Abstract
Water deficit stress is one of the severe limitations of crop growth especially in arid and semiarid regions of the world as it effect the plant growth at all stages of development. In the present study, four safflower genotypes Esfahan native, Esfahan-14, PI537,598 and IL111 were tested for their growth and crop yield under water-deficit stress. A detailed biochemical analysis was carried out at various levels of irrigation to find out the genotypic variation and the activity of several enzymes known to play significant role under drought stress. A split plot experiment based on randomized complete blocks design was conducted at three levels of irrigation: 100 % (normal water requirement for safflower), 75 % and 50 %. A significant increase in the activity of SOD, CAT, GPX enzymes and the levels of ABA and proline was observed with an increase in the water stress level in the leaves of all the genotypes investigated. The highest increase in the activities of antioxidant enzymes and proline and ABA content with reduced electrolyte leakage was observed in the relatively drought tolerance native Esfahan cultivar. These results suggest that the cultivars that exhibit highest levels of antioxidant enzymes activity and proline and ABA content under water deficit conditions may provide better drought tolerance in safflower.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water deficit stress severely limits crop growth especially in arid and semiarid regions of the world as it affects all stages of plant growth and development (Shamim et al. 2009). Drought stress can adversely affect root and shoot growth resulting in reduced leaf surface area (Sajedi et al. 2010). Exposure to environmental stresses such as drought stress, heat stress, cold stress, salt stress and plant diseases often leads to the production of reactive oxygen species (Hossain et al. 2009), and other toxic compounds that diminish a plant’s performance (Cheong et al. 2003). Reactive oxygen species (ROS) are highly toxic to plant cells and in an absence of any protective mechanism they can react with proteins, lipids and DNA and this can inactivate an antioxidant defense system (El-Shabrawi et al. 2010). Plants have a battery of enzymatic and non-enzymatic scavenging pathways or detoxification systems that working together function as an extremely efficient system to counter the deleterious effects of ROS (Yao et al. 2009; Hossain et al. 2010).
Higher plants have active oxygen-scavenging systems consisting of several antioxidant enzymes, such as SOD, CAT, ascorbate peroxidase and glutathione reductase and some low molecules of non-enzyme antioxidants, such as ascorbic acid and reduced glutathione (Bowler et al. 1992). Within a cell, SOD constitutes the first line of defense against ROS and MAD and has been known as the end product of peroxidation of membrane lipids. Water deficit stress that increases generation of ROS is responsible for stress-dependent peroxidation of membrane lipids (Alscher et al. 2002). The results of numerous studies suggest that alleviation of oxidative damage and increased resistance to water stress or salt stress is often correlated with more efficient antioxidant and glyoxalase systems (Aghaei et al. 2009). Accumulation of this amino acid in cells has been reported for different abiotic stresses such as drought (Hsu et al. 2003), and UV-B radiation (Chris et al. 2006). Proline synthesis has also been proposed as a non-enzyme means to scavenge OH• radicals and as an alternative sink for energy to regulate the state of cellular redox.
The plant hormone abscisic acid (ABA) plays a key role in many developmental processes and stress responses to environmental stimuli (Weiner et al. 2010). Cellular dehydration during seed maturation and vegetative growth stages induces an increase in endogenous ABA levels, which controls many dehydration-responsive genes (Yasunari et al. 2011). Increased ABA levels in response to osmotic stresses, such as drought and high salinity, induce the expression of many genes that appear to play multifaceted roles in response to dehydration and tolerance to water stress in both plant tissue and seed (Yamaguchi-Shinozaki and Shinozaki 2006). ABA-enhanced antioxidant enzyme activity induces tolerance of many crops to cold and water stresses (Zhou et al. 2005). The beneficial effects of enzymatic and non-enzymatic systems on plant growth under water deficit are yet to be investigated. Therefore, the purpose of this work was to determine the response of biochemical activities in safflower cultivars under water deficit stress.
Material and methods
Experimental procedure
A field experiment was performed at the Research Station of Islamic Azad University, Arak Branch, Iran, in 2009. The site was located at 34°59′N latitude, 49°43′E longitude at an altitude of 1,757 m above the sea level. This region has a semi-arid climate (250 mm rainfall yearly) (Table 1). Physical and chemical properties of the soil were determined from depths of 0–30 and 30–60 cm, using standard laboratory methods. The soil at the site of the experiment was clay loam with a clay type of montmorillionite, low in nitrogen (0.06–0.07 %), low in organic matter (0.56–060 %) and alkaline in reaction with a pH of 7.9 and Ec = 0.66 dSi m−1. The texture of the soil was sand/loam with 10 % neutralizing substances.
A split plot experiment based on randomized complete blocks design was conducted with four replicates. Tested in the main plots were four irrigation levels; 100 % (I1), 75 % (I2) and 50 % (I3) crop water requirement and the following safflower cultivars were tested in the sub plots; Esfahan-14 (V1), Esfahan native (V2), PI-537,598 (V3) and IL-111 (V4). Full irrigation was determined according to requirements and daily measurements of evaporation from basin pans. Daily evaporation from each basin pan was calculated by the following equation V = S × H
- V:
-
Daily evaporation from basin pan
- S:
-
Area of basin pan
- H:
-
Rate of evaporated water
Potential of evapotranspiration was obtained from a multiple of the coefficient of basin pan and evaporated water. Rate of entered water to each plot was calculated by the following equation (Alizadeh 2002).
Safflower coefficients were 0.35, 1.15 and 0.25 at initiation, middle and final growth, respectively (Alizadeh 2005). Irrigation water efficiency was taken as 80 %. Plots were irrigated using polyethylene tubes in which amounts of water were controlled using a water contour. Water consumption during the growth season of safflower were 6,200, 4,650 and 3,100 m3/ha for 100 %, 75 % and 50 % crop water requirements, respectively. Water deficit started from the initial stem stage.
Soil preparation was by plowing in the fall using perpendicular disks. Safflower seeds were planted 5 cm apart in 6-m rows, with a 60 cm spacing. To prevent any likely side effects, the two sidelines between the plots were left unplanted. Seeds were hand-sown on 5th of May 2009. Seeds of safflower (Carthamus tinctorius L.) were collected from Seed and Plant Improvement Institute, Karaj, Iran. Amounts of fertilizer were determined according to results of a soil testing analysis and one third of N and all of P were applied at seeding and the remaining N was applied twice during the vegetative stage. Ten sample plants were harvested from the middle of each plot at physiological maturity.
Enzymatic bioassay
Sampling
For the measurement of antioxidant activity and biomarker contents, three leaf samples were collected at the flowering stage from the sampled plants. They were then washed, frozen and stored using liquid N2 at −80 °C for further analyses.
Extract preparation and antioxidant bioassay
Each leaf sample (0.5 g) was ground in a mortar with a pestle in 5 ml of 50 mM phosphate buffer (pH 7.8) at 4 °C. The homogenate was centrifuged at 13,000 × g for 15 min. The supernatant was recovered for determinations of SOD and CAT activities as well as concentrations of lipid peroxides, expressed as total thiobarbituric acid-reacting substances (TBARS) as previously described (Guo et al. 2005). The 3-ml reaction solution of SOD contained 13 μM methionine, 63 μM ρ-nitro blue tetrazolium chloride, 1.3 μM riboflavin, 50 mM phosphate buffer (pH 7.8), and enzyme extract. The reaction solution was incubated for 10 min under fluorescent light with 80 μmol m−2 s−1. Absorbance was determined at 560 nm with a spectrophotometer (Model UV-2010, Hitachi, Japan). One unit of SOD activity was defined as the amount of enzyme required for inhibition of photochemical reduction of ρ-nitro blue tetrazolium chloride by 50 %. The SOD activity of an extract was expressed as SOD units per milligram of protein.
The 3-ml reaction solution of CAT contained 15 mM H2O2, 50 mM phosphate buffer (pH 7.0), and 50 μl of enzyme extracts. The reaction was initiated by adding enzyme extract. The decrease of absorbance of H2O2 (extinction coefficient 0.00394 mM−1 cm−1) within 1 min at 240 nm was recorded. One unit of CAT activity was defined as the amount of enzyme required for oxidize 1 μmol of H2O2 per minute. CAT activity of an extract was expressed as CAT units per milligram of protein.
Glutathione peroxidase (GPX) activity was measured by spectrophotometric method according to Drotar et al. (1985) with some modification using a microplate reader (Synergy HT BIO-TEK, USA). The reaction mixture (250 μl) contained 2 mM glutathione, 1 mM NADPH, 1 mM EDTA, 2 mM t-butyl hydroperoxide and 0.5 U of glutathione reductase in 100 mM sodium phosphate buffer, pH 7.0 and 10 μg of extracted proteins. The rate of NADPH oxidation was measured at 340 nm over a time period 15 min. To test the effects of peroxidase, GPX inhibitors, 1 mM azide, 10 mM mercaptosuccinic acid (MSA) or 100, μM ethacrynic acid (EA) was added to the reaction mixture.
Levels of membrane damage were determined by the MAD measurement as the end product of peroxidation of membrane lipids (De Vos et al. 1991). In brief, samples were homogenized in an aqueous solution of trichloroacetic acid (10 % w/v), and aliquots of the filtrates were heated in 0.25 % thiobarbituric acid. Amounts of MAD were determined from absorbance at 532 nm, followed by correction for non-specific absorbance at 600 nm. The concentration of MAD was determined using the extinction coefficient of MAD (ε =155 mM cm−1).
The plant tissue homogenate was centrifuged at 5,000 g for 60 min to remove debris. O,O′_Dityrosine was recovered by gradient elution from the C-18 column (Econosil C18, 250 mm × 10 mm). The composition of eluent varied linearly from acetonitrile-water-TFA (1:99:0.02) to acetonitrile-water-TFA (20:80:0.02) over 25 min. The gradient was started 5 min after the injection. A flow rate of 4 ml/min was used. O,O′_Dityrosine was analyzed by reversed-phase HPLC with simultaneous UV detection (280 nm) and fluorescence detection (ex. 280 nm, em. 410 nm). A phenomenex inertsil ODS 2 (150 mm × 4.6 mm, 5 μm) HPLC column (Bester, Amsterdam, The Netherlands), equipped with a guard column, was used for these analyses. A gradient was formed from 10 mM ammonium acetate, adjusted to pH 4.5 with acetic acid and methanol, starting with 1 % methanol with an increasing level to 10 %, over 30 min. The flow rate was 0.8 ml/min and a standard sample of dityrosine was prepared according to the method cited in Amado et al. (1984).
The free proline content was extracted from 0.5 g leaf samples in 3 % (w/v) aqueous sulphosalycylic acid and estimated using ninhydrin reagent according to the method cited in Bates et al. (1973). The absorbance of fraction with toluene aspired from the liquid phase was read at 520 nm. Proline concentration was determined using a calibration curve and expressed as μmol proline g−1 FW.
Amounts of ABA from leaf tissue were measured in crude aqueous extracts of leaf discs (20–30 mg FW) using a solid-phase radioimmunoassay based on a monoclonal antibody (DBPA1) raised against free (S)-ABA. Physicochemical methods were used to validate radioimmunoassay results with crude extracts of non-stressed and stressed bean tissue (Vernieri et al. 1989).
Electrolyte leakage was measured on the youngest fully expanded leaves at the flowering stage. Samples were harvested one day before irrigation. A special steal puncture was designed with a 30 mm inner diameter for quick punching of leaf discs. Excised samples (15 discs) were immediately placed in test tubes containing 10 cc mannitol solution having −2 bar osmotic potential. Electric conductivity of each tube was measured after 24 h at 25 ° C with a EC- meter (Aman et al. 2005). Vant Hoff formula was used to obtain mannitol solution having −2 bar osmotic potential (Martinez et al. 2004).
Statistical analysis
Data were subjected to analysis of variance using SAS and means were compared using Duncan’s multiple range test.
Results
Results indicated that water deficit stress and cultivar had significant affects on grain yield, SOD, CAT, GPX activity, MAD and DT content. Water deficit stress significantly enhanced the activity of antioxidants compared with the control treatment and resulted in significant reduction in grain yield. SOD activity ranged from 1151 to 1809 U/mg protein from the control to the highest level of drought. For CAT and GPX the corresponding ranges were 106.8 to 157.1 and 184.1 to 259.2 U/mg protein, respectively. It was also determined that, 75 % and 50 % crop water requirement decreased grain yield by 21.8 % and 9.7 % respectively compared with the control.
MDA and DT contents on the basis of μmol/mg protein fresh weight ranged from 68.21 to 48.05 and 86.82 to 66.04 μmol/mg protein, respectively. For grain yield the corresponding values were in the range of 1198 (control treatment) to 936 kg ha−1 (50 % crop water requirement). The effect of cultivar on the activity of antioxidants, MAD and DT contents and grain yield was significant.
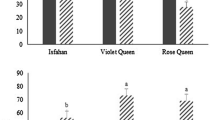
The highest records of activity of SOD, CAT, GPX were 196.6, 187.8, 260.2 U/mg protein respectively and the lowest content of MAD and DT were 54.99 and 33.90 μmol/mg protein, recorded in Esfahan native cultivar. Interestingly, it resulted in significant increase in safflower grain yield of 1402.88 kg ha−1 in Esfahan native cultivar. The lowest activity of antioxidant enzymes and the highest content biomarkers of MAD and DT were obtained from IL111 cultivar resulting in a significant reduction in safflower grain yield.
The interaction effects of water deficit stress and cultivar was significant on grain yield, and activity of CAT, GPX and DT content. Mean comparison of interaction effects indicated that, at 100 safflower water requirement, the highest activity of SOD (1,607 U/mg protein), CAT (145.9 U/mg protein), GPX (214.6 U/mg protein) and the lowest DT (42.20 μmol/mg protein) and MAD (69.07 μmol/mg protein) content were observed from Esfahan native cultivar. The same trend was observed for 75 % and 50 % water requirement treatments, demonstrating the maximum activity of antioxidant enzymes and minimum DT and MAD for Esfahan native cultivar (Table 2). In addition, at 100 %, 75 % and 50 % water requirements, the highest safflower grain yields were 1,583.05, 1,340.9 and 1,284.1 Kg ha−1 for Esfahan native cultivar, respectively (Fig. 1).
Results indicate that the effect of water deficit stress and cultivar on ABA and proline content was significant. Water deficit stress significantly enhanced ABA and proline content in leaves of the safflower plant. In water deficit stress, 50 % water requirement increased ABA and proline contents by 24.7 % and 37 % respectively, as compared with 100 % water requirement. According to mean comparison of treatments, the highest and lowest ABA and proline contents were observed from Esfahan native and IL111 cultivars, respectively. Means comparison of interaction effects indicated that although water deficit stress and cultivar did not affect ABA, the highest ABA and proline content at 100 %, 75 % and 50 % water requirement, were obtained from Esfahan native and Esfahan-14 cultivars, respectively (Table 2).
Results of this study showed significant correlation between grain yield with proline, ABA, SOD, CAT and GPX under water deficit, it also had negative correlation with MAD and DT.
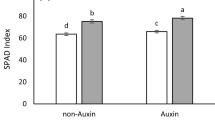
Results indicate that the effect of cultivar on cell electrolyte leakage was significant. The lowest electrolyte leakage was 2,710 μs.cm−1 obtained from Esfahan native cultivar. Electrolyte leakage in Esfahan native cultivar decreased compared with Esfahan 14, PI-537,598, IL-111 cultivars by 9.2, 9 and 19 %, respectively. Although interaction effects of treatments on electrolyte leakage were not significant, water deficit stress could increase electrolyte leakage in all of the cultivars. The lowest electrolyte leakage in full irrigation, 75 % and 50 % water requirement was from Esfahan native cultivar (Fig. 2).
Discussion
When plants are subjected to different stresses, antioxidant activity increases. To alleviate stress, by degrading the byproducts of that stress, plants produce free radicals such as superoxide and peroxide, which are not favorable to plant growth (Rahimizadeh et al. 2007). The enzymatic antioxidant system is one of these protective mechanisms that includes activity of superoxide dismutase, which can be found in various cell compartments, it catalyses the isproportion of the O2 radicals H2O2 and O2 (Hegedus et al. 2001).
According to these results, water deficit stress significantly increased the activity of SOD, CAT and GPX in leaves compared with control plants. These results are in agreement with results reported by Soleimanzadeh et al. (2010) in sunflower and Sajedi et al. (2010) in corn.
Tohidi-Moghadam et al. (2009) reported that canola (Brassica napus L.) under water deficit stress showed a significant increase in SOD, CAT and GPX activities in leaves compared with the control, and those plants with higher levels of antioxidants demonstrated better resistance to water stress and produced higher yield and dry matter allocation to the grain filling process evident from the harvest index.
It seems that an increase in SOD, CAT and GPX activities under conditions of water deficit stress could prevent oxidative damage; therefore a simultaneous increase in activity of these enzymes contributes to a decrease of deleterious effects of H2O2 under drought stress. Pasternak et al. (2005) reported that H2O2 can be removed using the ascorbate-glutathione cycle [ascorbic acid (ASA)-GSH cycle] in which APX and SOD are the key enzymes. The findings of this study are consistent with results reported by Sreenivasulu et al. (2000) in foxtail millet. Smironff and Colombe (1998) reported that MDA accumulation is often used as an indicator of lipid peroxidation. It was reported that MDA content increased in the early period of drought but then decreased. Results showed that the highest activity of antioxidant enzymes and the lowest content of MAD and DT under optimum and water deficit stress were obtained from Esfahan native cultivar. Oveysi et al. (2010) reported that drought stress via stomatal closure, reduction in leaf area and photosynthesis and a shortening of the seed filling period limited the carbohydrate supply for wheat seeds. Water deficit, will effect seed weight as one of the yield components. A decrease in grain yield can be attributed to reduced leaf expansion, reduced flux of assimilates to growing organs and accelerated leaf senescence.
According to means comparison of treatments, water deficit stress could increase proline and ABA contents in leaves. Evidence of increased proline content in leaves under water deficit stress in this research is in agreement with results of other research (Tatar and Gevrek 2008). Trotel-Aziz et al. (2003) reported that proline synthesis relies on increased transcription of D1-pyrroline-5-carboxylate synthesis and prevention of its degradation requires inactivation of the proline dehydrogenase enzyme. The beneficial role of proline in plant stress tolerance has been suggested by earlier correlative studies and was recently confirmed by genetic as well as transgenic studies, demonstrating that proline can increase a plant’s tolerance to abiotic stress. This can be partly attributed to the ability of osmolytes to scavenge reactive oxygen species, although the underlying mechanism is presently unclear (Hong et al. 2000).
Ludewing et al. (1988) reported that ABA was already shown to accumulate rapidly and to high levels in plants subjected to water and osmotic stress. Means comparison of interaction effects indicated that water deficit stress and cultivar did not affect ABA, however, under stressed conditions the highest ABA was obtained from Esfahan native and Esfahan-14 cultivars, respectively. This may indicate that plant resistance was activated due to a protective factor under water stress. In addition, the production of osmolytes and membrane stability can determine a plant species’ resistance to drought stress (Moussa and Abdel Aziz 2008). The findings of this research were consistent with results reported by Zhou et al (2005) in Stylosanthes guianensis.
The trait of electrolyte leakage showed that plants under stress conditions compared with 100 % water requirement had higher electrical conductivity that indicated low cytoplasmic membrane stability. It appears that under moderate water stress there was increased root growth in deep layers of the soil and increasing water absorption prevented severe loss of leaf water potential, and this caused less damage to cell membranes. Jabbari et al. (2006) expressed the cause of cell wall damage as a result of drought: in drought stress condition, stomatal closure reduced carbon dioxide fixation, while light dependent reactions and electron transport continued at normal levels. Under such circumstances there is a limited amount of NADP to accept electrons. So oxygen acts as an electron receptor, and this alternative response may lead to accumulation of toxic oxygen compounds such as, superoxide radicals, hydrogen peroxide and hydroxyl radicals.
Conclusion
Results indicated that water deficit stress could increase activity of SOD, CAT, GPX enzymes, ABA and proline contents in leaves of safflower but, dityrosin and malondialdehyde contents and grain yield decreased. The increase in tolerance to drought stress in Esfahan native cultivar is associated with antioxidant enzyme activity, proline and ABA contents that resulted in a significant decrease in electrolyte leakage from safflower leaves. According to these results, it may be suggested that increased activity of antioxidant enzymes and proline and ABA contents can reduce the harmful effects of reactive oxygen species and improve plant drought tolerance, therefore antioxidant enzymes and proline and ABA can be taken as indices of drought tolerance in plants.
Abbreviations
- SOD:
-
EC 1.15.1.1 Superoxide dismutase
- CAT:
-
EC 1.11.1.6 Catalase
- GPX:
-
EC 1.11.1.9 Glutathione peroxidase
- APX:
-
Ascorbate peroxidase
- MDA:
-
Malondialdehyde
- DT:
-
Dityrosine
- ABA:
-
Abscisic acid
- ROS:
-
Reactive oxygen species
References
Aghaei K, Ehsanpour AA, Komatsu S (2009) Potato responds to salt stress by increased activity of antioxidant enzymes. J Integr Plant Biol 51:1095–1103
Alizadeh A (2002) Designing of irrigation system. University of Mashhad Ferdowsi press, Iran, p 655
Alizadeh A (2005) Soil, water, plant relationship. University of Imam Reza press, Iran, p 470
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutase in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Amado R, Aeschbach R, Neukom H (1984) Dityrosine: in vitro production and characterization. Meth Enzymol 107:377–388
Aman YA, Habibi D, Mashhadi Akbar Boujar M, Khodabandeh N (2005) Antioxidant enzyme as index for select of different genotypes of sunflower for drought tolerance. Iran J Agron Plant Breed 1:1–11
Bates LS, Waldern RP, Treare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bowler C, Van Montago M, Inze D (1992) Superoxide dismutase and stress tolerance. Ann Rev Plant Physiol Plant Mol Biol 43:83–116
Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S (2003) CLB1, a calcium sensor that differentially regulates salt, drought, and cold responses in arabidopsis. Plant Cell 15:1833–1845
Chris A, Zeeshan M, Abraham G, Prasad SM (2006) Proline accumulation in Cylindrospermum sp. Environ Exp Bot 57:154–159
De Vos C, Schat H, De Waal M, Vooijs R, Ernst W (1991) Increased to copper-induced damage of the root plasma membrane in copper tolerant Silene cucubalus. Plant Physiol 82:523–528
Drotar A, Phelps P, Fall R (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Guo Z, Tan H, Zhu Z, Lu S, Zhou B (2005) Effect of intermediates on ascorbic acid and oxalate biosynthesis of rice and in relation to its stress resistance. Plant Physiol Biochem 43:955–962
Hegedus A, Erdei S, Horvath G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093
Hong JH, Choi HJ, Kang J, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53–64
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272
Hsu SY, Hsu YT, Kao CH (2003) The effect of polyethylene glycol on praline accumulation in rice leaves. Biol Plant 46:73–78
Jabbari F, Ahmadi A, Poustini K, Alhzadeh H (2006) Survey of relation activity of antioxidant enzyme with cell membrane stability and chlorophyll of bread wheat cultivars of resistance and sensitive to drought stress. J Agric Sci 2:50–56
Ludewing M, Dorffling K, Seifert H (1988) Abscisic acid and water transport in sunflowers. Planta 75:325–333
Martinez JP, Lutts S, Schank A, Bajji M, Kinet JM (2004) Is osmotic adjustment required for water stress resistance in the Meditrranean shrub Atriplex halimus L. J Plant Physiol 161:1041–1051
Moussa HR, Abdel Aziz M (2008) Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Aust J Crop Sci 1:31–36
Oveysi M, Mirhadi MJ, Madani H, Nourmohamadi G, Zarghami R, Madani A (2010) The impact of source restriction on yield formation of corn (Zea mays L.) due to water deficiency. Plant Soil Environ 56:476–481
Pasternak T, Rudas V, Potters G, Jansen MAK (2005) Morphogenic effects of abiotic stress: reorientation of growth in Arabidopsis thaliana seedlings. Environ Exp Bot 53:299–314
Rahimizadeh M, Habibi D, Madani H, Mohammadi GN, Mehraban A, Sabet AM (2007) The effect of micronutrients on antioxidant enzymes metabolism in sunflower (Helianthus annuus L.) under drought stress. Helia 30:167–174
Sajedi NA, Ardakani MR, Rejali F, Mohabbati F, Miransari M (2010) Yield and yield components of hybrid corn (Zea mays L.) as affected by mycorrhizal symbiosis and zinc sulfate under drought stress. Physiol Mol Biol Plant 16:343–351
Shamim A, Rashid A, Muhammad YA, Ashraf M, Ejaz AW (2009) Sunflower (Helianthus annuus L.) response to drought stress at germination and seedling growth stages. Pak J Bot 41:647–654
Smironff F, Colombe N (1998) Drought influences the activity of enzymes of the chloroplast hydrogen peroxide system. J Exp Bot 39:1097–1108
Soleimanzadeh H, Habibi D, Ardakani MR, Paknejad F, Rejali F (2010) Effect of potassium levels on antioxidant enzymes and malondialdehyde content under drought stress in sunflower (Helianthus annuus L.). Am J Agric Bio Sci 5:56–61
Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol Plant 109:435–442
Tatar O, Gevrek MN (2008) Influence of water stress on proline accumulation, lipid peroxidation and water content of wheat. Asian J Plant Sci 7:409–412
Tohidi-Moghadam HR, Shirani-Rad AH, Nour-Mohammadi G, Habibi D, Mashhadi-Akbar-Boojar M (2009) Effect of super absorbent application on antioxidant enzyme activities in canola (Brassica napus L.) cultivars under water stress conditions. Am J Agric Bio Sci 4:215–223
Trotel-Aziz P, Niogret MF, Deleu C, Bouchereau A, Aziz A, Larher FR (2003) The control of proline consumption by abscisic acid during osmotic stress recovery of canola leaf discs. Physiol Plant 117:213–221
Vernieri P, Perata P, Armellini D, Bugnoli M, Presentini R, Lorenzi R, Eccarelli N, Alpi A, Tognoni F (1989) Solidphase radioimmunoassay for the quantitation of abscisic acid in plant crude extracts using a new monoclonal antibody. J Plant Physiol 134:441–446
Weiner JJ, Peterson FC, Volkman BF, Cutler SR (2010) Structural and functional insights into core ABA signaling. Curr Opin Plant Biol 13:495–502
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yao X, Chu J, Wang G (2009) Effects of drought stress and selenium supply on growth and physiological characteristics of wheat seedlings. Acta Physiol Plant 31:1031–1036
Yasunari F, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajedi, N.A., Ferasat, M., Mirzakhani, M. et al. Impact of water deficit stress on biochemical characteristics of safflower cultivars. Physiol Mol Biol Plants 18, 323–329 (2012). https://doi.org/10.1007/s12298-012-0129-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-012-0129-3