Abstract
The effects of several silicates (talcum powder (TP), calcium silicate (CS), sodium silicate (SS), and potassium silicate (PS)), in comparison with other amendments (quicklime (QL) and potassium dihydrogen phosphate (PDP)) on cadmium (Cd) uptake by three dicotyledonous crops (Amaranthus hypochondriacus L. Cv. ‘K112’, Amaranthus tricolor L., and Brassica oleracea var. albiflora Kuntze) were investigated in Cd–contaminated soil. The effects of both application methods of amendments (singly and combined) and timing of application were also evaluated. Sodium silicate was the most effective in reducing crop Cd uptake and translocation, which was diminished by 51 % in roots, 53 % in stems, and 72 % in leaves on average. Application of CS amendment showed greater efficiency than PDP amendment in decreasing Cd uptake by crops and resulted in increased biomass. Potassium silicate only slightly decreased shoot Cd concentration. Combination of PDP and SS was able to overcome the inhibitory effect of SS on crop yield while decreasing Cd concentrations in roots, stems and leaves of the tested crops by average rates of 52, 65, and 68 % respectively. Applications of SS and PS significantly reduced the root-to-shoot Cd transfer factor. We found that Si accumulation in crops was not associated with lower Cd concentration, indicating that Si in crops may play a major role in alleviating metal stress rather than inhibiting crop Cd accumulation. We suggested that the inhibitive effect of silicates on crops Cd uptake was majorly attributed to the properties of the silicates, those were their specific effects on soil pH and cations, which increased Cd adsorption by soil and suppressed Cd uptake from soil solution by increasing the relative dissolved concentrations of competing cations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd), one of the most toxic heavy metals, is listed in the top 10 of the 2011 priority hazardous substances by the American Agency for Toxic Substance and Disease Registry (ATSDR 2011). High levels of Cd can occur in soils either naturally as a consequence of Cd-rich parent materials or because of anthropogenic activities such as mining, smelting, solid-waste disposal, application of phosphate fertilizers or sewage sludge, and atmospheric deposition (from waste incineration, fossil fuel combustion) (Assche 1998; Boularbah et al. 2006; Sun et al. 2008). Cadmium has relatively high mobility in the environment resulting in higher bioavailability for plant uptake than other metals. Consequently, Cd poses a greater threat than most toxic metals to the food chain (Kabata and Pendias 2001). A variety of studies have reported that food crops are capable of accumulating relatively high levels of Cd from soil (Chen et al. 1999; Cobb et al. 2000; Zhuang et al. 2009), such as amaranth (Chunilall et al. 2005; Fan and Zhou 2009; Li et al. 2012) and Chinese kale (Jinadasa et al. 1997; Moir and Thornton 1989; Tan et al. 2011). People who consume food crops grown in Cd contaminated soil are at risk of an elevated Cd exposure. Therefore, it is important to control the Cd content in crops to ensure food safety.
Different actions can be undertaken to reduce or eliminate the accumulation of Cd by plants. In contaminated farmlands, in situ immobilization of heavy metals using different soil amendments is a cost-effective strategy to alleviate soil heavy metal pollution (Lee et al. 2009; McGowen et al. 2001). Potentially effective amendments include alkaline substances (e.g., lime, fly ash, calcium carbonate, and manganese oxide), phosphates (e.g., diammonium phosphate, phosphate rock, hydroxyapatite), organic material (e.g., green manure, animal excrement, and peat), and other plant fertilizers (Sarwar et al. 2010). However, these amendments may not be effective in all cases. For example, neither lime (Bolan et al. 2003; Maier et al. 1997) nor phosphate (Hong et al. 2008; Tan et al. 2011) has a consistently positive effect on soil Cd immobilization. There is still a need for a range of more efficient and economical approaches for coping with metal toxicity in plants that may occur in large areas.
Several siliceous materials have been applied to in situ stabilization of Cd in soils, including sodium silicate (Feng et al. 2010; Nwugo and Huerta 2008a), potassium silicates (Shi et al. 2010; Zhang et al. 2008), and some silicon-rich materials like steel sludge and furnace slag (Chen et al. 2000; Gu et al. 2011). Numerous studies have demonstrated that Si application can enhance resistance and tolerance to Cd in graminaceous plants such as rice (Nwugo and Huerta 2008b) and maize (Vaculik et al. 2009) that are well known as Si-accumulators. The possible mechanisms for inhibition of Cd transport in Gramineae plants mediated by Si include (1) restrain the apoplasmic transport of Cd by depositing Si on the surface of the cell wall of epidermis and/or endodermis (Shi et al. 2010). Thickening of the Casparian strips and the cell wall of xylem and pericycle may occur after Si deposition in the endodermis (da Cunha amd do Nascimento 2009). Silicon might be attributed to the enhancement of root apoplasmic barrier development by accelerating suberin lamellae deposition and enhancing the tertiary endodermal cell walls formation (Lukacova et al. 2013; Vaculik et al. 2009); (2) precipitation of Si metal complex in the cytoplasm and vacuoles (da Cunha amd do Nascimento 2009). In contrast, less work has been done on the possible role of Si in dicots that are rather poor Si accumulators (less than 1 % of the dry weight) (Neumann and zur Nieden 2001; Treder and Cieslinski 2005).
In this study, we evaluated the effects of several silicates on Cd uptake by crops in comparison to traditional lime and phosphate amendments. Most studies emphasized the interaction of silicon and phosphorus on plants growth (Ma and Takahashi 1990; Rothbuhr and Scott 1957), but rarely studied their interaction with respect to Cd uptake by plants. In addition, there is lack of understanding of the optimal timing of application of amendments to the soil in relation to preventing excessive Cd uptake by plants (Treder and Cieslinski 2005). Three commonly grown dicotyledonous crops, including grain amaranth (Amaranthus hypochondriacus L. Cv. ‘K112’), red amaranth (Amaranthus tricolor L.) and Chinese kale (Brassica oleracea var. albiflora Kuntze), being the food source with high health risk posed by Cd were selected. The aims of this research are to: (1) investigate the effects of several low-cost silicates on Cd immobilization in Cd-contaminated soil and on reduction of Cd uptake by dicotyledonous crops, (2) identify the possible mechanisms involved in silicate-mediated inhibition of Cd uptake by dicotyledonous crops, (3) examine the effect of placement method (alone or in combination) and timing of application of silicates on Cd transfer from soil to plants, and (4) study Si and P interactions with respect to Cd plant uptake.
Materials and methods
Pot experiments
The soil was collected from the surface layer (0–20 cm) of a vegetable garden, near a waste landfill site in the suburb of Guangzhou, China. The soil was air-dried, crushed, mixed thoroughly, and sieved to 1 cm. Chemical properties of the soil were: soil pH 6.3, organic matter 4.7 %, cation exchange capacity (CEC) 13 cmol kg−1, available Si 82 mg kg−1, available P 122 mg kg−1, and total Cd 6.1 mg kg−1. Pot experiments were set up outdoors in the South China Botanical Garden (Guangzhou, China) beginning in January 2011. The experiment was coincident with the dry season.
-
Experiment 1:
Contaminated soil (7.5 kg per pot) was transferred into each of 84 plastic pots (35 cm diameter × 20 cm deep). Basic fertilizers were applied at a rate of 0.2 g kg−1 N and 0.2 g kg−1 K2O soil by adding 2.26 g urea and 3.22 g KNO3 per pot. The amendments included four silicates (talc, calcium silicate, sodium silicate and potassium silicate), phosphate, lime, and control. Non-amended treatment was used as the control. Talc, calcium silicate, and potassium silicate were added at the rate equivalent to the same Si content of sodium silicate. The doses of all soil amendments and timing of application are shown in Table 1. All the amendments were firstly ground into powder. The amendments were separately mixed with the soils to obtain homogeneity and were then equilibrated for 10 days with constant water status (80 % of field capacity). During the incubation period, the soils were thoroughly mixed every 3 days. After soil incubation, 20 seeds of crops (grain amaranth, red amaranth and Chinese kale) were initially sowed to each of the pots and later shinned to six uniform seedlings (2 cm high).
Table 1 Treatments and material of different amendments used in pot experiment -
Experiment 2:
This experiment was performed to study the effect of combined application of silicate (both sodium and potassium) with phosphate and timing of application of amendments on the inhibition of Cd uptake by plants. As shown in Table 1, the combined amendments were added at two different stages, including before sowing (the same as experiment 1) and after 30 days of growth. Grain amaranth and red amaranth were initially sown in each of the amended pots and later shinned to six uniform seedlings.
All treatments were replicated four times. All pots were kept outdoors and regularly watered to keep soil water holding capacity at a level between 70 and 90 %. The crops were harvested for analysis after a 60-day period of growth.
Sample analysis
Soil pH values were measured using a pH meter (Mettler Toledo FE20) with a water solid ratio of 2.5:1, while available soil Cd was determined using 0.1 M CaCl2 solution (McLaughlin et al. 2000) on the day before sowing.
The crops were harvested and separated into roots, stems, and leaves, and rinsed with distilled water. The samples were oven-dried for 72 h at 70 °C, weighed, and ground to pass a 100-mesh sieve. After digestion of the samples in HNO3-HClO4 (4:1), Cd concentrations in the plant digests were determined using flame atomic absorption spectrometry (FAAS, Hitachi Z-5300). Concentrations of Ca2+, Mg2+, K+, Cu2+, Zn2+, and Mn2+ in the digests were measured by inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 2000). Silicon concentrations in the plants were determined by gravimetric method (Dong 1997). To ensure the precision of analytical procedures, a national standard plant material (poplar leaf GBW07604) was used and blanks were also included in digestion batches.
Statistical analysis
Data from plant and soil samples were statistically analyzed using one-way ANOVA at a significance level of p < 0.05 using SPSS 11.6 software. Duncan’s new multiple range test was used to detect any significant differences between means of different treatments. Simple correlation analysis and linear regression analysis were used to test the relation between soil pH and available Cd.
Results
The effect of single amendments on crop Cd uptake
Plant growth and biomass production
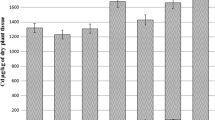
Plants did not show obvious Cd toxicity symptoms such as deformation or yellowing or leaf senescence. The effects of different amendment (talcum powder (TP), calcium silicate (CS), sodium silicate (SS), potassium silicate (PS), quicklime (QL), and potassium dihydrogen phosphate (PDP)) on biomass yields of grain amaranth, red amaranth, and Chinese kale are shown in Fig. 1. The results showed that, in most cases, application of TP, SS, and QL reduced the dry biomass of tested crops. Compared to the control (non-amended plants), addition of SS markedly reduced root and shoot dry weight of grain amaranth by 66 and 56 %, respectively. However, the root and shoot biomass of both red amaranth and Chinese kale showed no statistically significant differences between the SS treatment and the control. Treatment with CS increased biomass of the three crops, having an effect on biomass similar to that of the PDP treatment.
Dry weight of crops under different treatments. Error bars represent ±SE of quadruplicates. Bars with the same letter within a parameter are not significantly different (p > 0.05). CK non-amended treatment, TP talcum powder, CS calcium silicate, SS, sodium silicate, PS potassium silicate, QL quicklime, PDP potassium dihydrogen phosphate
Cadmium accumulation in plants
The magnitude of amendment effects on Cd concentration in the three crops followed the order: SS > QL > CS ≥ PDP > PS ≈ CK ≥ TP (Fig. 2). Thus, SS was the most effective of the six amendments in reducing Cd in crops, with the average reductions in root, stem, and leaf concentrations of the three crops being 51, 53, and 72 %, respectively, when compared to the control crops (no amendment treated crops). The effect of QL on Cd uptake in grain amaranth was similar to that of the SS treatment, with the reduction in root, stem, and leaf concentrations being 52, 74, and 74 %, respectively. However, the relative Cd reductions in shoots of red amaranth and Chinese kale in response to the QL treatment were about half that of the SS amendment. The application of CS also decreased Cd concentration in the three tested crops as compared to the control, with the greatest reduction of 45 % in leaves of grain amaranth. Phosphate treatment (PDP) had similar effect to CS treatment on reducing shoot Cd concentrations, while the opposite trend was observed for roots.
Cadmium transfer factors in plants
The transfer factors of Cd from root to shoot (TF) of the three crops under different treatments are shown in Table 2. Results showed that all the TF values depended on treatments and crop species. Among the four treatments with silicates, SS and PS treatments greatly reduced TF of all the crops. For red amaranth, the TF was significantly lower (0.91) in the SS treatment than that in the control (2.5). Application of PS reduced the TF value of Chinese kale from 0.98 (control) to 0.44. The CS treatment reduced TF values for grain amaranth only. By contrast, the addition of TP did not significantly change the TF value of any of the tested crops. On the other hand, the effect of PDP was similar to that of PS. QL treatment affected TF differently in the three crops.
Silicon accumulation in crop leaves
Grain amaranth and red amaranth accumulated much more Si in leaves than Chinese kale (Fig. 3). Silicon concentration in the crops was influenced by the type of amendments. Except for impacts of QL and CS treatment on Si in leaves of Chinese kale, application of the amendments increased Si concentration in the leaves of the crops. Addition of SS resulted in the highest Si concentration in the leaves of grain amaranth, which was 1.8-fold higher than that of the control. In Chinese kale, the highest Si concentration was observed with the PS amendment, which was 4.4-fold higher than that of the control. Treatment of PDP ranked first (in red amaranth) or second (in grain amaranth and Chinese kale) in increasing Si accumulation.
Bioavailability of mineral nutrients and ratios of bioavailable nutrient concentrations to Cd
Table 3 shows the concentrations of six mineral nutrients (Ca, Mg, K, Cu, Zn, Mn) in leaves of grain amaranth and Chinese kale. Result showed that application of the amendments affected the bioavailability of mineral nutrients. However, macronutrients (Ca, Mg, K) were less affected by the amendments than micronutrients (Cu, Zn, Mn), except for the SS treatment. In most cases, added CS, SS, and QL into the soil significantly decreased Cu, Zn, and Mn uptake by crops. Meanwhile, Cu, Zn, and Mn concentrations were increased by PS treatment in Chinese kale but decreased in grain amaranth.
Applications of amendments affected the concentration ratios of nutrients (Ca, Mg, K, Cu, Zn, Mn)/Cd in leaves of the crops (Fig. 4). Compared to the control, application of SS and QL amendments significantly increased Ca/Cd, Mg/Cd, K/Cd, and Cu/Cd ratios and decreased Zn/Cd and Mn/Cd ratios in leaves of the crops. Similar trends were obtained in the crops under CS amendment, but with smaller changes in Ca/Cd, Mg/Cd, K/Cd, Cu/Cd, and Zn/Cd ratios. On the other hand, both the applications of PS amendment and PDP amendment significantly increased the K/Cd and Mn/Cd ratios, while the TP treatment did not affect any of the concentration ratios of nutrients/Cd.
The effect of placement method and timing of application
Plant growth and biomass
Figure 5 shows the effects of combined application of SS + PDP and PS + PDP on the biomass of grain amaranth and red amaranth. Soil amendment with SS + PDP applied before sowing had no significant effect on dry biomass of either crops, whereas PS + PDP significantly increased root and shoot dry weight of red amaranth and grain amaranth by 60–67 and 30–36 %, respectively, as compared to the control. After 30 days of growth, SS + PDP (30 days) applied to the soil decreased biomass of both crops, whereas PS + PDP (30 days) performed differently, increasing biomass of grain amaranth and decreasing biomass of red amaranth.
Dry weight of grain amaranth and red amaranth. Error bars represent ±SE of quadruplicates. CK non-amended treatment, SS + PDP SS and PDP composition added before sowing, PS + PDP PS and PDP composition added before sowing, SS + PDP (30 days) SS + PDP added in 30 days after sowing, PS + PDP (30 days) PS + PDP added in 30 days after sowing
Cadmium concentration in grain amaranth and red amaranth
As shown in Fig. 6, the two types of combined amendments significantly reduced Cd concentration in the shoots of grain amaranth and red amaranth. Compared to the control, application of SS + PDP was more efficient in decreasing Cd concentration in the crops than PS + PDP, with average Cd reductions of 52 % for roots, 65 % for stems and 68 % for leaves. The application of SS + PDP (30 days) decreased Cd uptake by the crops, but the decrease was less than for the same amendments applied at the beginning. Application of PS + PDP (30 days) decreased Cd accumulation in shoots of the crops; again, the decrease was less than when the amendments were applied at the beginning.
Cadmium accumulation in grain amaranth and red amaranth. Error bars represent ±SE of quadruplicates. CK non-amended treatment; SS + PDP SS and PDP composition added before sowing, PS + PDP PS and PDP composition added before sowing, SS + PDP (30 day) SS + PDP added at 30 days after sowing, PS + PDP (30 days) PS + PDP added at 30 days after sowing
Effects of amendments on soil pH and available Cd
The pH values and the concentrations of available Cd of the soils are shown in Table 4. Soil pH increased from 6.3 in the non-amended soil to 7.7 in QL-treated soil, while available Cd in the soil decreased from 1.7 for non-amended control to 0.31 for the QL treatment. Application of amendments significantly increased soil pH and decreased the concentration of available Cd, with the exception of PDP. One week after the amendments were added, soil pH values followed the order: QL ≈ SS > SS + PDP > CS ≥ PS ≈ PS + PDP > TP ≈ CK ≈ PDP. The soil concentration of available Cd followed the order: QL < SS < SS + PDP < CS ≤ PS ≈ TP < PS + PDP < CK.
Correlation analysis indicated significant effects of soil pH on soil available Cd (r = −0.929, P < 0.001). The correlation between pH and available Cd can be described by the following regression equation (Fig. 7).
Discussion
It has been suggested that Si has a positive effect on growth and biomass of Si accumulators such as rice (Zhang et al. 2008) and maize (Liang et al. 2005) grown in both soil and hydroponic media. However, the results of the present study showed that Si application (except for CS (calcium silicate)) did not increase the biomass of any of the three tested dicotyledons species. This agrees with other studies showing no growth promoting effects in leafy vegetables (Chinese cabbage and lettuce) (Wang et al. 2012) and sweet basil (Putwattana et al. 2010) grown in contaminated soil. Moreover, the effect of amendments on crop yield differed with plant genus. Amaranthaceae crops, especially red amaranth, were more sensitive to the effects of the different amendments than Chinese kale (Fig. 1). Great differences in response to the application of Si have been observed not only between species but also within species. For example, Kulikova and Lux (2010) studied the effect of Si on five Zea mays L. hybrids, and found that growth promoting effects on shoots and roots in the Si + Cd treatment was only achieved for one hybrid. Thus, Si-mediated enhancement of plant biomass production is not a universal phenomenon in either Si accumulators or non-Si accumulators including dicotyledonous plants.
Different silicates used in this experiment showed various effects in reducing Cd accumulation by crops (Fig. 2). Among the four tested silicates, SS (sodium silicate) was the most effective amendment in decreasing Cd concentrations in plants, which agrees well with studies finding a decrease in both root and shoot Cd accumulation in rice using Na2Si3O7 treatment under hydroponic condition (Nwugo and Huerta 2008b) and in maize by adding Na2SiO3·9H2O to soil (Liang et al. 2005). Liming is a well-known and proven practice for controlling uptake of Cd by plants (Bolan and Duraisamy 2003). However, in this experiment, liming was not as effective as the SS treatment, with the decrease in Cd concentrations of red amaranth and Chinese kale by liming being about half that achieved by the SS treatment (Fig. 2). Calcium silicate had a somewhat smaller effect (16–43 %) than the Cd reduction (38–60 %) in grain and straw of rice reported by Li et al. (2008). Cadmium concentrations in the PS (potassium silicate) treated crops were consistent with reports that application of PS increased Cd retention in roots (endodermis and epidermis) while reducing Cd translocation to shoots in strawberry grown in soil (Treder and Cieslinski 2005) and pakchoi (Brassica chinensis L.) cultivated in hydroponics (Song et al. 2009).
An important finding of this study is that higher Si in crops did not always result in lower crops Cd concentration, indicating that greater Si accumulation in plants did not directly contribute to lower Cd accumulation. For example, the PS amendment resulted in the highest Si concentrations in leaves of Chinese kale, but did not decrease the Cd concentration in leaves and even increased the Cd concentrations in roots by two fold, as compared to the control (Fig. 3). We suggest that Si in the dicotyledonous crops played a greater role in alleviating metal stress than in restricting Cd transport from soil to plants. da Cunha and do Nascimento (2009) also found not only deposition of both silica and Cd in the cell wall of the epidermis, endodermis, pericycle, and xylem of roots and in mesophyll cell wall of leaves in maize but also higher Cd accumulation after Si addition to soil, suggesting that Si played a major physiological role in alleviating Cd stress and achieving cell detoxification in maize by co-precipitation of a Si metal complex.
Several studies have suggested that Si could restrict the transport of Cd from roots to shoots by retaining Cd in the root, a mechanism which may function not only in monocotyledon species such as rice (Zhang et al. 2008) and wheat (Rizwan et al. 2012) but also in dicotyledonous species such as peanut (Shi et al. 2010) and pak choi (Song et al. 2009). We found not only higher Si concentrations, but also lower TF values in PS or SS treated crops than in the control (Table 2). It is claimed that Si bound to the cell walls exhibits a high affinity for Cd (Wang et al. 2000). We suggest that Cd silicate precipitation may be promoted in root cells of the silicate-treated plants due to the relatively high Cd and silicate concentrations in the root solution. Furthermore, application of silicate may increase phosphate uptake by plants, as the addition of silicates to soil can increase phosphate desorption by competition (Lee et al. 2004; Roy et al. 1971) due to the similar chemical structures and properties of orthosilicic acid and orthophosphoric acid (Obihara and Russell 1972). This interaction of silicate and phosphate has occurred in our study, as increased Si concentrations were observed in leaves of the crops receiving PDP (potassium dihydrogen phosphate) treatments. High phosphate supply induces plants to form inositol phosphates which is then stored in the roots. Inositol phosphates complex strongly with heavy metals such as Cd, Cu, and Zn due to their anion charge (Persson et al. 1998; Turner et al. 2002), and thus may reduce the transport of Cd from root to plant top.
We thus conclude that the inhibitive effect of silicate on Cd uptake by crops is substantially indirect, that is, dependent on their initial effect on soil, including increased soil pH and introduction of relatively high concentrations of competing cations into soil solution. These changes in soil chemistry induce Cd adsorption and reduce Cd competitiveness for plant uptake.
Soil pH is considered a critical factor controlling the mobility of Cd in soils (Bolton and Evans 1996; Eriksson 1989; Li et al. 2008) and affecting plant Cd uptake (Singh and Myhr 1998). Results from this study showed that SS was the most effective in reducing Cd bioavailability compared to other silicates; this is likely due to a substantial soil pH increase (from 6.3 to 7.7) after the application of SS (Table 4). The correlation analysis between soil available Cd and soil pH (Equation 1) also showed that soil pH increase was a major reason for the reduction in soil available Cd, a result consistent with the reports of Chen et al. (2000) and Liang et al. (2005).
Soil pH changes could strongly affect available Cd and absorption of cations in the soil. Different metal ions have adsorption curves uniquely dependent on soil pH due to their different chemical properties (Gomes et al. 2001; Li 2001). Thus, modifying soil pH could also change the adsorption capacities of different metal ions and their concentrations in soil solution. Results showed that the application of amendments not only affected the bioavailable Cd, but also affected the bioavailability of mineral nutrients (Table 3). Plant nutrients are not only required for better plant growth and development, but also helpful to alleviate heavy metal stress. Significant change in ratios of bioavailable mineral nutrient concentrations to Cd (Ca/Cd, Mg/Cd, K/Cd, Cu/Cd, Zn/Cd, Mn/Cd) resulted from the soil amendments (Fig. 4). We suggest that changes in the bioavailable mineral nutrient status in the soil induced by modification of soil pH and exogenous addition of nutrients is another factor that directly affects Cd uptake by plants. Increasing the bioavailable nutrient/Cd ratios in soil (e.g., Ca/Cd, Mg/Cd, K/Cd and Cu/Cd) could result in lower Cd uptake by plants due to ion competition. Song et al. suggested that the main reason that Si reduced uptake and transport of Cd in maize and rice could be that Si enhanced uptake of Ca ions into plants (Song et al. 2009). In our study, much higher bioavailable Ca/Cd, Mg/Cd, K/Cd, and Cu/Cd ratios under SS or CS treatment were found, leading to suppression of Cd uptake by more cations competing for exchange sites with Cd ions at the root surface (Bolan et al. 2003). Although the bioavailable Zn/Cd and Mn/Cd ratios decreased in the silicate-amended soils, competition of these micronutrients with Cd is probably much weaker than that of the macronutrients.
Our results are consistent with other research showing that the combined application of silicate and phosphate to soil could promote plant growth as a consequence of the interaction of silicate and phosphate (Ma and Takahashi 1990). The combined application of SS and PDP amendment in this study was not only as effective as the sole application of SS amendment in decreasing Cd uptake by crops, but also solved the inhibitory effect on crop yield caused by the sole addition of SS amendment. The application of PS + PDP treatment also showed a significant effect in decreasing shoot Cd concentration and increasing biomass of crops: the combined treatment was more effective than the sole application of PS or PDP. The combined application of silicates and phosphate might be beneficial to nutrient balance by both having an inhibitory effect on Cd uptake and improving the fertilizer effect on crop growth. However, the application of silicates with phosphate (30 days after sowing) was not a more effective practice to reduce Cd uptake than application before sowing (Fig. 6). This phenomenon is probably caused by the facts that the more sensibility of crops to the effect of the amendments added after sowing (for 30 days) and full penetration and absorption among soil, crops, and amendments when Si were added 10 days before sowing. These results indicated that timing of application was one of the important factors influencing the effect on Cd uptake by plants.
Conclusion
The maximum reduction of Cd accumulation in plants was observed with the SS treatment. The application of SS combined with PDP before sowing not only decreased Cd uptake by crops but also overcame the inhibitory effect of SS on crop yield. Thus, optimum placement and timing of amendments can enhance Cd retention in soil and improve plant growth. Higher Si in crops did not resulted in lower Cd concentrations, however, application of SS and PS restricted Cd transport from root to shoot, suggesting that the beneficial role of Si may be related more to alleviation of metal stress than to the inhibition of Cd uptake by plants. The effect of the specific silicates on soil pH and concentrations of competitive cations in the soil solution are important in governing soil Cd availability in soil.
References
Assche FV (1998) The relative contribution of different environmental sources to human cadmium exposure and the EU cadmium risk assessmen. NiCad 98, Prague, Czech Republic, September 21–22
ATSDR (2011) 2011 priority list of hazardous substances that will be the subjuct of toxicological profiles (Detailed Data Table). Agency for Toxic Substances and Disease Registry, Atlanta
Bolan NS, Adriano DC, Mani PA, Duraisamy A (2003) Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 251:187–198
Bolan NS, Duraisamy VP (2003) Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: a review involving specific case studies. Aust J Soil Res 41:533–555
Bolton KA, Evans LJ (1996) Cadmium adsorption capacity of selected Ontario soils. Can J Soil Sci 76:183–189
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, Morel JL (2006) Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817
Chen HM, Zheng CR, Tu C, Zhu YG (1999) Heavy metal pollution in soils in China: status and countermeasures. Ambio 28:130–134
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234
Chunilall V, Kindness A, Jonnalagadda SB (2005) Heavy metal uptake by two edible Amaranthus herbs grown on soils contaminated with lead, mercury, cadmium, and nickel. J Environ Sci Health B 40:375–384
Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E (2000) Accumulation of heavy metals by vegetables grown in mine wastes. Environ Toxicol Chem 19:600–607
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197:323–330
Dong M (1997) Survey, Obervation and Analysis of Terrestrial Biocommunities. Standard Press of China, Beijing, p 163 (in Chinese)
Eriksson JE (1989) The influence of pH, soil type and time on adsorbtion and uptake by plants of Cd added to the soil. Water Air Soil Pollut 48:317–335
Fan HL, Zhou W (2009) Screening of amaranth cultivars (Amaranthus mangostanus L.) for cadmium hyperaccumulation. Agr Sci China 8:342–351
Feng JP, Shi QH, Wang XF, Wei M, Yang FJ, Xu HN (2010) Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci Hortic-Amsterdam 123:521–530
Gomes PC, Fontes MPF, da Silva AG, Mendonca ED, Netto AR (2001) Selectivity sequence and competitive adsorption of heavy metals by Brazilian soils. Soil Sci Soc Am J 65:1115–1121
Gu HH, Qiu H, Tian T, Zhan SS, Deng THB, Chaney RL, Wang SZ, Tang YT, Morel JL, Qiu RL (2011) Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere 83:1234–1240
Hong CO, Lee DK, Kim PJ (2008) Feasibility of phosphate fertilizer to immobilize cadmium in a field. Chemosphere 70:2009–2015
Jinadasa KBPN, Milham PJ, Hawkins CA, Cornish PS, Williams PA, Kaldor CJ, Conroy JP (1997) Survey of cadmium levels in vegetables and soils of greater Sydney, Australia. J Environ Qual 26:924–933
Kabata A, Pendias H (2001) Trace elements in soils and plants. CRC Press, Boca Raton
Kulikova ZL, Lux A (2010) Silicon influence on maize, Zea mays L., hybrids exposed to cadmium treatment. B Environ Contam Toxicol 85:243–250
Lee SH, Lee JS, Choi YJ, Kim JG (2009) In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 77:1069–1075
Lee YB, Hoon C, Hwang JY, Lee IB, Kim PJ (2004) Enhancement of phosphate desorption by silicate in soils with salt accumulation. Soil Sci Plant Nutr 50:493–499
Li HX (2001) Soil chemistry. Higher Education Press, Beijing, pp 167–211 (in Chinese)
Li NY, Fu QL, Zhuang P, Guo B, Zou B, Li ZA (2012) Effect of fertilizers on Cd uptake of Amaranthus hypochondriacus, a high biomass, fast growing and easily cultivated potential Cd hyperaccumulator. Int J Phytorem 14:162–173
Li P, Wang XX, Zhang TL, Zhou DM, He YQ (2008) Effects of several amendments on rice growth and uptake of copper and cadmium from a contaminated soil. J Environ Sci-China 20:449–455
Liang YC, Wong JWC, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Lukacova Z, Svubova R, Kohanova J, Lux A (2013) Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul 70:89–103
Ma JF, Takahashi E (1990) Effect of silicon on the growth and phosphorus uptake of rice. Plant Soil 126:115–119
Maier NA, McLaughlin MJ, Heap M, Butt M, Smart MK, Williams CMJ (1997) Effect of current-season application of calcitic lime on soil pH, yield and cadmium concentration in potato (Solanum tuberosum L.) tubers. Nutr Cycl Agroecosyst 47:29–40
McGowen SL, Basta NT, Brown GO (2001) Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J Environ Qual 30:493–500
McLaughlin MJ, Bell MJ, Wright GC, Cozens GD (2000) Uptake and partitioning of cadmium by cultivars of peanut (Arachis hypogaea L.). Plant Soil 222:51–58
Moir AM, Thornton I (1989) Lead and cadmium in urban allotment and garden soils and vegetables in the United-Kingdom. Environ Geochem Health 11:113–119
Neumann D, zur Nieden U (2001) Silicon and heavy metal tolerance of higher plants. Phytochemistry 56:685–692
Nwugo CC, Huerta AJ (2008a) Silicon-induced cadmium resistance in rice (Oryza sativa). J Plant Nutr Soil Sci 171:841–848
Nwugo CC, Huerta AJ (2008b) Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 311:73–86
Obihara CH, Russell EW (1972) Specific adsorption of silicate and phosphate by soils. J Soil Sci 23:105–117
Persson H, Turk M, Nyman M, Sandberg AS (1998) Binding of Cu2+, Zn2+ and Cd2+ to inositol tri-, tetra-, penta, and hexaphosphates. J Agr Food Chem 46:3194–3200
Putwattana N, Kruatrachue M, Pokethitiyook P, Chaiyarat R (2010) Immobilization of cadmium in soil by cow manure and silicate fertilizer, and reduced accumulation of cadmium in sweet basil (Ocimum basilicum). ScienceAsia 36:349–354
Rizwan M, Meunier JD, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209:326–334
Rothbuhr L, Scott F (1957) Study of the uptake of silicon and phosphorus by wheat plants, with radiochemical methods. Biochem J 65:241–245
Roy AC, ALi MY, Fox RL, SMVA JA (1971) Influence of calcium silicate on phosphate solubility and availability in Hawaiian latosols. In: Symposium on Soil Fertility Evaluation. New Delhi, pp 757–765
Sarwar N, Saifullah MSS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agr 90:925–937
Shi GR, Cai QS, Liu CF, Wu L (2010) Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul 61:45–52
Singh BR, Myhr K (1998) Cadmium uptake by barley as affected by Cd sources and pH levels. Geoderma 84:185–194
Song A, Li ZJ, Zhang J, Xue GF, Fan FL, Liang YC (2009) Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J Hazard Mater 172:74–83
Sun Y, Li Z, Guo B, Chu G, Wei C, Liang Y (2008) Arsenic mitigates cadmium toxicity in rice seedlings. Environ Expl Bot 64:264–270
Tan WN, Li ZA, Qiu J, Zou B, Li NY, Zhuang P, Wang G (2011) Lime and phosphate could reduce cadmium uptake by five vegetables commonly grown in South China. Pedosphere 21:223–229
Treder W, Cieslinski G (2005) Effect of silicon application on cadmium uptake and distribution in strawberry plants grown on contaminated soils. J Plant Nutr 28:917–929
Turner BL, Paphazy MJ, Haygarth PM, McKelvie ID (2002) Inositol phosphates in the environment. Philos T Roy Soc B 357:449–469
Vaculik M, Lux A, Luxova M, Tanimoto E, Lichtscheidl I (2009) Silicon mitigates cadmium inhibitory effects in young maize plants. Environ Exp Bot 67:52–58
Wang LJ, Wang YH, Chen Q, Cao WD, Li M, Zhang FS (2000) Silicon induced cadmium tolerance of rice seedlings. J Plant Nutr 23:1397–1406
Wang YH, Ai SY, Tang MD, Li MJ, Yao JW, Luo YJ (2012) Effect of application of silicon amendment on Cd over-standard soils of vegetable fields. Sci Agric Sin 45:3310–3317 (in Chinese)
Zhang CC, Wang LJ, Nie Q, Zhang WX, Zhang FS (2008) Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ Exp Bot 62:300–307
Zhuang P, McBride MB, Xia HP, Li NY, Lia ZA (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407:1551–1561
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 40871221 and No. 41301571), and the Research Fund Program of Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology (2013K0008). We thank Dr. Yong Shen for his help during the experiment and Dr. Jorge Paz-Ferreiro for his valuable suggestions on the manuscript. We thank Prof. Elena Maestri, the editor, and three anonymous reviewers for their comments on an early version of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Huan-ping Lu and Ping Zhuang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, Hp., Zhuang, P., Li, Za. et al. Contrasting effects of silicates on cadmium uptake by three dicotyledonous crops grown in contaminated soil. Environ Sci Pollut Res 21, 9921–9930 (2014). https://doi.org/10.1007/s11356-014-2947-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2947-z