Abstract
The objective of this study was to compare capacity of antioxidant enzymes between Khon Kaen 3 (KK3), a drought-tolerant, and Suphanburi 72 (SP72), a drought-sensitive sugarcane variety. Young sugarcane plants were grown under controlled conditions. Water stress was imposed at 4 leaf stage by withholding water supply for 4, 6 and 8 days as compared to control, receiving adequate water supply. First fully expanded leaves were harvested and proteins were extracted for assaying the activities of ascorbate peroxidase (APX), peroxidase (POX) and catalase (CAT). APX was a major antioxidant system in leaves of both the sugarcane varieties as it accounted for 65 % in KK3 and 69 % in SP72 in scavenging H2O2. POX accounted for 27 and 23 % in KK3 and SP72, respectively in the removal of H2O2. Negligible scavenging activities of CAT were observed in both sugarcane varieties. APX activities in KK3 leaves were induced and maintained during progressive water stress and were 15 % higher than that in SP72. POX activities in KK3 were 30 % greater than that in SP72. In conclusion, drought tolerance in KK3 was, at least partially, due to the greater activities of APX and POX under water deficit stress as compared to those of SP72.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is widely grown in rain-fed areas of tropics and subtropics (Fischer et al. 2008), where the conditions are prone to drought. Water stress is a major constraint limiting crop growth and yields, and these limitations are attributed mainly to decreased rates of photosynthesis (Chaves et al. 2009). A decline in photosynthetic capacity leads to conditions of excess light energy and increased generation and accumulation of reactive oxygen species (ROS) becomes inevitable (Asada 2006). ROS can cause extensive damages to photosynthetic machineries and other cellular components that may result in death of plant cells and tissues (Takahashi and Badger 2011; Møller et al. 2007). However, plants have evolved elaborate systems of enzymatic and non-enzymatic antioxidants that allow scavenging of ROS and protection of plant cells from oxidative damages during environmental stresses. Non-enzymatic antioxidants include lipid-soluble and membrane-associated tocopherols, water soluble ascorbic acid and glutathione. Enzymatic constituents are superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX) and glutathione reductase (Gill and Tuteja 2010). Water stress enhanced activities of POX, CAT and SOD in leaves of bread wheat (Triticum aestivum L), and greater activities of POX and CAT were found in drought-tolerant variety. However, there were non-significant differences in SOD activity between the drought-tolerant and drought-sensitive cultivars (Wu et al. 2012). In velvet bentgrass (Agrostis canina L.), a drought-tolerant cultivar, the activities of APX and CAT under water stress were greater than the control during 7–21 days of water stress and then decreased significantly on day 28 after water stress. However, SOD activities appeared to be consistently lower under progressive water stress (DaCosta and Huang 2007). In tall fescue (Festuca arundinacea Schreb.), the activity of SOD increased, while CAT and POX activities remained unchanged during most period of surface drying (Fu and Huang 2001). These indicate that the involvement of different antioxidant enzymes in scavenging ROS may vary depending on plant species and severity of water stress or stress duration. Up regulation of transcripts of some antioxidant (Kido et al. 2012) and of antioxidant proteins (Ngamhui et al. 2012) have also been reported for sugarcane. However, activities of antioxidant enzymes in different varieties of sugarcane in response to different regimes of water deficit stress have not been well documented.
The objective of this study was to compare antioxidant enzyme activities between drought-tolerant and drought-sensitive varieties of sugarcane.
Materials and methods
Plant materials and growth conditions
Three replicated experiments were carried out under controlled environments. For each set of experiments, fresh cane stalks of KhonKaen 3 (KK3), a drought-tolerant variety and Suphanburi 72 (SP72), a drought-sensitive variety were planted in plastic pots each having a diameter and depth of 25 and 28 cm, respectively, and containing 9 kg of air-dried soil. Treatments comprised of 3 levels of water deficit stress, viz., withholding water supply for 4, 6, and 8 days as compared to the adequate water supply (control) under three replications. After planting, the pots were kept in a growth chamber (Model 630, Contherm Scientific Ltd., New Zealand) with an irradiance of 720 µmol m−2 s−1, under a 12 h light/dark cycle, 35/30 °C day/night temperature, and 80 % constant relative humidity.
Water deficit stress treatment, soil and plant samplings
Soil moisture content in the pots was maintained at the full water-holding capacity by daily manual watering until the plants had four fully expanded leaves when water stress was imposed. On sampling dates, soil moisture content (SMC) in the pots was determined using a soil core sampler (Auger set for heterogeneous soil, model 08.02, Eijkelkamp, Agrisearch Equipment, Netherlands). The distal 10 cm of all leaf blades were used for measuring leaf water potential (LWP) using a pressure bomb (model 3005 HGPL, Soil Moisture Equipment Corp., Santa Barbara, CA, USA). The remaining part of first fully expanded leaves from plants in all treatments were harvested, frozen in liquid nitrogen and kept at −80 °C until protein extraction.
Enzyme extract preparation
Three hundred mg of frozen leaf material without midribs were ground in liquid nitrogen with a mortar and pestle, and further extracted with 100 mM potassium phosphate buffer, pH 7.0, containing 0.5 mM ethylenediaminetetraacetic acid disodium salt (Na2EDTA) pH 8.0 and 2 % polyvinyl pyrrolidone (PVP) at 4 °C. The homogenate was centrifuged at 12000×g at 4 °C for 30 min and supernatants used as crude enzyme extract.
Estimation of protein concentration
Total protein concentration of samples was determined using Quick Start™ Bradford Protein Assay (Bio-Rad Laboratories, Inc., CA) according to the manufacturer’s instruction using bovine serum albumin as a standard. All samples were re-suspended and left for 15 min before the absorbance was measured at 595 nm using a Thermo Scientific Genesys™ 10S UV/Visible spectrophotometer. Each protein sample was estimated in triplicate.
Assay of antioxidant enzymes
For optimization of APX and CAT activities 4, 6, 8 and 10 µg of protein ml−1 of reaction mixture, and durations for enzyme assay were maintained at 1, 2, and 3 min for APX and CAT, and 1–5 min for POX. Each enzyme assay was run in triplicate.
The results from optimization of APX, POX and CAT indicated that 4 µg proteins in 1 ml reaction mixture with 1 min reaction time were optimum for APX and CAT activities and 5 min for POX activity.
Ascorbate peroxidase activity was assayed by measuring the rate of decrease in absorbance of ascorbate at 290 nm according to a modified method of Nakano and Asada (1981). A total volume of 2.5 ml assay reaction mixture contained 100 mM potassium buffer pH 7.0, 0.1 mM Na2EDTA pH 8.0, 0.5 mM ascorbic acid, 0.1 mM hydrogen peroxide (H2O2) and 4 µg protein. The decrease in absorbance at 290 nm was recorded spectrophotometrically at an interval of 5 s up to 1 min. The background rate of non-enzymatic ascorbic acid oxidation was measured at the same time by replacing the protein extract with the extraction buffer. This rate was subtracted from the reaction rate in the presence of the protein extract to correct for the rates of non-enzymatic ascorbic acid oxidation. The extinction coefficient of 2.8 mM−1 cm−1 for ascorbic acid was used in calculating the enzyme activity.
Peroxidase was assayed according to the methods of Chance and Maehly (1955). The rate of POX activity was measured by the rate of oxidation of guaiacol to tetraguaiacol. POX activity was calculated using absorption for tetraguaiacol (extinction coefficient = 26.6 mM−1 cm−1). The activity was assayed in a 2.5 ml reaction mixture containing 100 mM potassium buffer pH 6.6, 16 mM guaiacol, 10 mM H2O2 and 4 µg protein. The increase in absorbance was recorded at an interval of 5 s up to 5 min at 470 nm. The background rate of non-enzymatic tetraguaiacol formation was measured at the same time by replacing the protein extract with the extraction buffer. This rate was subtracted from the reaction rate in the presence of the protein extract to correct for the rates of non-enzymatic tetraguaiacol formation.
Catalase activity was assayed according to Chance and Maehly (1955). The activity of CAT was determined by the decrease in absorbance at 240 nm caused by the decomposition of H2O2 (extinction coefficient = 40 mM−1 cm−1). A total volume of 2.5 ml reaction mixture contained 100 mM potassium buffer pH 7.0, 100 mM H2O2 and 4 µg protein. The decrease in absorbance was recorded at an interval of 5 s up to 1 min. The background rate of non-enzymatic H2O2 decomposition was measured at the same time by replacing the protein extract with the extraction buffer. This rate was subtracted from the reaction rate in the presence of the protein extract to correct for the rates of non-enzymatic H2O2 decomposition.
Statistical analysis
Means and standard errors were calculated with Excel 2007. The graphs were drawn using Microsoft Office 2007 software.
Results and discussion
Withholding water supply caused a significant reduction in SMCs in the pots and LWPs of sugarcane plants. SMC in KK3 pots under 4, 6, and 8 days of water holding was 5.8, 6.1 and 5.9 %, respectively as compared to 10.1, 10.8 and 10.9 % in the pots under adequate water supply sampled on the corresponding dates. Changes in SMCs in SP72 pots were similar to those in KK3 pots. SMCs in SP72 pots under water deprivation for 4, 6, and 8 days were 7.4, 6.5 and 6.4 %, respectively.
Under adequate water supply, LWPs of KK3 was maintained between −0.2 and −0.3 MPa during three dates of sampling. However, withholding water supply for 4, 6 and 8 days resulted in decline in LWPs in KK3 to −1.2, −1.4 and −1.8 MPa, respectively. LWP in SP72 under water stress for 4, 6 and 8 days decreased to −1.9, −2.0 and −2.6 MPa, respectively, while under adequate water supply on the same sampling dates, LWPs of SP72 was between −0.15 and −0.4 (Table 1). KK3, a drought-tolerant variety, had higher LWPs than SP72, a drought-sensitive variety. Drought tolerance in KK3 may be partly due into its capacity to maintain high water status under water stress. Under water deficit stress, shoot growth is more sensitive than photosynthesis and as a consequence, soluble sugars and amino acids, particularly proline, accumulate in shoot tissues (Muller et al. 2011). Accumulation of these solutes leads to osmotic adjustment, which results in increased water retention and turgor maintenance (Zhang et al. 1999).
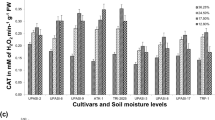
In addition to significant differences in maintaining water status between two sugarcane varieties, there were also significant changes in the activities of some antioxidant enzymes in response to water deficit. The activity of APX in leaves of KK3 after withholding water supply for 4, 6 and 8 days was 2.98, 3.18 and 3.25 µmol mg−1 protein min−1, respectively, while under adequate water supply activity was 2.39, 2.84 and 2.82 µmol mg−1 protein min−1, respectively (Fig. 1A). In SP72 activity of APXs progressively declined after 4 days of withholding water supply (Fig. 1A). There was significant increases in APX activities in leaves of SP72 in response to withholding of water for 4 and 8 days, and the activities were 2.82 and 2.63 µmol mg−1 protein min−1, respectively, whereas the activities in leaves of plants with adequate water supply were 2.46 and 2.02 µmol mg−1 protein min−1, respectively. There were non-significant changes in APX activities when watering was withheld for 6 days and the activity of APX in water-stressed and controlled plants were 2.34 and 2.29 µmol mg−1 protein min−1 (Fig. 1A). APX activity accounted for by 65 % in KK3 and 69 % in SP72 in scavenging H2O2. APX activities in KK3 leaves were induced and maintained during progressive water stress and were 15 % higher than that in SP72.
POX activities in leaves of KK3 were consistently and significantly greater than those of SP72 (Fig. 1B). POX accounted for 27 and 23 % in KK3 and SP72, respectively in the removal of H2O2. On an average, POX activity of KK3 variety was 30 % greater than that of SP72 variety. POX activities in leaves of KK3 and SP72 increased significantly when watering was withheld up to 6 days. In case of KK3 POX activity was 1.46 and 1.30 µmol mg−1 protein min−1 at 4 and 6 days, respectively. On the same sampling dates, POX activities under adequate water supply were 1.22 and 1.15 µmol mg−1 protein min−1, respectively. There were non-significant differences in POX activities in leaves of KK3 under controlled and water-stressed conditions after withholding water for 8 days and the activities were 1.12 and 1.14 µmol mg−1 protein min−1, respectively. Withholding water supply for 4 or 8 days did not have significant effect on POX activities in leaves of SP72. POX activity in controlled and water stressed plants when watering was withheld for 4 or 8 days were 0.87 and 0.90 or 0.76 and 0.82 µmol mg−1 protein min−1, respectively (Fig. 1B). APX activity in KK3 and SP72 were 2.3 and 1.4 fold greater than POX activities, respectively.
The responses of CAT activity to increasing water stress was very low in both sugarcane varieties, and on an average activity of CAT was only 14 and 12 % of APX activity under adequate water supply and water stress, respectively. Although some significant differences were detected in CAT activities in response to withholding water supply, the magnitudes of differences were so small. In KK3 and SP72, CAT activities were between 0.33 and 0.39, and 0.29 and 0.35 µmol mg−1 protein min−1, respectively (Fig. 1C).
Under water stress condition, excessive light energy invariably results in the generation of ROS in chloroplasts and over-reduced electron transport chains in mitochondria also produce superoxide anions (Mittler et al. 2004). Superoxide radicals are converted to H2O2 by SOD and H2O2 needs to be scavenged to avoid its use in the formation of highly toxic hydroxyl radicals via the metal-dependent Fenton reaction (Mittler et al. 2004). APXs, glutathione peroxidases (GPXs), peroxiredoxins (PrxRs), and CAT detoxify H2O2. APX operate in ascorbate–glutathione cycle in chloroplasts, cytosol, mitochondria, apoplast and peroxisomes. In two sugarcane varieties, APX had the highest activity among H2O2 scavenging enzymes and the activity increased under water stress, suggesting that APX networks were inducible. The activity of APX accounted for more than 65 % in both sugarcane varieties. During C3 photosynthesis, major sources of H2O2 generation are chloroplasts, peroxisomes (Foyer and Noctor 2003) and mitochondria (Rasmusson et al. 1998). However, in C4 photosynthesis, photorespiration could be negligible (Edwards and Walker 1983), and the major sites of H2O2 production are chloroplasts and mitochondria. As extensive generation of H2O2 occurs in photosynthetic tissues, and the activity of APX is induced by water stress, it is most likely that networks of APX could be a major system responsible for removing H2O2 in sugarcane leaves. Greater activity of APX in KK3 than SP72 suggests that drought tolerance may be associated with greater capacity of APX networks. POX activity was only 42 and 34 % of APX activity in KK3 and SP72, respectively, and accounted for only 23 to 27 % in two sugarcane varieties, suggesting that APX could be predominant networks, while POX could be a minor system for scavenging H2O2 in sugarcane leaves. Activity of APX is regulated at transcriptional and post-translational levels. Water stress up-regulated the expression of APX genes (Sečenji et al. 2010). Post-translational modification of APX occurred by S-nitrosylation, and salinity stress increased both APX activity and S-nitrosylated APX (Begara-Morales et al. 2014). CAT activity was the lowest among H2O2 scavenging enzymes in sugarcane leaves. As sugarcane is a C4 plant, photorespiration is minimized by CO2 concentration mechanism within bundle sheath cells (Edwards and Walker 1983), thereby minimizing the requirement of CAT. A decrease in CAT activity under water stress could be accounted for by an increase in activity of APX.
References
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology, 141(2), 391–396.
Begara-Morales, J. C., Sánchez-Calvo, B., Chaki, M., Valderrama, R., Mata-Pérez, C., López-Jaramillo, J., et al. (2014). Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. Journal of Experimental Botany, 65(2), 527–538.
Chance, B., & Maehly, A. C. (1955). Assay of catalases and peroxidases. Methods in Enzymology, 2, 764–775.
Chaves, M. M., Flexas, J., & Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, 103(4), 551–560.
DaCosta, M., & Huang, B. (2007). Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. Journal of the American Society for Horticultural Science, 132(3), 319–326.
Edwards, G., & Walker, D. (1983). C 3 , C 4 : Mechanisms, cellular and environmental regulation of photosynthesis. Berkeley: University of California Press.
Fischer, G., Nachtergaele, F., Prieler, F., Teiseira, E., van Velthuizen, H. T., Verelst, L., & Wiberg, D. (2008). Global agro-ecological zones assessment for agriculture (GAEZ 2008). Laxenberg: IIASA.
Foyer, C. H., & Noctor, G. (2003). Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum, 119(3), 355–364.
Fu, J., & Huang, B. (2001). Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environmental and Experimental Botany, 45(2), 105–114.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930.
Kido, É. A., Ferreira Neto, J. R. C., de Silva, R. L. O., Pandolfi, V., Guimarães, A. C. R., Veiga, D. T., et al. (2012). New insights in the sugarcane transcriptome responding to drought stress as revealed by supersage. The Scientific World Journal,. doi:10.1100/2012/821062.
Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in Plant Science, 9(10), 490–498.
Møller, I. M., Jensen, P. E., & Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annual Review of Plant Biology, 58(1), 459–481.
Muller, B., Pantin, F., Génard, M., Turc, O., Freixes, S., Piques, M., & Gibon, Y. (2011). Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Botany, 62(6), 1715–1729.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22(5), 867–880.
Ngamhui, N., Akkasaeng, C., Zhu, Y. J., Tantisuwichwong, N., Roytrakul, S., & Sansayawichai, T. (2012). Differentially expressed proteins in sugarcane leaves in response to water deficit stress. Plant Omics, 5(4), 365–371.
Rasmusson, A. G., Heiser, V., Zabaleta, E., Brennicke, A., & Grohmann, L. (1998). Physiological, biochemical and molecular aspects of mitochondrial complex I in plants. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1364(2), 101–111.
Sečenji, M., Hideg, É., Bebes, A., & Györgyey, J. (2010). Transcriptional differences in gene families of the ascorbate–glutathione cycle in wheat during mild water deficit. Plant Cell Reports, 29(1), 37–50.
Takahashi, S., & Badger, M. R. (2011). Photoprotection in plants: A new light on photosystem II damage. Trends in Plant Science, 16(1), 53–60.
Wu, G. Q., Zhang, L. N., & Wang, Y. Y. (2012). Response of growth and antioxidant enzymes to osmotic stress in two different wheat (Triticum aestivum L.) cultivars seedlings. Plant Soil and Environment, 58, 534–539.
Zhang, J., Nguyen, H. T., & Blum, A. (1999). Genetic analysis of osmotic adjustment in crop plants. Journal of Experimental Botany, 50(332), 291–302.
Acknowledgments
The authors are grateful for funding provided by Royal Golden Jubilee Ph.D. Program (RGJ), Thailand Research Fund (Grant No. PHD/0117/2551). N. Ngamhui was a RGJ scholarship recipient. We are thankful to Mrs. Taksina Sansayawichai, Ms. Amarawan Tippayarak (KhonKaen Field Crops Research Center) and Mr. Manit Suknimit (Suphan Buri Agricultural Research and Development Center, Office of Agriculture Research and Development Region 5) for their kindness providing all knowledge about sugarcane and sugarcane stalks that were used in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ngamhui, N., Tantisuwichwong, N., Roytrakul, S. et al. Relationship between drought tolerance with activities of antioxidant enzymes in sugarcane. Ind J Plant Physiol. 20, 145–150 (2015). https://doi.org/10.1007/s40502-015-0155-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-015-0155-6