully washed in running tap water and then in de-ionized water to remove soil matter completely. The leaf number per plant in the control as well as treatments was counted. Each plant was separated into leaf, stem and root. Root length, shoot length, fresh weight (FW) and dry weight (DW) were recorded. The separated plant parts were dried in an oven at 40 °C for 12 h, powdered and stored for Cu analysis by AAS. The powdered samples (250 mg) of root and shoot were weighed out from control to treatment groups, and digested in the microwave digestion system with 10 mL of binary acid mixture, 65% HNO3 and HClO4 in the ratio 4:1. After digestion, the samples were cooled, filtered, made up to 25 mL using de-ionized water and the accumulation of Cu in the respective samples were done by AAS.
BCF and TF were calculated as follows:
-
BCF = Concentration of metal in plant root/Initial concentration of metal in substrate.
-
TF = Metal concentration in shoot/Metal concentration in root (Yoon et al. 2006).
Preparation of sample for free radical scavenging and other biochemical assays
Fresh plants as a whole were taken from the control as well as treatment groups, shade-dried and powdered. Each sample (10 g) was sequentially extracted in a soxhlet using 300 mL of solvents including hexane, chloroform, acetone, methanol and water. A preliminary qualitative analysis of all the extracts was carried out for alkaloids, flavanoid, tannin, saponin, glycosides and phenolics as per Sasidharan et al. (2011). The methanolic and aqueous extracts were selected for further in vitro antioxidant assays such as DPPH and FRAP.
DPPH and FRAP assays
The in vitro antioxidant activities of different concentrations of plant extracts from control to various treatment groups were determined (50–400 µg mL−1) based on standard procedure (Blois 1958), and expressed in mg ascorbic acid equivalents (AAE) g−1 of leaf extract, using ascorbic acid standard (5–20 µg mL−1). Ferric reducing power of the plant extract was determined using the standard method (Benzie and Strain 1996) with slight modifications. FRAP values were expressed as mM Fe2+ equivalents g−1 of sample. All the measurements were done in triplicate.
Biochemical assays
Total phenolic content in methanolic leaf extract was carried out according to the modified Folin–Ciocalteau method (Herald et al. 2012) and expressed as mg gallic acid equivalents (GAE) g−1 FW. Total flavanoid content was determined according to the micro titre plate assay (Herald et al. 2012). The methanolic extracts of different concentrations (25, 50, 100, 200, 400 µg mL−1) were used to measure the flavanoid content. Quercetin was used as a standard (10–100 µg L−1) to generate a calibration curve and the values were expressed as mg quercetin equivalents (QE) g−1 FW. The chlorophyll content and carotenoid were determined by the standard method (Arnon 1949), and expressed in mg g−1 FW. Proline concentration in the control as well as Cu-treated plant extract was determined spectrophotometrically (Bates et al. 1973) and expressed as µmol g−1 FW. Lipid peroxidation was measured spectrophotometrically by determining the malondialdehyde (MDA) content in the samples using extinction coefficient of 155 mM−1 cm−1 and expressed as µmol g−1 FW (Hodges et al. 1999).
Assay of antioxidant enzymes
Fresh leaves (500 mg) of each of the control as well as treatment groups were homogenised separately in 5 mL of extraction buffer containing 100 mM potassium phosphate buffer (pH 7.8), 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1% (w/v) poly vinyl pyrrolidone, (PVP), filtered through a cheese cloth and centrifuged at 15,000×g for 12 min at 4 °C. The supernatant was collected and used for enzymatic antioxidant assays. The protein content of the supernatants was measured (Brafdord 1976). The superoxide dismutase (SOD) activity was assayed as per the standard procedure (McCord and Fridovich 1969). The change in absorbance was calculated for 560 and 532 nm and the enzyme activity was expressed as Units mg−1 protein. One unit of SOD activity can be defined as the amount of protein needed to inhibit 50% of photo reduction of NBT. The catalase (CAT) activity was determined based on decrease in absorbance at 240 nm, for every 30 s up to 2 min (Aebi 1984) and expressed as units mg−1 protein, using the molar extinction coefficient of 39.4 mM−1 cm−1. The ascorbate peroxidase (APX) activity was measured (Nakano and Asada 1981) with an increase in absorbance at 290 nm and expressed as units mg−1 protein, with molar extinction coefficient of 2.8 mM−1 cm−1. Glutathione reductase (GR) activity was recorded based on the increase in absorbance for 5 min at 412 nm (molar extinction coefficient of 26.6 mM−1 cm−1) and expressed in units mg−1 protein (Foster and Hess 1980).
TEM assays
A middle leaf section of 2 mm length, was taken from the control as well as the highest treatment dose (800 mg kg−1 soil), fixed in 4% glutaraldehyde (v/v) in 0.2 mol L−1 phosphate buffer saline (PBS), pH 7.2 for 7 h and post fixed in 1% OSMIUM tetroxide for 1 h. Then the fixed specimen was treated with 0.2 mol L−1 of PBS (pH 7.2) for 2 h and dehydrated using graded ethanol series (50, 60, 70, 80, 90, 95 and 100%) followed by acetone. The samples were filtered and embedded in Spurr’s resin. Ultra thin sections were prepared and the image was viewed under Technai TEM at an accelerating voltage of 60 kV (Hong-yun et al. 2005).
Statistical analysis
All the analyses were performed in triplicate and the results were recorded as mean ± standard deviation (SD, n = 3). The experimental data were statistically analysed using one-way analysis of variance (ANOVA) by Duncan’s new multiple range tests (DMRT) using SPSS version 17.
Results
Growth parameters
The overall growth characteristics of the plant under all treatments (Fig. 1) revealed its capacity to tolerate increasing amount of Cu (50–800 mg kg−1) in soil. The effect of Cu on growth parameters such as number of leaves, length of shoot and root, FW and DW of each plant sample were recorded (Table 2). No significant difference in the leaf number was observed among the control and treatment groups.
Root and shoot length
In Eclipta alba L., root length was significantly increased from the control by 14, 27, 32 and 42% and that of shoot length by 8, 12, 15 and 14.7% to treatment levels up to 400 mg kg−1 of Cu in soil (Table 2). A negative impact of Cu (71% decrease in root length) was noticed in plant roots exposed to 800 mg kg−1 in soil (Fig. 2). The current observation (Table 2) can be viewed as a critical tolerance limit of the plant root to Cu stress.
The shoot length of Eclipta alba L. recorded its maximum at 400 mg kg−1 and minimum at 800 mg kg−1 of Cu in soil. However, in shoot length, no significant difference (Table 2) was observed from that of the control to the highest treatment level.
Plant biomass
A significant increase (p < 0.05) in fresh weight of the shoot (13, 14, 15 and 18%) was observed (Table 2), in treated plants up to 400 mg kg−1 of Cu in soil. However, fresh weight of the root increased significantly from the control to the treatment levels of 100–400 mg kg−1. But a significant (p < 0.05) reduction (18%) in fresh weight was observed in roots exposed to 800 mg kg−1 of Cu in soil.
The DW of shoot increased significantly (p < 0.05) by 19% at 400 mg kg−1, but decreased significantly (28%) at 800 mg kg−1 of Cu in soil (Table 2), when compared to the control. Likewise, DW of the root increased significantly (22%) at 400 mg kg−1 and reduced significantly (21%) at 800 mg kg−1 of Cu in soil.
Chemical analysis of Cu in plant and soil
The accumulation of Cu by the shoot and root of Eclipta alba L. increased significantly with increasing concentrations of Cu in soil (Table 3). The highest shoot accumulation of Cu was observed at 400 mg kg−1 soil, whereas the root accumulation reached its maximum at 800 mg kg−1 treatment level.
After harvesting, copper content of different soil samples (Table 3) was found significantly different in accordance with the treatment levels. A significant reduction in BCF was observed from the control (Table 4) to the treatment levels of Cu in soil. But the TF was significantly increased (p < 0.05) up to the treatment level of 100 mg kg−1 of Cu in soil than the control. No significant change in TF was observed in the plant exposed to 200 and 400 mg kg−1, but decreased significantly at 800 mg kg−1 of Cu in soil. It may also be noted that the TF of Eclipta alba L. was found to be greater than 1 in treatment levels up to 400 mg kg−1 of Cu in soil.
Free radical scavenging activity
Comparison of IC50 value (DPPH) and the FRAP equivalents of the methanolic leaf extract of the control and the Cu treated plants (Table 5) revealed that, maximum antioxidant activity was exhibited by plants treated with 200 mg kg−1 of Cu in soil. Moreover, at 400 and 800 mg kg−1 of Cu in soil, no significant change in antioxidant capacity was observed compared to the control.
Effect of Cu on biochemical parameters
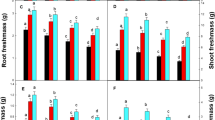
The analysis of pigment content in Eclipta alba L. (Fig. 3a, b) revealed a significant increase in total chlorophyll and carotenoid (28, 29%) in 50 mg kg−1 of Cu treated plants only, compared to the control and reduced significantly thereafter.
A significant increase in MDA (73, 117, 143, 161 and 185%) was observed in Eclipta alba L. (Fig. 3c), from control to various levels of treatments. The proline content also increased (105, 260, 391, 471 and 254%) significantly (p < 0.05) with increasing levels of Cu in soil (Fig. 3d).
In Eclipta alba L., the flavanoid and phenolic compounds increased significantly (flavanoids by 28, 48, 87, 128 and 152% and phenolics by 15, 47, 120, 144 and 169%) from control to the treatment groups (Fig. 3e, f).
Effect of copper on antioxidant enzyme activities and protein content
The enzymatic antioxidants estimated in relation to Cu stress were SOD, CAT, APX and GR (Fig. 4). The activity of SOD (Fig. 4a) in Eclipta alba L. was concentration dependent. The highest SOD activity was shown by plants that received 800 mg kg−1 of Cu in soil. The CAT activity (Fig. 4b) decreased significantly with respect to the increasing metal concentrations, and declined markedly at 800 mg kg−1 of Cu in soils. A significant increase (p < 0.05) in peroxidase activity (Fig. 4c) was observed in the treated plants, compared to the control. Maximum APX activity was observed in 800 mg kg−1 treated groups. GR activity also showed a similar trend (Fig. 4d) with respect to increasing Cu levels in soil. The activity of GR reached its maximum at 200 mg kg−1 of Cu in soil.
Copper treatment resulted in a significant increase in protein content (Fig. 4e), only in 400 and 800 mg kg−1 of Cu, when compared to the control.
TEM assays
Transmission electron microscopy (TEM) was used to determine the localisation of Cu inside the plant as well as the intracellular changes occurred in relation to metal detoxification mechanism. The resin embedded specimens of the control and 800 mg kg−1 Cu exposed plant leaves were compared. In the control plant leaf, many chloroplasts with a properly defined structure were visible. The chloroplast was observed as the most affected organelle in the plant leaf. The thylakoid arrays in the granum, starch grains in the chloroplast, mitochondria, vacuoles and golgi bodies were evident (Fig. 5a, b). But in 800 mg kg−1 treated plant leaf, the chloroplast was found distorted with disassembled thylakoid arrays (Fig. 5c–f). The membrane vesiculation and cytoplasmic vacuolation was visible (Fig. 5d, e) in plant leaf exposed to Cu treatment, compared to the control. A tendency for the withdrawal of plasma membrane from its cell wall was observed in leaf cells of Cu treated plants (Fig. 5f), over the control plant leaf. A large amount of electron dense bodies of reduced metal particles were found deposited in the cell wall (Fig. 6g–j) as well as in the vacuoles (Fig. 6k–l).
Discussion
Growth parameters
The difference in growth pattern and biochemical responses in Eclipta alba L. revealed its capacity to overcome Cu stress in soil. Eclipta alba L. is a dicot plant with columnar growth pattern. Therefore, measurement of length of the main shoot and that of taproot as well as shoot length, root length and biomass (Table 2) are meaningful information of its growth response to any kind of environmental stress. In general, the different levels of Cu treatments demonstrated a beneficial role in the growth of the plant in soil, (Fig. 1) except visible root toxicity at the highest dose. The result indicated plant’s capacity to tolerate Cu stress in soil to an optimum level. A similar response of increase in root length at smaller doses of Cu is already reported (Sako et al. 2016). The selection of a native tolerant species which can survive the toxic conditions is the main criteria for the selection of plants for phytoremediation studies. However, the exact potentials of the plant to tolerate or resist the Cu stress can be revealed from its biochemical responses.
A significant increase (p < 0.05) in length and biomass of both shoot and root was found in Eclipta alba L. (Table 2) at low levels of Cu in soils. A similar effect has been widely studied (Nair et al. 2014; Chen et al. 2015). A significant decrease in root length and root biomass with darkening of root hairs (Fig. 2) was observed in the highest dose of 800 mg kg−1, while shoot biomass remains unchanged. In general, increase in root length at initial doses can be viewed as a supporting factor for better absorption of water and minerals in plants and thereby increasing the plant growth. Therefore, Cu at low level in the soil was found beneficial to the plant. Moreover, the level between 400 and 800 mg kg−1 of Cu in soils appeared as a critical limit to the plant. A similar darkening of root hairs was also observed in Lupinus luteus exposed to Cu in a liquid culture medium (Mourato and Martins 2009).
Analysis of copper in soil and plant
The significantly high amount of Cu observed in different soil samples after harvest were in accordance with the levels of treatment of the metal in these soil samples. The observed trend of accumulation of Cu in different parts of Eclipta alba L. (Table 3) proved its ability to survive in Cu contaminated soils. A similar accumulation pattern is already reported in other plants such as Lemna trisulca L. (Prasad et al. 2001) and Phyllostachys pubescens (Chen et al. 2015). The current study proved that the plant was able to absorb the added Cu in the soil, but the transport of Cu from root to shoot decreased, when the concentration reached certain critical levels. The concentration dependent accumulation of Cu by the plant appeared as a positive sign of its potential for extracting Cu from the soil.
The BCF value reveals the potential of a plant species to concentrate metals from soil into roots, whereas translocation factor (TF) reveals its capacity to translocate it to the above ground parts. The plants exhibiting BCF or TF values ≥1 are considered as hyper accumulators suitable for phytoextraction (Yoon et al. 2006). In Eclipta alba L. (Table 4), BCF value was <1 in all the treatment groups, whereas the TF was >1 at treatment levels except the highest one, when compared to the control. A similar observation in BCF and TF is already reported (Michaud et al. 2008; Chen et al. 2015). Since the BCF of Eclipta alba L. is <1, it would not come under the category of hyper accumulator. But TF value >1 revealed its capacity to extract Cu from soil and translocate into roots and finally into shoots. Moreover, maximum translocation of Cu from root to shoot was exhibited up to 400 mg kg−1 soil. The dose dependent accumulation of Cu also attributed a positive sign for the phytoextraction of Cu from soil into roots or shoots. In general, metal tolerance and high biomass yield are the two important factors to be considered for phytoremediation potential, which was found constructive in Eclipta alba L. It may be noted that many soil factors also influence the transport of metals from soil to plant. Therefore, the plant deserves further investigation to confirm its potential in the management of metal contamination.
Free radical scavenging assays
The FRAP and DPPH assays (Table 5) showed a significant increase in quenching DPPH in treated ones, compared to the control, which was visible from the progressive increase in free radical scavenging activity. The least concentration of the plant extract to achieve the IC50 value was obtained under treatment of 200 mg kg−1 of Cu in soil. No significant change was observed in antioxidant activity of further treatments, than the control, which revealed that the plant antioxidant activity was not affected by excess Cu in soil.
The ferric reducing antioxidant power of the extract (FRAP value) was compared with the absorbance of 1 mM ferrous sulphate and expressed as ferrous equivalents (FE) in µg mL−1 extract. The FRAP value remains unchanged in Cu treated groups, compared to the control, which showed that the reducing property of the plant was not affected by Cu stress in soil. An earlier report on Serbian poplar clones showed a slight increase in FRAP value when subjected to Cu toxicity, but the effect was not significant (Trudic et al. 2013). The FRAP assay is used as a measure to combat Cu induced oxidative stress in Brassica juncea L. (Szôllôsi et al. 2011). It was observed that Eclipta alba L. was found to be capable of resisting Cu stress, by means of its metal reducing property, even at elevated levels of Cu in soil.
Biochemical parameters
The change in pigment contents can be used as a parameter to show the impact of Cu on plant physiology, especially photosynthesis (Yruela 2005). In the current study, the inhibitory effect of Cu on pigment contents (Fig. 3a, b), could be due to the significant loss of chlorophyll by Cu induced peroxidation of chloroplast membrane or pigment degradation. A similar decrease in pigment content from Cu stress is already reported in eight populations of Haumaniastrum katangense (Peng et al. 2012).
The lipid peroxidation is an indication of membrane damage by biotic or abiotic stress (Sytar et al. 2013). The Cu exposed Eclipta alba L. exhibited a marked increase in MDA content (Fig. 3c). Hence the enzymatic antioxidant machinery get stimulated to overcome the Cu induced lipid peroxidation. This is an important characteristic feature exhibited by metal accumulating and tolerant plants to overcome the stress. Increased MDA production under Cu stress is also widely reported (Lu et al. 2010; Chai et al. 2014).
The proline accumulation in plants is another indication of plant defence mechanism to various abiotic stress including heavy metals (Schat et al. 1997). However, an increase in proline, which plays an important role in the process of membrane stabilisation, free radical scavenging, enzyme protection and cytosolic acidity regulation as well as in non-enzymatic free radical detoxification, reveals the capacity of plants to resist metal toxicity (Rejeb et al. 2014). In the study, proline accumulation significantly increased in treated plants (Fig. 3d) with respect to the increasing Cu concentrations in soil. The current study emphasised the stimulatory role of proline as a means of plant defence mechanism, to alleviate Cu toxicity in soil, by stabilising the membrane damage. A similar stimulation of proline synthesis has already been reported (Thounaojam et al. 2012).
In Eclipta alba L., the significant increase in phenolics and flavanoid, from control to treated plants with respect to increasing Cu levels in soil (Fig. 3e, f) showed its positive biochemical response in relation to Cu accumulation. The current observation supported a stimulatory role of secondary metabolites against Cu stress, and suggests further analysis on the involvement of different functional groups of bio molecules, which could be a part of plant tolerance mechanism. A similar increase in flavanoid and phenolics against Cu induced toxicity has already been reported in Nephrolepis biserrata (Khatun et al. 2008; Manan et al. 2015).
Antioxidant enzymes
The defensive role of various enzymatic antioxidants was determined in the study. The most effective SOD causes dismutation of superoxide radical into H2O2 and oxygen. The elevated levels of SOD is an indication of plant defence mechanism against the over production of reactive oxygen species induced by heavy metals (Ahmad et al. 2010; Madejón et al. 2009). The present observation proved that the generated enzymatic antioxidant defence mechanism in Eclipta alba L. was essential to cope with the free radical production induced by Cu. In Eclipta alba L., the significantly (p < 0.05) increased activity of SOD (Fig. 4a) than the control shows the plant’s tolerant capacity to dismutate superoxide radicals. A similar effect has been reported widely (Srivastava et al. 2006; Thounaojam et al. 2012; Ali et al. 2016).
The principal scavenging enzyme, catalase (CAT), which can dismutate toxic H2O2 produced during endogenous or exogenous stress conditions, is another indication of plant response to metal stress (Ahmad et al. 2010). The present data suggest a significant decrease in CAT activity at the given concentrations of Cu, when compared to the control (Fig. 4b), which might be due to the inhibitory role of Cu. The accumulated Cu in plant tissues showed a negative effect on CAT activity, which could be due to either over production of H2O2 or due to the excessive activation of APX. A similar result is obtained in Lemna trisulca L. and also in white lupin and soybean plants (Prasad et al. 2001; Sanchez-pardo et al. 2012).
The ascorbate peroxidase (APX) involved in scavenging of H2O2 in ascorbate–glutathione cycle, plays another important role in plant defence mechanism against different abiotic stress (Ahmad et al. 2010). The significantly increased APX activity reported in the current study (Fig. 4c) showed the defensive role of APX in overcoming Cu stress in soil by the plant. Increase in peroxidase activity as an indication of the adaptive tolerance of plants to Cu stress has been previously reported (Sanchez-pardo et al. 2012; Thounaojam et al. 2012). Thus, APX activity in Eclipta alba L. proved to be beneficial for the detoxification of H2O2 induced from Cu toxicity.
The glutathione reductase (GR)—a potential enzyme of ascorbate–glutathione pathway—is another effective scavenger of free radicals (Ahmad et al. 2010). The significant increase in GR activity (Fig. 4d) observed in treated plants over control, showed the stimulatory role of the enzyme in overcoming Cu induced stress in the plant. A similar response in GR activity is already reported (Thounaojam et al. 2012).
The present investigation confirmed that initial Cu treatments did not cause a change in protein content (Fig. 4e), over the control. However, increase in protein content at treatment levels of 400 and 800 mg kg−1 might be attributed to the synthesis of some stress related proteins, which can be analysed by further proteomics studies. A similar increase in protein content with response to Cu stress in soil has been previously reported (Doganlar 2013).
TEM analysis
The large amount of electron dense bodies (metal particles) found deposited near the inner side of the cell wall, cell membrane and also outside and inside of the chloroplast membrane (Fig. 6g–i) of the treated plants stands as evidence for high ability of Eclipta alba L. to take up, accumulate and translocate Cu into its leaves, whereas the damage of cell organelles remains evidence to the adverse effect of the metal in its cells. The chloroplast was found as the most affected organelle in response to Cu stress when compared to the control. Disorientation of chloroplast with disrupted thylakoid arrays in the granum was observed in 800 mg kg−1 Cu treated plant leaf (Fig. 5c–f) compared to the control (Fig. 5a, b). The results were in accordance with Cu tolerance in Salix purpurea (Hakmaoui et al. 2007). No change has been observed in other organelles like mitochondria or peroxisomes. The vacuolation observed in TEM analysis revealed this as a means of metal sequestration (Fig. 6j–l), or metal detoxification mechanism by the plant to overcome Cu toxicity. This agrees with previous observations in this regard (Hong-yun et al. 2005; Chen et al. 2015). Thus, TEM analysis confirmed intracellular distribution of metal particles in treated plants as well as organelle changes occurred in them, which are evidence to the adaptive mechanism in plants, in relation to Cu stress in soil.
Conclusion
Eclipta alba L.—a fast growing and high biomass yielding tropical wasteland medicinal herb—growing abundantly on heavy metal contaminated roadsides, was hypothesized as a plant with high phytoremediation potentials. Experimental evidence for its response to increasing levels of Cu in soil is revealed for the first time. Present experimental evidence suggests that the plant has certain promising features for metal decontamination studies in soils. The tolerance of Eclipta alba L. to Cu and its detoxification capabilities are assessed on the basis of its ability to survive, accumulate and sequestrate excess Cu in soil. Response of the plant in terms of enzymatic antioxidants and secondary metabolites showed its biochemical mechanism to overcome Cu stress in soil. The ultra structural changes and intracellular Cu accumulation supported detoxification process of the plant by vacuolar sequestration of the metal. Disorientation of chloroplast structure revealed Cu toxicity to its photosynthetic apparatus. The present study necessitates further investigations including molecular response of the plant to assess its phytoremediation potential.
Author contribution statement
Both the authors have equal contribution to the development of this article and nobody else has claim of contribution in this regard. CC has carried out this research work as part of her Ph.D. thesis under the strict supervision and guidance of the JGR.
Abbreviations
- APX:
-
Ascorbate peroxidase
- AAS:
-
Atomic absorption spectrophotometer
- BCF:
-
Bio-concentration factor
- CAT:
-
Catalase
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DW:
-
Dry weight
- FRAP:
-
Ferric reducing antioxidant potential
- GAE:
-
Gallic acid equivalents
- GR:
-
Glutathione reductase
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- QE:
-
Quercetin equivalents
- SOD:
-
Superoxide dismutase
- TF:
-
Translocation factor
- TEM:
-
Transmission electron microscopy
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad P, Umar S, Sharma S (2010) Mechanism of free radical scavenging and role of phytohormones in plants under abiotic stress. In: Ashraf M, Ozturk M, Ahmad MSA (eds) Plant adaptation and phytoremediation, Springer, Dordrecht, Heidelberg, London, pp 100–118. doi:10.1007/978-90-481-9370-7
Ali S, Rizwan M, Ullah N, Bharwana SA, Waseem M, Farooq MA, Abbassi GH, Fareed M (2016) Physiological and biochemical mechanisms of silicon-induced copper stress tolerance in cotton (Gossypium hirsutum L.). Acta Physiol Plant 38:1–11. doi:10.1007/s11738-016-2279-3
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–12
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239:70–76
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 29:1199–1200
Brafdord MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chai M, Shi F, Li R (2014) Growth and physiological responses to copper stress in a halophyte Spartina alterniflora (Poaceae). Acta Physiol Plant 36:745–754. doi:10.1007/s11738-013-1452-1
Chaney RL, Malik M, Li YM, Brown SL, Eric P, Angle JS, Baker AJM (1997) Phytoremediation of soil metals. Curr Opin Biotechnol 8:279–284
Chen M, Ma L (2001) Comparison of three aqua regia digestion methods for twenty Florida soils. Soil Sci Soc Am J 65:491. doi:10.2136/sssaj2001.652491x
Chen J, Shafi M, Li S, Wang Y, Wu J, Ye Z, Peng D, Yan W, Liu D (2015) Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Nat Publ Group. doi:10.1038/srep13554
Cunningham S, Berti W, Huang J (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Doganlar ZB (2013) Metal accumulation and physiological responses induced by copper and cadmium in Lemna gibba L. minor and Spirodela polyrhiza. Chem Speciat Bioavailab 25(2):79–88. doi:10.3184/095422913X13706128469701
Ent A, Baker AJM, Pollard AJ, Schat H (2012) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil. doi:10.1007/s11104-012-1287-3
Foster JG, Hess JL (1980) Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Hakmaoui A, Ater M, Boka K, Baron M (2007) Copper and cadmium tolerance, uptake and effect on chloroplast ultrastructure. Studies on Salix purpurea and Phragmites australis. Verlag der Zeitschrift fur Naturforschung. Tubingen 62:417–426
Herald TJ, Gadgil P, Tilley M (2012) High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J Sci Food Agric 92:2326–2331. doi:10.1002/jsfa.5633
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hong-yun P, Xiao-e Y, Sheng-ke T (2005) Accumulation and ultrastructural distribution of copper. J Zhejiang Univ Sci 6B:311–318. doi:10.1631/jzus.2005.B0311
Hussain I, Khan H (2010) Investigation of heavy metals content in medicinal plant, Eclipta alba L. J Chem Soc Pak 32(1):28–34
Jackson ML (1958) Soil chemical analysis, Prentice Hall of India, New Delph, pp 38–134
Khatun S, Ali MB, Hahn EJ, Paek KY (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot 64:279–285. doi:10.1016/j.envexpbot.2008.02.004
Lu Y, Li X, Liu Y, CuiY PanYT (2010) Seedlings growth and antioxidative enzymes activities in leaves under heavy metal stress differ between two desert plants: a perennial (Peganum harmala) and an annual (Halogeton glomeratus) grass. Acta Physiol Plant 32:583–590. doi:10.1007/s11738-009-0436-7
Madejón P, Ramírez-Benítez JE, Corrales I, Barceló J, Poschenrieder C (2009) Copper-induced oxidative damage and enhanced antioxidant defenses in the root apex of maize cultivars differing in Cu tolerance. Environ Exp Bot 67:415–420. doi:10.1016/j.envexpbot.2009.08.006
Manan FA, Mamat DD, Samad AA, Ong YS, Ooh KF, Chai TT (2015) Heavy metal accumulation and antioxidant properties of Nephrolepis biserrata growing in heavy metal-contaminated soil. Glob NEST J 17:1–10
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzyme function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Michaud AM, Chappellaz C, Hinsinger P (2008) Copper phytotoxicity affects root elongation and iron nutrition in durum wheat (Triticum turgidum durum L.). Plant Soil 310:151–165. doi:10.1007/s11104-008-9642-0
Mourato MP, Martins LL (2009) Physiological responses of Lupinus luteus to different copper concentrations. Biol Plant 53:105–111
Nair PMG, Kim SH, Chung IM (2014) Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: physiological and molecular level responses of in vitro grown plants. Acta Physiol Plant 36:2947–2958. doi:10.1007/s11738-014-1667-9
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Peng H, Wang-müller Q, Witt T (2012) Differences in copper accumulation and copper stress between eight populations of Haumaniastrum katangense. Environ Exp Bot 79:58–65. doi:10.1016/j.envexpbot.2011.12.015
Prasad MNV, Malec P, Waloszek A, Bojko M, Strzałka K (2001) Physiological responses of Lemna trisulca L. (duckweed) to cadmium and copper bioaccumulation. Plant Sci 161:881–889
Raskin I, Smith R, Salt D (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8:221–226
Ray JG, George J (2009) Phytosociology of roadside communities to identify ecological potentials of tolerant species. J Ecol Nat Environ 1:184–190
Ray JG, George J (2011) Nickel in soils and resilient plants on roadsides of Kerala, South India. Grunthosnavsthvo (Soil Sci) 12(1–2):12–23
Reichman SM (2002) The responses of plants to metal toxicity : a review focusing on copper, manganese and zinc. Ameef, Prahan, pp 1–54
Rejeb KB, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284. doi:10.1016/j.plaphy.2014.04.007
Sako A, Kandakar J, Tamari N, Higa A, Yamaguchi K, Kitamura Y (2016) Copper excess promotes propagation and induces proteomic change in root cultures of Hyoscyamus albus L. Plant Physiol Biochem 103:1–9. doi:10.1016/j.plaphy.2016.02.032
Sanchez-pardo B, Fernández-pascual M, Zornoza P (2012) Copper microlocalisation, ultrastructural alterations and antioxidant responses in the nodules of white lupin and soybean plants grown under conditions of copper excess. Environ Exp Bot 84:52–60. doi:10.1016/j.envexpbot.2012.04.017
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY, Bedong-semeling J, Nasi BA (2011) Extraction, isolation and characterization of bioactive compounds from plant extracts. Afr J Tradit Complement Altern Med 8(1):1–10
Schat H, Sharma SS, Vooijs R (1997) Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vuigaris. Physiol Plant 101:477–482
Srivastava S, Mishra S, Tripathi RD, Dwivedi RD, Gupta DK (2006) Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (L.f.) Royle. Aquat Toxicol 80:405–415. doi:10.1016/j.aquatox.2006.10.006
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzalka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35:985–999. doi:10.1007/s11738-012-1169-6
Szôllôsi R, Kálmán E, Medvegy A, Petô A, Varga SI (2011) Studies on oxidative stress caused by Cu and Zn excess in germinating seeds of Indian mustard (Brassica juncea L.). Acta Biol Szeged 55(1):175–178
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Panda SK (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39. doi:10.1016/j.plaphy.2012.01.006
Trudic B, Kebert M, Popovic MB, Štajner D, Orlovic S, Galovic V, Pilipovic A (2013) The effect of heavy metal pollution in soil on serbian poplar clones. Izvomi Znanstveni clanci Sumarski list 5–6:287–296
Yang Z, Chu C (2011) Towards understanding plant response to heavy metal stress. In: Shanker A, Venkateswarlu B (eds) Abiotic stress in plants: mechanisms and adaptations. INTECH, Rijeka, pp 59–78. doi:10.5772/24204
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464. doi:10.1016/j.scitotenv.2006.01.016
Yruela I (2005) Copper in Plants. Braz J Plant Physiol 17:145–156
Acknowledgements
The authors gratefully acknowledge utilization of instrumentation facilities at the DBT-MSUB centre in the School of Biosciences, AAS at the Institute of Intensive Research in Basic Sciences of Mahatma Gandhi University, Kottayam and TEM facility at the All India Institute of Medical Sciences, New Delhi for successful completion of this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S Srivastava.
Rights and permissions
About this article
Cite this article
Chandrasekhar, C., Ray, J.G. Copper accumulation, localization and antioxidant response in Eclipta alba L. in relation to quantitative variation of the metal in soil. Acta Physiol Plant 39, 205 (2017). https://doi.org/10.1007/s11738-017-2508-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2508-4