Abstract
The current investigation was conducted to elucidate the potential modulatory functions of both enzymatic and non-enzymatic scavenging elements of three Iranian basil (Ocimum basilicum L.) cultivars in response to different water-deficit stress treatments [i.e., control (W1: 100 % FC), mild (W2: 75 % FC), moderate (W3: 50 % FC), and severe (W4: 25 % FC)]. In general, the growth parameters, viz., plant height, number of lateral branches, number of flowers in the inflorescence per plant, and dry and fresh weights of leaves and inflorescence followed by yield were considerably affected by water-deficit stress levels (p ≤ 0.05), though some fluctuations were observed among three cultivars. Under severe water-deficit stress (W4), total chlorophyll content overall increased, while a pronounced reduction in the carotenoid content was observed by boosting of water-deficit stress intensities. Apart from some quantitative variations, ROS-scavenging enzymes, such as SOD, CAT, APX, GPX, and PPO, exhibited different behaviors versus different levels of water-deficit stress in the basil cultivars, concluding that their modulation could be a cultivar-dependent mechanism and stress-dependent mechanism. Among different metabolites detected in the essential oil of basil cultivars, both methyl chavicol and squalene were superior in the cultivars 2 and 3, while in cultivar 1, linalool and squalene were the predominant constituents, under water deprivation conditions. Taking all the features studied here into consideration, presumably, cultivar 1 is qualified enough to nominate as the most tolerant basil cultivar, could be accordingly utilized as a promising source/material for breeding programs of basil under drought stress, and possibly other abiotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a member of abiotic stress category, water-deficit stress has been regarded as the most pivotal factor restricting total plant growth and productivity, thereof, worldwide (Bray 1997; Lei et al. 2006). From a global scale perspective, water-deficit stress is rapidly promoting in both arid and semi-arid areas, including Iran. Hence, deep and precise understanding of all or at least most of the possible molecular and physiological mechanisms governing on the plant tolerance versus water-deficit stress is environmentally an unavoidable and crucial task.
It is believed that water-deficit stress provokes broad spectrum of common reactions in plants, leading to cellular dehydration and, consequently, various osmotic alterations (Bartels and Sunkar 2005; Farooq et al. 2012). Despite their usefulness under normal magnitudes, the fact has widely been accepted that overproduction of reactive oxygen species (ROS; as one of the consequences of environmental stresses) is too harmful for the normal activity of plant cellular machinery (Gill and Tuteja 2010; Sharma et al. 2012). ROS are generally clustered into the following two main categories: the first one consists of free radicals, such as hydroxyl radical (•OH) and superoxide anion (O •−2 ), while the second one takes account of non-radical molecules, such as singlet oxygen (1O2), hydrogen peroxide (H2O2), etc., as reviewed comprehensively by Sharma et al. (2012) and Gill and Tuteja (2010).
To conquer destructive consequences of higher proportions of ROS, including uncontrollable damages on cell wall, and the synthesis of chlorophyll, proteins, nucleic acids, and so forth (Noctor et al. 2014), plants commonly recruit two different scavenging systems called non-enzymic and enzymic antioxidants. The former includes Ascorbate (AsA), glutathione (GSH), phenolic constituents, non-protein amino acids, alkaloids, and α-tocopherols, while the latter encompasses catalase (CAT), superoxide dismutase (SOD), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), glutathione peroxidase, GPX; guaiacol peroxidase, GOPX, and glutathione-S-transferase (GST) (Apel and Hirt 2004; Foyer et al. 2006). Furthermore, under stress conditions, several antioxidant activities have been also recorded for both isoprenoids volatile compounds (e.g., hemiterpenes, homoterpenes, monoterpenes, and sesquiterpenes) and phenylpropanoids (Loreto and Velikova 2001; Vickers et al. 2009; Pollastri et al. 2014; Loreto et al. 2014). The isoprenoids group are assumed as the prominent category of natural products with more than 55,000 known compounds structurally diversified (Chang et al. 2010). In plants, countless protective functions have been recorded for volatile isoprenoids against various abiotic stresses, including thermal (high temperature) and oxidative stress, as comprehensively reviewed by Vickers et al. (2009) and Loreto et al. (2014). Similarly, phenylpropanoids are potentially capable to moderate adverse consequences of ROS through an antioxidant-based system, and are almost exclusively synthesized versus diverse abiotic stresses, including drought (Fini et al. 2012; Agati and Tattini 2010; Castellarin et al. 2007).
Basil (Ocimum basilicum L.; family Lamiaceae), as an annual, ornamental, culinary, herbaceous and aromatic plant, is climatologically distributed profusely in the tropical and subtropical regions, including Iran, and currently regarded as one of the globally most important medicinal plant, particularly for the production of essential oil (Simon et al. 1990; Ekren et al. 2012). Until now, from the essential oil of O. basilicum, in excess of 140 different constituents have been recorded (Hiltunen and Holm 2006), among which the main components include volatile isoprenoids (monoterpenes and sesquiterpenes) and phenylpropanoids, such as limonene, camphor, 1,8-cineole, linalool, eugenol, and methyl chavicol (Gang et al. 2001; Lee et al. 2005; Sajjadi 2006; Ekren et al. 2012). Though the magnitudes of essential oil of the plant may be attributable to the disparities in chemotypes, aroma, leaf and flower colors, growth and developmental phases of plant tissues, and the geographical origin of the plants alongside environmental circumstances (Carović-Stanko et al. 2010; Ekren et al. 2012; Ghasemi Pirbalouti et al. 2013), water supply can also augment the overall quantity of essential oil as well as the main individual constituents of essential oils. For instance, in basil, linalool and methyl chavicol contents can be positively affected by severe water shortage, albeit the ratio of total sesquiterpenes decreased under the same condition (Simon et al. 1992; Omidbaigi et al. 2003; Khalid 2006; Radácsi et al. 2010; Ekren et al. 2012). Water-deficit stress have also exhibited significant negative impacts on the plant height and yield of purple basil, while a remarkable growth occurred regarding the essential oil compositions (Singh et al. 2010; Ekren et al. 2012; Forouzandeh et al. 2012). In sweet basil, the amounts of linalool, methyl chavicol, 1,8-cineole, and trans-α-bergamotene, as the major constituents of essential oil were markedly changed under water-deficit stress (Omidbaigi et al. 2003; Radácsi et al. 2010).
Irrespective of many studies with the aim of determining the composition of essential oil under diverse environmental cues, including salinity, extreme temperature, and water-deficit stress (Liu et al. 2011a, b; Nowak et al. 2010; Pavarini et al. 2012; Alavi-Samani et al. 2015), according to the best of our knowledge, there is a research gap in the identification of the possible function(s) of volatile compounds as a measure of non-enzymatic oxidative defense systems and its association with antioxidant enzyme activities under water stress to protect basil against oxidative damages. To examine this, three geographically different Iranian cultivars of sweet basil were employed first, and subsequently, a number of drought-induced variables were measured in response to different levels of water-deficit stress at the full bloom stage.
Materials and methods
Plant materials and culture conditions
In general, three cultivars of O. basilicum, originating from geographically two different areas of Iran [i.e., Amol (Cultivar 2 and 3) and Jahrom (Cultivar 1) cities], were employed as starting plant materials. The seeds of each cultivar were subjected first to sterilization process with submerging in 10 % sodium hypochlorite for 5 min and immediately washed thrice using sterilized autoclaved water to remove disinfection liquid. Afterwards, the germination process of the seeds was undertaken at temperature ranging from 20 to 30 °C, under natural light condition, in an isolated nursery located at the research greenhouse of Faculty of Agriculture, University of Tehran, Karaj, Iran, with the beginning time of mid May, 2014 (35°49′57″N, 50°59′29″E). For each cultivar, three out of ten-day-old seedlings were transferred into individual pots containing sandy-loam soil and allowed to continue their growth under optimum circumstances for four additional weeks. Some physical and chemical characteristics of the soil are presented in Table 1.
Water-deficit stress treatment
When the basil seedlings reached 10 cm in plant height, seedlings were transplanted into new plastic pots. All the seedlings were irrigated thrice/week with applying no water-deficit stress until they adapted to the soil circumstances and reached six leaf stages. In applying water-deficit stress treatment, four different stress levels were adjusted first in relation to field capacity (FC) through withholding water, nominated then as W1: 100 % FC, W2: 75 % FC, W3: 50 % FC, and W4: 25 % FC, and subsequently, the healthy and uniform seedlings were transferred into each pot (Omidbaigi et al. 2003; Khalid 2006; Moeini Alishah et al. 2006). Notably, all the control plants were maintained under optimal irrigation conditions (W1: 100 % FC). For each pot, the ratio of soil moisture was measured through the calculation of the soil water content percentage (Yadav et al. 2014). The work was performed in a factorial scheme (3 × 4) based on a completely randomized design with two factors (i.e., three cultivars and four water-deficit stress treatments) and three replications. Sampling of the three cultivars was performed at full bloom stage (from May to June, 2014), after applying 29 days water scarcity stress treatment. The shoot samples of each treatment were harvested carefully and subsequently detached into two parts: the first one immediately frozen in liquid nitrogen and stored at −80 °C until further analyses, while the second one allowed to dry completely at 25–30 °C for the GC–MS analysis.
Plant growth parameters
For each cultivar, a number of growth parameters, including plant height, length of lateral branches, number of secondary branches per plant, and flowers in the inflorescence, were recorded at the time of harvesting. Meanwhile, both dry and fresh weights of the leaves and inflorescence of each basil cultivar were measured, as well.
Determination of the RWC
The measurement of relative water content (RWC) was performed in the full flowering stage from middle leaves under the top of the shoots (three replications per treatment), using the following formula (Slatyer 1967):
where FW, SW, and DW indicate “fresh weight” (i.e., the individual weight of fresh middle leaf sample immediately upon harvesting), “saturated weight” (the individual weight of each harvested leaf sample upon immersing in an equal amount of distilled water at 4 °C for 24 h), and “dry weight” (the individual weight of each harvested leaf sample upon drying in an oven at 70 °C for 72 h), respectively.
Chlorophyll content
The measurement of leaf chlorophyll content as well as total carotenoids was conducted according to the method described by (Arnon 1949; Davies 1976). In this context, 400 mg of fresh leaves of each sample were carefully fragmented into tiny segments in 80 % acetone. The resultant extracts were individually centrifuged at 8,000g for 5 min and chlorophyll concentration total carotenoids were spectrophotometrically determined in terms of the absorbance at the wavelengths of 470, 645, and 663 nm using Shimadzu UV-160 spectrophotometer. Calculations were carried out through the following formulas:
where V and W indicate volume of the extract and weight of leaf segments, respectively.
Measurement of H2O2 content
So as to quantify the H2O2 content, the methodology of Loreto and Velikova (2001) was employed. Briefly, fresh leaf fragments (~0.35 g) were homogenized in an ice bath with 5 mL of 0.1 % TCA. The resultant homogenate was centrifuged at 12,000g for 15 min, and then, 0.5 mL of the supernatant was added to 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide. For each extract treatment, the absorbance of the corresponded supernatant was individually calculated at 390 nm using a Shimadzu UV-160 spectrophotometer. The proportion of H2O2 content of each sample, in the following, was calculated based on a standard calibration curve formerly acquired with different concentrations of H2O2 and eventually expressed as µmol of H2O2 per gram of fresh leaf weight of each plant sample.
Determination of total protein content and antioxidant enzyme activity
Frozen leaves of each cultivar under study were homogenized in the extraction buffer (50 mM Tris–HCl, pH 7.8, and containing 10 % glycerol) with a mortar and pestle, and subsequently centrifuged at 12,000g for 15 min at 4 °C. The resultant supernatant was collected and utilized immediately for the determination of enzyme activity. Total protein concentration was determined according to the Bradford method (1976) using the commercial protein assay (BioRad, USA).
SOD activity was spectrophotometrically assayed on the basis of the inhibition of photoreduction of nitroblue tetrazolium (NBT) at 560 nm, as formerly developed by McCord and Fridovich (1969). The reaction mixture consisted of 100 mM Tris HCl buffer (pH 7.8), 0.1 mM EDTA, 12 mM methionine, 75 µM NBT, and 0.025 % Triton X-100. The 50 µL of enzyme extract and 11 µL of 2 µM Riboflavin were added to 2.9 mL of the reaction mixture. Reactions were conducted in 30 min at 25 °C under light intensity of 276 µmol m−2 s−1. One unit of SOD was defined as the amount of the enzyme that would inhibit 50 % of NBT photoreduction. SOD activity was expressed as unit mg−1 of protein.
CAT activity was determined in terms of H2O2 oxidation according to the method of Aebi (1984). The reactions solution (3 mL) consisted of 50 mM phosphate buffer (pH 7.0), 5.0 µL of 30 % H2O2, and 50 µL of crude enzyme extract. Changes in absorbance at 240 nm were recorded every 20 s for 4 min by means of Shimadzu UV-160 spectrophotometer. The activity of CAT enzyme was defined as nmol of H2O2 decomposed min−1 mg−1 of protein.
The assay of APX activity was accomplished according to Nakano and Asada (1981), using ascorbic acid as a substrate. The reaction mixture contained 1.5 mL of 0.5 M sodium phosphate buffer (pH 7.0), 600 µL of 0.1 mM EDTA, 400 µL of 0.5 mM ascorbic acid, and 100 µL of enzyme extract. The initiation process of ascorbate oxidation occurred via the addition of 400 µL of 30 % H2O2, and the decrease at 290 nm was recorded after 20 s for 60 s using Shimadzu UV-160 spectrophotometer. APX activity was expressed in nmol of ascorbate oxidized min−1 mg−1 of protein.
Regarding GPX activity, the reaction mixture consisted of 3 µL of guaiacol and 10 µL H2O2 (30 %) in 3 mL of phosphate buffer (pH 7.0) (Dionisio-Sese and Tobita 1998). The reaction was initiated through adding 50 µL of crude extract, and absorbance was recorded after 4 min at interval of 20 s. GPX activity was expressed as nmol of guaiacol oxidized min−1 mg−1 of protein.
Polyphenol oxidase (PPO) activity was determined as described by Kar and Mishra (1976) with some minor modifications. Assay system consisted of 50 µL of 0.01 M pyrogallol in 3 mL of phosphate buffer (pH 7.0), and enzyme extract initiated the reaction, absorbance was recorded at 420 nm for 3 min at interval of 20 s. The PPO activity was defined as nmol of purpurogallin obtained min−1 mg−1 of protein.
Isolation and chemical analysis of essential oils
Fresh plants were collected from each treatment and dried at 25–30 °C after the harvest. Shoot biomass dried from each pot of treatment was pooled and hydro-distilled for 3 h using a Clevenger-type system. Samples were dried with anhydrous sodium sulfate and kept in amber vials at 4 °C until the chromatographic analysis. The quantitative analysis of the chemical composition of the essential oils was performed using a gas chromatograph coupled with a mass spectrophotometer (GC–MS) (Agilent technologies 7890A), equipped with 5975 C mass selective detector. A HP5M5 column of dimension 30 m × 0.25 mm and film thickness 0.25 µm was used for the chromatographic resolution. Oven temperature was programmed to remain at 60 °C for 3 min, then an increase at a rate of 3 °C min−1 until 150 °C was reached; since then, temperature was programmed at 3 °C min−1 until reaching 260 °C, and this temperature was maintained for 3 min; the injector and detector temperature were maintained at 230 and 250 °C, respectively. Hydrogen was used as carrier gas with a flow rate of 1 mL min−1 and split ratio of 1:40. The components of the oils were identified by their retention indices relative to C8–C25 n-alkanes and compared with standard published data (Adams 2007) and commercial library (Wiley).
Statistical analysis
The results were subjected to statistically analyze using the analysis of variance (ANOVA) to discriminate significant differences (defined as p ≤ 0.05). The data calculations were performed with the SAS software and shown as the mean ± standard deviation (SD). The significant differences between treatments were compared by Duncan’s multiple range tests.
Results
Effect of water-deficit stress on plant growth parameters
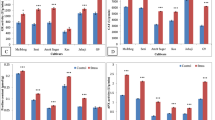
As shown in Table 2, growth parameters, viz., plant height, number of lateral branches, number of flowers in the inflorescence per plant, and dry and fresh weights of leaves and inflorescence followed by yield were considerably affected by water-deficit stress levels (p ≤ 0.05). Under normal conditions (W1), cultivar 2 showed lower levels of fresh and dry weight than the others. Furthermore, the plant height and number of lateral branches declined under water-deficit stress circumstances in all the three cultivars under study (Fig. 1). The same trend occurred for the number of flowers in the inflorescence in parallel with increasing the severity of stress levels. Fresh and dry aerial biomasses were strictly diminished under water shortage stress, particularly for the plants sampled from W4. Considering the above results, the plant growth and yield were eventually found to be dramatically restricted under the water-deficit stress conditions.
Effect of water-deficit stress on RWC
The RWC values of the three cultivars under study changed significantly as a result of varying in irrigation contents (Fig. 2). As expected, the highest ratios of RWC (an average of 96 %) were obtained for the control plants (W1). Under the water stress conditions, the RWC of the entire cultivars decreased, falling to its minimum value in 25 % FC (W4). Meanwhile, the occurrence of RWC was significantly reduced for the plant samples harvested from W2 as compared with their corresponding controls in all the three cultivars. A comparison of RWC values in the three cultivars at the highest level of water-deficit stress showed that cultivar 1 had the highest leaf RWC under severe drought stress, could be accordingly marked as the most tolerant basil cultivars under water-deficit stress rather than the others.
Effect of water-deficit stress on relative water content (RWC) (%) of the three Iranian cultivars of O. basilicum L. Bars are mean values of three replicates ± standard deviation. W1 100 % FC (control), W2 75 % (mild water stress), W3 50 % (moderate water stress), and W4 25 % FC (severe water stress). Cul 1 cultivar 1, Cul 2 cultivar 2, Cul 3 cultivar 3
Effect of water-deficit stress on plant pigments
As shown in Fig. 3a–c, Chl a, Chl b, and total chl concentrations increased first and then declined under water-deficit stress levels in cultivars 2. The highest Chl a concentration was found in cultivar 2 growing at 75 % of field capacity (W2). Under W4 treatment, the total chl content increased as compared with their corresponding in the cultivars 1 and 3. Furthermore, Chl a and Chl b had an apparent increase in cultivar 1 and 3 as compared with their control in the W4 treatment of stress. On the contrary, a pronounced reduction in the carotenoid content was observed by the increment of water-deficit stress. Furthermore, decrease of cartenoid content in cultivar 1 was detected more than the cultivars 2 and 3 under water-deficit stress treatments (Fig. 3d).
Effect of water-deficit stress on plant pigments of the three Iranian cultivars of O. basilicum L. Chl a (a), Chl b (b), total Chl (c), and carotenoids (d). Bars are mean values of three replicates ± standard deviation. Different letters (a–c) indicated above the bars represent statistically significant difference at p ≤ 0.05 (Duncan’s multiple range test). W1 100 % FC (control), W2 75 % (mild water stress), W3 50 % (moderate water stress), and W4 25 % FC (severe water stress). Cul 1 cultivar 1, Cul 2 cultivar 2, Cul 3 cultivar 3
H2O2 content fluctuations
Under normal conditions, H2O2 content of cultivar 1 and 2 was approximately similar (p ≤ 0.05), as the level of H2O2 was shown to remain unchanged in the first three levels of water-deficit stress. Afterwards, the level of H2O2 significantly increased in cultivar 2 under severe stress. According to Fig. 4, as the stress severity increased the level of H2O2 rose considerably in cultivar 3 as compared with the two remaining cultivars.
Effect of water-deficit stress levels on H2O2 content of the three Iranian cultivars of O. basilicum L. Bars are mean values of three replicates ± standard deviation. Different letters (a–c) indicated above the bar represent statistically significant difference at p ≤ 0.05 (Duncan’s multiple range test). W1 100 % FC (control), W2 75 % (mild water stress), W3 50 % (moderate water stress), W4 25 % FC (severe water stress). Cul 1 cultivar 1, Cul 2 cultivar 2, Cul 3 cultivar 3
Changes in activities of antioxidant enzymes
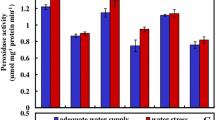
As shown in Fig. 5a, SOD activity significantly increased in cultivar 3 under W2. The maximum SOD activity was observed in cultivar 3 for both control and stress conditions. In fact, water-deficit stress led to small increase or no effect on SOD activity in cultivar 1 and 2. In addition, CAT activity in the three cultivars differentially increased. In cultivar 2, CAT was more active than the other two cultivars, but according to Fig. 5b, its activity was unaffected by the increase of water stress, excepting an increase in Cultivar 3 under W2. APX activity was increased with 50 and 75 % of field capacity only in cultivar 1. In cultivar 2, APX activity did not change, while W3 decreased APX activity. In cultivar 3, APX activity was statistically unaffected in W2 and W3 (Fig. 5c). The three cultivars exhibited a low GPX activity for W1 (Fig. 5d). More precisely, GPX activity substantially increased under W4 treatment of the water-deficit stress condition. Although in cultivar 1, GPX activity showed a sharp increase in W4 treatment. In general, GPX responded with a substantial effect in activity. In response to water-deficit stress, PPO exhibited some fluctuations. In normal conditions, PPO activity in cultivar 1 and 3 was similar with the exception of this treatment group in cultivar 2 (Fig. 5e). Briefly, different levels of water-deficit stress brought about a significant increase in PPO activity in cultivar 1 as PPO activity increased in severe stress only in cultivar 1. However, no significant difference was detected in PPO activity in cultivar 1 between W3- and W4-level-deficit water stresses. PPO activity was not significantly different under water-deficit stress conditions in cultivar 3. In addition, PPO activity in cultivar 2 increased in W2, but decreased in W3 and W4.
Effect of water-deficit stress on enzyme activity of the three Iranian cultivars of O. basilicum L. SOD activity (a), CAT activity (b), APX activity (c), GPX activity (d), and PPO activity (e). Bars are mean values of three replicates ± standard deviation. Different letters (a–c) indicated above the bar represent statistically significant difference at p ≤ 0.05 (Duncan’s multiple range test). W1 100 % FC (control), W2 75 % (mild water stress), W3 50 % (moderate water stress), and W4 25 % FC (severe water stress). Cul 1 cultivar 1, Cul 2 cultivar 2, Cul 3 cultivar 3
Effect of water-deficit stress on the essential oil composition
Under both control and water-deficit stress conditions, the magnitude and composition of essential oil compounds varied in the three cultivars under study (Table 3), albeit methyl chavicol, squalene, linalool, α-bergamotene, germacrene D, α-caryophyllene, eicosane, and γ-cadinene were found as the main constituents in all the cultivars throughout water-deficit stress, excepting methyl chavicol as not found in cultivar 1. Furthermore, in cultivar 3, α-bergamotene, γ-cadinene, and linalool were not detected. According to our results, the majority (81.49–100 %) of essential oil constituents under normal and stress conditions were identified using the GC–MS analysis in the all three cultivars. The occurrences of linalool and squalene contents in essential oil of cultivar 1, methyl chavicol, squalene, and epi-bicyclose squiphellandrene contents in essential oil of cultivar 2, methyl chavicol, squalene, and eicosane in essential oil of cultivar 3 were dramatically influenced by increasing stress intensity. Nonetheless, some constituents, interestingly, were induced in low levels and nearly unchanged under water-deficit stress in all the three cultivars. Notably, germacrene D, β-elemene, cis-α-bisabolene, and γ-cadinene (in %) in the essential oil of cultivar 1 and α-bergamotene followed by γ-cadinene in the essential oil of cultivar 2 and α-caryophyllene, germacrene D alongside Cis-α-bisabolene in the essential oil of cultivar 3 were marginally decreased under water-deficit stress. On average, the essential oil contains 39.14–76.54 % methyl chavicol in cultivar 2 and 3, 1.84–45.6 % linalool in cultivar 1 and 2, and 8.33–20.73 % squalene in all the three cultivars under water-deficit stress treatments.
Discussion
In nature, unavoidably, plants are struggling to immense biotic and abiotic stresses, including water-deficit stress, leading often to the generation of a wide range of bioactive compounds to diminish the detrimental consequences into minimum scales (Sandalio et al. 2009). Meanwhile, ROS generation as well as its modulation through plant cellular machinery is a critical factor, mainly as they are capable enough to exert substantial negative effects on various physiological and biochemical mechanisms of living cells (Sandalio et al. 2009). Therefore, a number of enzymatic and non-enzymatic mechanisms (see above) are commonly recruited by plants to moderate the extra accumulation of ROS which are undoubtedly toxic (but in higher amounts) and may result in apoptosis or cell death. With this in mind, the possible reaction(s) of different basil cultivars were assessed in terms of the accumulation/production of some volatile metabolites as non-enzymatic antioxidants plus a number of scavenging enzymes under water shortage stress.
Considering the possible physiological consequences of cellular water-deficit stress, the metrics RWC has been regarded as the most applicable measure of plant water status (Khanna et al. 2014; Talbi et al. 2015; Vaz et al. 2016). The amounts of leaf RWC remained somewhat unchanged (~90 %) for W1 and W2 in all the basil cultivars (see above), but as stress intensified, its value significantly decreased in all the three cultivars, as the highest diminution occurred in cultivar 3, concluding that it is more susceptible versus water-deficit stress than the others two cultivars of basil. The differences in RWC have been suggested to be due to the variations in cell wall elasticity, as cell wall of tolerant cultivars is more vigorous than that of sensitive ones (Kramer and Boyer 1995; Martínez et al. 2007; Hessini et al. 2009).
On the other hand, upon applying W3 and W4, chlorophyll content of cultivar 2 was reduced as compared with W1, indicating that it has possibly low capacity for light perception. In fact, it has been suggested that drought stress may result in irreparable damages on photosynthetic pigments, and consequently, thylakoid membrane would be unavoidably destroyed (Bijanzadeh and emam 2010; Din et al. 2011; Kannan and Kulandaivelu 2011). Therefore, photosynthetic capacity would be declined under drought stress. In cultivar 1 and 3, however, considerable increase in chlorophyll content occurred under W4 treatment, suggesting that these two cultivars have evolved a protective mechanism against water stress-induced damages, possibly via alterations in the genomic capability for the synthesis of protective pigments (Batra et al. 2014). Similarly, drought stress was positively provoked the growth of chlorophyll contents in various plant species (Megdiche et al. 2008; Mafakheri et al. 2010; Pirzad et al. 2011; Ashraf and Harris 2013; Batra et al. 2014), representing a stimulated growth of chlorophyll contents under water-deficit stress. The reason of increased chlorophyll contents in cells could be explainable by the chloroplast development under water stress, as it may facilitate enhanced generation of antioxidant defense systems derived from the more excessive excited energy produced by photosynthetic apparatus (Chang et al. 1997; Talbi et al. 2015).
In addition to chlorophyll contents, carotenoids also appear to play key roles under drought stress. For instance, they could be employed as a precursor in signaling as well as a protective element for photosynthetic machinery under different biotic and abiotic stresses (Ashraf and Harris 2013). Such features could be due to the compounds with conjugated double bonds, so that energy transfer would be easily taken place under diverse stress conditions (Cantrell et al. 2003). Here, our results indicated that different basil cultivars exhibited different responses for carotenoid contents. As a result, both chlorophyll and carotenoid contents could be utilized as reliable selection criteria for breeding programs of the plant, as the same was reported by Ziaf et al. (2009) in pepper.
Moreover, the fact is currently well known that virtually, all environmental stresses stimulate or involve oxidative stress somewhat through the generation and accumulation of ROS (Sharma et al. 2012). Under stress conditions, when ROS overproduced, H2O2 especially can diffuse across biological membranes and severely damage plant metabolism (Tripathi 2010; Mubarakshina et al. 2010). Here, the rapid rate of H2O2 accumulation rose in cultivar 3 in parallel with increasing the water-deficit stress levels. Comparing with the other cultivars, it appears that cultivar 3 may also create genomic organization, since increased levels of H2O2 occurred under higher levels of water-deficit stress, concluding that cultivar 3 is possibly a drought-sensitive cultivar of basil. Moreover, under control condition, H2O2 accumulation was not similar in the three cultivars (Fig. 4). It can be deduced that plants may have different genetic capacities in response to stress, so that significant changes were observed in H2O2 contents and antioxidant enzymes activities nearly in all the stress levels. It is worth noting that only in the cultivar 3, there was a significant difference between the control and stress water levels, which led to a significant increase in H2O2 content and may lead to instability of cell membrane. Under high levels of stress, stress-susceptible plant revealed increased amounts of H2O2, the initial relative tolerance, but their surveillance would be threatened for a long period (Luna et al. 2004; Nazari et al. 2012). These characteristics are correlated to the drought-sensitive plants (Nazari et al. 2012; Murshed et al. 2013). Even though exposure to sub-lethal levels of some stresses activated the most imperative elements in the scavenging system, the great accumulation of H2O2 demonstrates the lack of antioxidant activity (Mittler 2002). In this regard, comparing with the other two cultivars, higher levels of SOD activity were observed in cultivar 3 either for control or under water-deficit stress (Fig. 5a), which is in accordance with the high level of H2O2. H2O2 is commonly generated from β oxidation of fatty acids and photorespiration, as well as a byproduct of SOD activity, and due to its toxicity must be eradicated by the conversion to H2O via subsequent reactions (Foyer et al. 2006; Gill and Tuteja 2010). Higher activity of CAT, APX, GPX, and PPO regulates H2O2 levels in cell and promotes the membranes stability. In a direct manner, CAT activity catalyzes H2O2 conversion through breaking it down to produce both water and oxygen in the peroxisome (Willekens et al. 1997; Sharma and Dubey 2005; Sharma et al. 2012). Considering CAT activity, the current data demonstrated that all the basil cultivars had similar trends as water stress levels increased. However, this enzyme activity was higher in cultivar 2 and exhibited a significant difference as compared with the others. Our data indicated that cultivar 2 harbored higher levels of H2O2 in W4. It has been suggested that induction of Cat activity could be activated in response to water-deficit stress (Abedi and Pakniyat 2010; Murshed et al. 2013; Wei et al. 2013; Talbi et al. 2015). The increase in APX activity could be a sign that plants react to ROS accumulation. APX scavenges H2O2 by reducing it at the expense of ascorbate in plant (Mittler 2002; Sharma et al. 2012). Water stress-induced stimulation of APX activity was more pronounced in W2 and W3 for cultivars 1. Hence, it plays a key role in scavenging H2O2 in the cultivar 1. Nonetheless, at higher water stress treatment (W4), other enzymes may cooperate or replace APX activity. From the results obtained, overall, it could be concluded that elevated levels of CAT and APX may be related to water stress-induced tolerance activation in cultivar 1.
The important induction of GPX activity has been reported with various stressful conditions, including drought, salinity, and metal toxicity (Sajedi et al. 2012; Mishra et al. 2013; Pandey et al. 2016). Our results showed a significant increase in GPX activity in the plants subjected to W4, implying the imperative role of GPX in efficient quencher of H2O2 in plant. However, a different response of GPX activity was also observed in the plants of three cultivars exposed to W2 and W3 treatments (Fig. 5d), possibly due to variation in genetic and biochemical capacities (Sajedi et al. 2012).
Unlike APX which is largely intracellular, PPO is more frequently secreted into the apoplast and involved in phenolic metabolism using H2O2 as a substrate (Passardi et al. 2005; Radwan et al. 2010). In this study, our data showed that PPO activity had a different trend to the water stress conditions. We observed significant increases in PPO activity in severe stress only in cultivar 1, alongside no significant changes in cultivar 3 under all the water-deficit stress levels. Furthermore, PPO activity in cultivar 2 increased in W2, but decreased in W3 and W4. It is probable that the elevated PPO activity in cultivar 1 under severe water-deficit stress could be due to an increased ROS-scavenging capacity and decreased membrane damage. Besides the critical role of PPO activity as a scavenger of H2O2, PPOs are involved in phenolic metabolism in normal plant growth and development, lignification, and cross linking of cell wall compounds (Fan et al. 2014).
On the basis of GC–MS data, the highest average levels of essential oil were methyl chavicol and squalene in cultivar 2 and 3 and linalool and squalene in cultivar 1. Methyl chavicol, linalool, and squalene appeared as the major constituents in essential oil components in our studied cultivar, agreeing with the previous reports (Gang et al. 2001; Ekren et al. 2012, Blank et al. 2012; pirmoradi et al. 2013). Nonetheless, the mean values of these constituents in the studied cultivars of basil were much higher than those recorded by Khalid (2006), Telci et al. (2006) and Ekren et al. (2012) under water-deficit stress conditions. Increased phenylpropanoids (methyl chavicol) biosynthesis may represent an impressible response of cultivar 2 and 3 to ROS scavenging under water-deficit stress. Several evidences exhibited that the most phenylpropanoids derivatives act as ROS detoxifying compounds in the adaptation of plants to water-deficit stress (Fini et al. 2012; Janz et al. 2010). Among the cultivars of basil studied, cultivar 3 had the highest squalene content in W1, whereas the oil from the other basil cultivars did not contain a measure of this chemical compound (Table 3), probably due to its cultivar origin. In our study, the amounts of some volatile isoprenoids include linalool, α-caryophyllene, germacrene D, β-elemene, α-bergamotene, α-farnesene, and γ-cadinene were decreased in cultivar 1 by increasing stress intensity, representing the association of isoprene emitting and environmental stress in this treatment, agreeing with Velikova (2008) and Way et al. (2011).
As mentioned above, the constituents amounts of the three cultivars varied under control condition, and even some of these compounds were not detected under water-deficit stress condition in the cultivars. The presence of some constituents, such as methyl eugenol, α-caryophyllene, germacrene D, cis-α-bisabolene, n-tetracosane, squalene, α-humulene, limonene, α-bergamotene, γ-cadinene, β-pinene, 1,8-cineole, β-ocimene, and α-farnesene, in all three cultivars, presumably, could be owing to their roles in water-deficit stress modulation in basil, agreeing with (Lavoir et al. 2009; Liu et al. 2011a, b; Manukyan 2011; Fini et al. 2012; Alavi-Samani et al. 2015; Sanchita et al. 2015). Thus, considering the current observations, it could be overall hypothesized that induction of volatile compounds may play pivotal roles in water-deficit stress modulation. It has been suggested that isoprenes can be recruited in plant defense system, not only through strengthening structure of cell membranes, but also owing to their possible antioxidant activities to moderate hazardous consequences of ROS generated under various environmental stresses (Loreto and Velikova 2001; Vickers et al. 2009; Pollastri et al. 2014; Loreto et al. 2014). In plants, scavenging process of the entire or majority of ROS (i.e., singlet oxygen, hydroxyl radicals, and H2O2) which is mediated by some volatile compounds of essential oil can support the unrivaled protective roles of such secondary metabolites under different environmental stresses (Vickers et al. 2009; Loreto et al. 2014). In aqueous solution, for instance, isoprene reacts with hydroxyl radicals to generate 2-methyltetrols, and it has been reported that isoprene scavenges hydroxyl radicals to protect from any stress led to oxidative damage (Santos et al. 2006). These effects related to reactions through the conjugated double-bond system in the isoprene molecule may serve to easily mediate electron and energy transfers, which give a ROS-scavenging ability to the molecule (Affeck and Yakir 2002; Vickers et al. 2009).
In cultivar 1, under W4, a drastic decrement occurred for the accumulation/production of several mono- and sesquiterpenes compounds. Under severe drought stress, as a consequence of the closure of stomata and reduction in the ratio of carotenoids, photosynthesis would be declined or completely prohibited, leading ultimately a significant reduction in stored carbon (Schnitzler et al. 2004; Brilli et al. 2007; Vickers et al. 2009). Under such situations, this small quantity of carbon is utilized to generate volatile isoprene through MEP and MVA pathways (Vickers et al. 2009; Loreto et al. 2014). It has also been proved that the effect of drought on the emission ratios of plant volatile compounds depends on the level of damage. In fact, the previous drought stress-based studies indicated that the emission of volatile isoprenoids will be intensified so long as they are combined with ROS (Loreto and Schnitzler 2010; Velikova et al. 2006). Under severe levels of environmental stress, an increase in the emission ratio of monoterpenes and isoprenes is expected to inhibit extra accumulation of H2O2 as well as other types of ROS (Velikova and Loreto 2005; Velikova et al. 2006; Loreto et al. 2014). Interestingly, the earlier works demonstrated that only volatile isoprenoids are able to exhibit protective functions under stress circumstances, but not triterpenes and other non-volatile isoprenoids (Helmig et al. 2004; Velikova et al. 2006). In our results, squalene, as a member of sesquiterpenes, increased dramatically under drought stress, agreeing with the mentioned hypothesis. Notably, as the precise quantification of sesquiterpenes is nearly impossible under environmental stresses, the contemporary efforts are mainly focused on the non-volatile isoprenoids (Vickers et al. 2009).
As mentioned above, water-deficit stress failed to considerably enhance H2O2 content in cultivar 1. In fact, high activities of GPX and APX enzymes and unchanged H2O2 level in different treatments of water-deficit stress in cultivar 1 were in accordance with decrease the volatile isoprenoids, actually to exhibit their antioxidant activities in the too complex protective network of the plant. On the subject of the increased tolerance to environmental stress in aromatic plants, the previous investigations demonstrated that antioxidant activities in isoprene-inhibited leaves are lower than in isoprene emitting, under high-temperature circumstances (Loreto and Velikova 2001; Loreto et al. 2014).
Earlier investigations demonstrated the overproduction of phenylpropanoids for the plants under environmental stresses, albeit their ROS-scavenging mechanisms are still unknown. Comparing with the controls, for both cultivars 2 and 3, considerable growth was detected for both methyl chavicol and methyl eugenol, connoting their H2O2 scavenging activities, though the amount of H2O2 increased in parallel with enhancement in the water-deficit stress levels. Similar observations have been recorded under salt, high light intensity, and drought stresses (Fini et al. 2012; Tattini et al. 2004; Agati et al. 2011). Subsequently, antioxidant enzyme activities decreased remarkably, indicating a possible negative association between the quantities of phenylpropanoids plus antioxidant enzymes and growth in H2O2 levels, agreeing with Fini et al. (2011, 2012). Considering such or similar interesting findings, it could be concluded that phenylpropanoids possibly are able to constitute a secondary antioxidant system, even temporally, as the same pointed out by Fini et al. (2012), whose research indicated the contrasting impacts of drought stress on phenylpropanoid biosynthesis and antioxidant enzymes activity in the leaves of Fraxinus ornus (Fini et al. 2012). Taking the results as a whole, it could be deduced that phenylpropanoids can be utilized as pivotal scavenging/protective vehicles, particularly when the dynamic coordinated antioxidant network of the plants are severely damaged under diverse environmental stresses.
Conclusions
The results of this study revealed that plant growth parameters followed by physiological status of the cultivars are generally reduced under water-deficit stress circumstances, although several significant variations were also detected among three Iranian cultivars of basil. In addition, the accumulation of H2O2 in cultivars 2 and 3 was significantly stimulated under different water-deficit stress levels, while moderate increase was taken place for cultivar 1. Interestingly, in all the three cultivars of basil, the scavenging enzymes, including SOD, CAT, APX, GPX, and PPO, exhibited different responses against different levels of water-deficit stress, albeit some fluctuations were also detected. Marginal reduction and/or unaffected changes in carotenoids contents as structural components of Photosystem II (PSII) were physiological responses in all the three cultivars of basil in response to water-deficit stress. On the other hand, it was concluded that water-deficit stress could be regarded as a stimuli to intensify the essential oil ratios of all the basil cultivars studied here. The main components of the essential oil were methyl chavicol and squalene in the cultivars 2 and 3 and linalool and squalene in cultivar 1 with the possible positive roles in water-deficit stress modulation, concluding that both quality and quantity of a given secondary metabolite could be affected by various factors, such as cultivar under study, environmental conditions, and so on. Interestingly, it could also be deduced that the compensation of low levels of antioxidant enzymes activity in cultivars 2 and 3 may occur through the increase in the ratios of both methyl chavicol and methyl eugenol under water deprivation conditions. As the last point of view, our finding demonstrated that an increase in the phenylpropanoid components of essential oil could encourage the role of ROS-scavenging enzymes, including SOD, CAT, APX, GPX, and PPO enzymes.
Author contribution statement
FK carried out the experimental works and drafted the manuscript. MR participated in the selection of basil cultivars and helped to analyze GC–MS data. JN assisted in manuscript revising and provided scientific helps. FSH conceived the study and helped to draft the manuscript. AB participated in the design of the study and performed the statistical analyses. HA participated in the design of the study and helped in the interpretation of GC–MS data. All the authors read and approved the final version of the manuscript.
Abbreviations
- ANOVA:
-
Analysis of variance
- FC:
-
Field capacity
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GPX:
-
Guaiacol peroxidase
- PPO:
-
Polyphenol oxidase
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SOD:
-
Superoxide dismutase
References
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Gent Plant Breed 46:27–34
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometery. Allured Publishing Corp, USA
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Affeck HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129:269–277
Agati G, Tattini M (2010) Multiple functional roles of flavonoids in photoprotection. New Phytol 186:786–793
Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M (2011) The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol 168:204–212
Alavi-Samani SM, Ataei Kachouei M, Ghasemi Pirbalouti A (2015) Growth, yield, chemical composition, and antioxidant activity of essential oils from two thyme species under foliar application of jasmonic acid and water deficit conditions. Hortic Environ Biotechnol 56:411–420
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 55:373–399
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Batra NG, Sharma V, Kumari N (2014) Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiate. J Plant Interact 9:712–721
Bijanzadeh E, Emam Y (2010) Effect of defoliation and drought stress on yield components and chlorophyll content of wheat. Pak J Biol Sci 13:699–705
Blank AF, Rosa YRS, Filho J, Santos C, Arrigoni-Blank M, Niculau E, Alves P (2012) A diallel study of yield components and essential oil constituents in basil (Ocimum basilicum L.). Ind Crop Prod 38:93–98
Bradford MM (1976) A rapid sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2:48–54
Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M (2007) Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol 175:244–254
Cantrell A, McGarvey DJ, Truscott TG, Rancan F, Böhm F (2003) Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch Biochem Biophys 412:47–54
Carović-Stanko K, Orlić S, Politeo O, Strikić F, Kolak I, Milos M, Satovic Z (2010) Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem 119:196–201
Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, Di Gaspero G (2007) Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ 30:1381–1399
Chang C, Locy R, Smeda R, Sahi S, Singh N (1997) Photoautotrophic tobacco cells adapted to grow at high salinity. Plant Cell Rep 16:495–502
Chang TH, Hsieh FL, Ko TP, Teng KH, Liang PH, Wang AHJ (2010) Structure of a heterotetrameric geranyl pyrophosphate synthase from mint (Mentha piperita) reveals intersubunit regulation. Plant Cell 22:454–467
Davies B (1976) Carotenoids. In: Goodwin TW (ed) Chemistry and biochemistry of plant pigments. Academic Press, London, pp 38–165
Din J, Khan SU, Ali I, Gurmani AR (2011) Physiological and agronomic response of canola varieties to drought stress. J Anim Plant Sci 21:78–82
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Ekren CS, Sönmez Ç, Özҫakal E, Kurttaş YSK, Bayram HG, Gürgülü H (2012) The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). J Food Agric Environ 109:155–161
Fan H, Ding L, Du Ch, Wu X (2014) Effect of short-term water-deficit stress on antioxidative systems in cucumber seedling roots. Bot Stud 55:46–53
Farooq M, Hussain M, Wahid A, Siddique KHM (2012) Drought stress in plants: an overview. In: Aroca R (ed) Plant responses, from morphplogical to molecular features. Springer, New York, pp 1–36
Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M (2011) Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal Behav 65:709–711
Fini A, Guidib L, Ferrini F, Brunetti C, Ferdinando M, Biricolti S, Pollastri S, Calamai L, Tattini M (2012) Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: an excess light stress affair? J Plant Physiol 169:929–939
Forouzandeh M, Fanoudi M, Arazmjou E, Tabiei H (2012) Effect of drought stress and types of fertilizers on the quantity and quality of medicinal plant Basil (Ocimum basilicum L.). Ind J Innov Dev 9:696–699
Foyer C, Trebst A, Noctor G (2006) Signaling and integration of defense functions of tocopherol, ascorbate and glutathione. In: Demmig-Adams B, Adams WW, Mattoo AK (eds) Photoprotection, photoinhibition, gene regulation, and environment. Springer, The Netherlands, pp 241–268
Gang DR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E (2001) An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol 125:539–555
Ghasemi Pirbalouti A, Mahdad E, Craker L (2013) Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chem 141:2440–2449
Gill S, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Helmig D, Bocquet F, Pollmann J, Revermann T (2004) Analytical techniques for sesquiterpene emission rate studies in vegetation enclosure experiments. Atmos Environ 38:557–572
Hessini K, Martínez JP, Gandour M, Albouchi A, Soltani A, Abdelly A (2009) Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ Exp Bot 67:312–319
Hiltunen R, Holm Y (2006) Essential oil of Ocimum. In: Hiltunen J, Holm Y (eds) Basil: the genus Ocimum. Harwood Academic Publisher, Amsterdam, pp 77–111
Janz D, Behnke K, Schnitzler JP, Kanawati B, Schmitt-Kopplin P, Polle A (2010) Pathway analysis of the transcriptome and metabolome of salt sensitive and tolerant poplar species reveals evolutionary adaption of stress tolerance mechanisms. BMC Plant Biol. doi:10.1186/1471-2229-10-150
Kannan ND, Kulandaivelu G (2011) Drought induced changes in physiological, biochemical and phytochemical properties of Withania somnifera Dun. J Med Plants Res 5:3929–3935
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Khalid KhA (2006) Influence of water stress on growth, essential oil, and chemical composition of herbs Ocimum sp.). Int Agrophy 20:289–296
Khanna SM, Choudhary P, Saini R, Jain PK, Srinivasan R (2014) Effect of water-deficit stress on growth and physiological parameters in chickpea cultivars differing in drought tolerance. Ann Biol 30:77–84
Kramer J, Boyer J (1995) Water relations of plants and soils. Academic Press, California
Lavoir AV, Staudt M, Schnitzler JP, Landais D, Massol F, Rocheteau A, Rodriguez R, Zimmer A, Rambal S (2009) Drought reduced monoterpene emissions from Quercus ilex trees: results from a throughfall displacement experiment within a forest ecosystem. Biogeosciences 6:863–893
Lee SJ, Umano K, Shibamoto T, Lee KG (2005) Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem 91:131–137
Lei Y, Yin C, Li C (2006) Differences in some morphological physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol Plant 127:182–191
Liu C, Liu Y, Guo K, Fan D, Li G, Zheng Y, Yu L, Yang R (2011a) Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ Exp Bot 71:174–183
Liu H, Wang X, Wang D, Zou Z, Lianga Z (2011b) Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind Crop Prod 33:84–88
Loreto F, Schnitzler JP (2010) Abiotic stresses and induced BVOCs. Trends Plant Sci 15:154–166
Loreto F, Velikova V (2001) Isoprene production by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Loreto F, Pollastri S, Fineschi S, Velikova V (2014) Volatile isoprenoids and their importance for protection against environmental constraints in the Mediterranean area. Environ Exper Bot 103:99–106
Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH (2004) Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot 56:417–423
Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC, Sohrabi Y (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4:580–585
Manukyan A (2011) Effect of growing factors on productivity and quality of lemon catmint, lemon balm and sage under soil less greenhouse production: I. drought stress. Med Aromat Plant Sci Biotechnol 5:119–125
Martínez JP, Silva H, Ledent JF, Pinto M (2007) Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur J Agron 26:30–38
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Megdiche W, Gharbi F, Jaleel C, Ksouri R, Abdelly C (2008) Photosynthesis, photosystem II efficiency of two salt-adapted halophytic seashore Cakile maritime ecotypes. Photosynthetica 46:410–419
Mishra P, Bhoomika K, Dubey RS (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250:3–19
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moeini Alishah H, Heidari R, Hassani A, Dizaji AA (2006) Effect of water stress on some morphological and biochemical characteristics of purple basil (Ocimum basilicum L.). J Biol Sci 6:763–767
Mubarakshina MM, Ivanonv BN, Naydov IA, Hillier W, Badger MR, Krieger-Liszkay A (2010) Production and diffusion of chloroplastic H2O2 and its implication to signalling. J Exp Bot 61:3577–3587
Murshed R, Lopez-Lauri F, Sallanon H (2013) Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L, cv. Micro-tom). Physiol Mol Biol Plants 19:363–378
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nazari M, Maali Amiri R, Mehraban FH, Khaneghah HZ (2012) Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation. Russ J Plant Physiol 59:183–189
Noctor G, Mandhi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164:1553–1555
Nowak M, Manderscheid R, Weigel HJ, Kleinwachter M, Selmar D (2010) Drought stress increases the accumulation of monoterpenes in sage (Salvia officinalis), an effect that is compensated by elevated carbon dioxide concentration. J Appl Bot Food Qual 83:133–136
Omidbaigi R, Hassani A, Sefidkon F (2003) Essential oil content and composition of sweet basil (Ocimum basilicum L.) at different irrigation regimes. J Ess Oil Bear Plants 6:104–108
Pandey P, Srivastava RK, Rajpoot R, Rani A, Pandey AK, Dubey RS (2016) Water deficit and aluminum interactive effects on generation of reactive oxygen species and responses of antioxidative enzymes in the seedlings of two rice cultivars differing in stress tolerance. Environ Sci Pollut Res Int 23:1516–1528
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Pavarini DP, Pavarini SP, Niehuesa M, Lopes NP (2012) Exogenous influences on plant secondary metabolite levels. Anim Feed Sci Tech 176:5–16
Pirmoradi MZ, Moghaddam M, Farhadi N (2013) Chemotaxonomic analysis of the aroma compounds in essential oils of two different Ocimum basilicum L. varieties from Iran. Chem Biodivers 10:1361–1371
Pirzad A, Shakiba MR, Zehtab-Salmasi S (2011) Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. J Med Plants Res 5:2483–2488
Pollastri S, Tsonev T, Loreto F (2014) Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J Exp Bot 65:1565–1570
Radácsi P, Inotai K, Sz Sárosi, Czövek P, Bernáth J, Németh É (2010) Effect of water supply on the physiological characteristic and production of Basil (Ocimum basilicum L.). Eur J Hort Sci 75:193–197
Radwan DEM, Fayez KA, Mahmoud SY, Lu G (2010) Modifications of antioxidant activity and protein composition of bean leaf due to bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiol Plant 32:891–904
Sajedi NA, Ferasat M, Mirzakhani M, Boojar MMA (2012) Impact of water-deficit stress on biochemical characteristics of safflower cultivars. Physiol Mol Biol Plants 18:323–329
Sajjadi SE (2006) Analysis of essential oils two cultivated basil (Ocimum basilicum L.) from Iran. DARU J Pharmaceut Sci 14:128–130
Sanchita, Singh R, Mishra A, Dhawan SS, Shirke PA, Gupta MM, Sharma A (2015) Physiological performance, secondary metabolite and expression profiling of genes associated with drought tolerance in Withania somnifera. Protoplasma. doi:10.1007/s00709-015-0771-z
Sandalio LM, Rodríguez-Serrano M, del Río LA, Romero-Puertas MC (2009) Reactive oxygen species and heavy metal toxicity. In: Rio LA, Puppo A (eds) Reactive oxygen species in plant signalling. Springer, Berlin, pp 175–189
Santos LS, Dalmazio I, Eberlin MN, Claeys M, Augusti R (2006) Mimicking the atmospheric OH-radical-mediated photooxidation of isoprene: formation of cloud-condensation nuclei polyols monitored by electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 20:2104–2108
Schnitzler JP, Graus M, Kreuzwieser J, Heizmann U, Rennenberg H, Wisthaler A, Hansel A (2004) Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiol 135:152–160
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Simon JE, Quinn J, Murray RG (1990) Basil: a source of essential oils. In: Janick J, Simon JE (eds) Advances in new crops. Timber Press, Portland, pp 484–489
Simon JE, Reiss-bubenheim D, Joly RJ, Charles DJ (1992) Water stress-induced alterations in essential oil content and composition of sweet basil. J Ess Oil Res 4:71–75
Singh S, Singh M, Singh AM, Kalra A, Yadav A, Patra DD (2010) Enhancing productivity of Indian basil (Ocimum basilicum L.) through harvest management under rainfed conditions of subtropical north Indian plains. Ind Crop Prod 32:601–606
Slatyer R (1967) Plant–water relationships. Academic Press, New York
Talbi S, Romero-Puertas MC, Hernández A, Terrón L, Ferchichi A, Sandalio LM (2015) Drought tolerance in a Saharian plant Oudneya africana: role of antioxidant defences. Environ Exp Bot 111:114–126
Tattini M, Galardi C, Pinelli P, Massai R, Remorini D, Agati G (2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol 163(3):547–561
Telci I, Bayram E, Yılmaz G, Avcı B (2006) Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochem Syst Ecol 34:489–497
Tripathi BN (2010) Stress metabolism of plants. Protoplasma. doi:10.1007/s00709-010-0196-7
Vaz M, Coelho R, Rato A, Samara-Lima R, Silva LL, Campostrini E, Mota JB (2016) Adaptive strategies of two Mediterranean grapevines varieties (Aragonez syn. Tempranillo and Trincadeira) face drought: physiological and structural responses. Theor Exp Plant Physiol. doi:10.1007/s40626-016-0074-6
Velikova V (2008) Isoprene as a tool for plant protection against abiotic stresses. J Plant Interact 3:1–15
Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperature and during the recovery from a heat stress. Plant Cell Environ 28:318–327
Velikova V, Loreto F, Tsonev T, Brilli F, Edreva A (2006) Isoprene prevents the negative consequences of high temperature stress in Platanus orientalis leaves. Fun Plant Biol 33:931–940
Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Hewitt CN (2009) Isoprene synthesis protects transgenic plants from oxidative stress. Plant Cell Environ 32:520–531
Way DA, Schnitzler J, Monson RK, Jackson RB (2011) Enhanced isoprene-related tolerance of heat- and light-stressed photosynthesis at low, but not high, CO2 concentrations. Oecologia 166:273–282
Wei H, Chao H, Xiaomin D (2013) TaASR1 a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ 36:1449–1464
Willekens H, Chamnongpol S, Davey M (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816
Yadav RK, Sangwan RS, Sabir F, Srivastava AK, Sangwan NS (2014) Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes and pathway gene expression in Artemisia annua L. Plant Physiol Biochem 74:70–83
Ziaf K, Amjad M, Pervez MA, Iqbal Q, Rajwana IA, Ayyub M (2009) Evaluation of different growth and physiological traits as indices of salt tolerance in hot pepper (Capsicum annuum L.). Pak J Bot 41:1797–1809
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G Bartosz.
Rights and permissions
About this article
Cite this article
Khakdan, F., Ranjbar, M., Nasiri, J. et al. The relationship between antioxidant compounds contents and antioxidant enzymes under water-deficit stress in the three Iranian cultivars of basil (Ocimum basilicum L.). Acta Physiol Plant 38, 226 (2016). https://doi.org/10.1007/s11738-016-2241-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2241-4