Abstract
The plant growth promoting bacteria can ameliorate the abiotic stressors through induced systemic tolerance in associated plants. The present work reports the efficiency of a plant growth promoting rhizobacterium (PGPR) Enterobacter sp. SBP-6 containing ACCD activity to stimulate the growth of the wheat plant under salinity stress conditions. Besides ACCD activity, the isolate was able to show other plant growth promoting (PGP) traits like phosphate solubilization, phytohormone production, nitrogen fixation, etc. The application of isolate SBP-6 to the wheat plants alleviated the inhibitory effects of gradient levels of salinity (150, 175, 200 mM NaCl) on various parameters of plant growth and photosynthetic pigments. Results of pot experiments revealed that inoculation with the test isolate significantly increased the plant biomass by 10–42 % as compared to their respective uninoculated control. An ability of the isolate to alleviate the effect of salt stress was also evident by significant increase of chlorophyll content (33–41 %), reduction in toxic Na+ ionic content (19–41 %), increase in K+ uptake (23–31 %), thereby favoring the K+/Na+ ratio and a significant decrease in leaf proline and malondialdehyde content in bacteria-treated plants exposed to salt stress. These results indicated that the selected isolate SBP-6 can be used for promoting the plant growth under salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among several abiotic environmental stressors, salinity is a major threat to the agricultural sustainability which adversely affects more than 800 million hectares of land worldwide (Tester and Munns 2008). Accumulation of high salt concentration results in major damages through the generation of reactive oxygen species, osmotic shock, ionic toxicity, stomatal closure and reduced leaf expansion (James et al. 2011). Increased soil salinity also adversely affects the soil microbial activities and crop productivity by limiting various macro and micronutrients. Therefore, there is a need to find eco-friendly and cost-effective approaches to deal with the threat of salinity stress to the agriculture. Application of the plant growth promoting rhizobacteria is one of the most suitable and sustainable approaches to combat the deleterious effects of salt and several other abiotic stressors including drought, salinity, nutrient deficiency, and temperature. The ability of PGPR to confer beneficial effects by enabling its host plants to tolerate or mitigate the effect of abiotic stressors is referred as ‘induced systemic tolerance’ (IST) (Yang et al. 2009). These PGPR prevent the deleterious effects of several environmental stressors. Plant growth stimulatory activity of PGPR on plants growing under salt and water stress (Dardanelli et al. 2008; Mayak et al. 2004; Nadeem et al. 2007), and nutrient deficiency (Egamberdiyeva 2007) have been reported in previous studies.
PGPR can ameliorate abiotic stresses and stimulate the plant growth directly by fixing atmospheric nitrogen, phytohormones production, and sequestration of iron by bacterial siderophores, phosphate solubilization or indirectly by inhibiting the disease-causing pathogenic organisms (Veerubommu and Kanoujia 2011). Moreover, certain PGPR can protect associated plants from the consequences of abiotic stressors through one or more mechanisms. One of the principal mechanisms by which many PGPR increase resistance of associated plants to abiotic stressors is the production of 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme that limit the deleterious level of ‘stress ethylene’. Under abiotic or biotic stress conditions, plants synthesize ethylene which at higher concentration termed as ‘stress ethylene’ inhibits elongation of plant shoot and root, causes chlorosis and leaf abscission, suppresses leaf expansion or promotes epinasty (Abeles et al. 1992). Under stress conditions, excess ACC exudes out from plant roots where ACC deaminase (ACCD) bacteria can sequester and degrade ACC to α-ketobutyrate (α-KB) and ammonia. Thus, PGPR with ACC deaminase activity reduce the negative effects of salinity stress (Glick 2007). Mayak et al. (2004) suggested that salt tolerant ACC deaminase bacteria help plants to overcome the deleterious effect of salt stressors. Hence, the presence of ACC deaminase activity could be one of the primary mechanisms by which bacteria support the plant growth under salt stress (Saleem et al. 2007). Experimental evidence suggested that ACCD rhizobacteria confer resistance to plants under various abiotic and biotic stress conditions like salinity, drought, pathogen attack, etc. (Glick 2007; Diagne et al. 2013; De Zelicourta et al. 2013).
Salinity stress increases the uptake of Na+ and decreases the uptake of K+ and Ca2+, which eventually causes a metabolic disturbance in plants (Yildirim et al. 2006). Previous reports have shown that the salinity also adversely affected absorption and transportation of Ca2+ in the plant (Zhang and Shi 2013; Liu et al. 2014). The above observation is supported from earlier finding which showed the antagonistic absorption of Na+ and K+ under saline condition (Grieve and Poss 2000). Therefore, an ability of plants to sustain the saline stress has been related to restricting or limiting the uptake of Na+ and promotes the continual uptake of K+ (Jeschke and Wolf 1988). Therefore, use of PGPR could be a beneficial step in the regulation of nutritional balance in plants subjected to salt stress (Kohler et al. 2009; Nadeem et al. 2009). An exopolysaccharide secreting bacterium Pseudomonas mendocina decreased the accumulation of Na+ uptake in plants growing in saline soil (Kohler et al. 2006). The reports of El-Komyet al. (2003) demonstrated that the inoculation of maize plant with Azospirillum sp. enhanced the uptake of K+ and Ca2+ in shoots which in turn, increased Ca2+ content and decreased leakage of K+ from the roots.
A number of other physiological changes in plants occur in response to salinity stress (Saharan and Nehra 2011). For adaptation to salt stress, plants accumulate various molecules in the form of osmolytes such as glycine betaine, proline, total soluble sugars, and antioxidative enzymes. Inoculation with PGPR increases the level of such molecules and antioxidative enzymes. The enhanced production of proline following inoculation of beneficial bacteria was observed in the abiotically stressed plants (Ait Barka et al. 2006). Similarly, Kohler et al. (2009) reported an increase in salt tolerance of lettuce plants by the enhanced production of antioxidative enzymes induced by inoculation with PGPR. Previous studies suggest that inoculation with PGPR enhance the salt tolerance in tomato (Mayak et al. 2004), red pepper (Siddikee et al. 2011) and groundnut (Saravanakumar and Samiyappan 2007).
The demand for wheat is growing approximately at the rate of 2 % per year worldwide (Rosegrant and Cline 2003). In India, wheat is grown under sub-tropical environments during mild winter which warms up towards grain filling stages of the crop. Wheat is a second most important cultivated cereals being used as staple food in India. However, productivity of wheat is severely affected by an increase in salinity, which lowers osmotic potential and decreases water availability in plants. Therefore, the present work was aimed to isolate the ACC deaminase producing bacteria capable of growing in the salt enriched medium, and to evaluate the various traits of such bacteria so that they can be utilized as plant growth promoting agents under salt stress. In addition, the enzyme activity was also characterized physiologically, and inoculation effect of SBP-6 on growth, osmolyte and ionic content of wheat plant growing under salt stress was also assessed.
Materials and methods
Isolation of bacteria
Bacteria were isolated from the rhizospheric soil of Jowar (Sorghum bicolor), a common plant growing in Shekhawati region (28°18′N, and 74°58′E) of Rajasthan, India. For the isolation of ACCD bacteria, 1 g of soil adhered to plant roots was inoculated into a flask containing 50 ml of DF (Dworkin and Foster) salt minimal media supplemented with 2.0 % ammonium chloride as a nitrogen source. Composition of DF medium was as follows (per litre): KH2PO4 4.0 g, Na2HPO4 6.0 g, MgSO4·7H2O 0.2 g, glucose 2.0 g, gluconic acid 2.0 g, citric acid 2.0 g, Trace elements: FeSO4·7H2O 1 mg, H3BO3 10 µg, MnSO4·H2O 11.19 µg, ZnSO4·7H2O124.6 µg, CuSO4·5H2O 78.22 µg, MoO310 µg, pH 7.2 (Dworkin and Foster 1958). The flask was incubated on a rotary shaker at 200 rpm for 48 h at 30 °C. After completion of incubation period, the sample was serial diluted (decimally) to 10−9 and 100 µl from each dilution was plated on DF agar medium supplemented with 3 mM ACC for 3 days at 30 °C. Following incubation, distinct bacterial colonies were picked and sub-cultured several times on DF-ACC agar plate to ensure its ability to use ACC as carbon and nitrogen sources. Finally, ACC utilizing bacterial isolate SBP-6 was selected and tested for ACCD activity and other plant growth promoting properties. Glycerol stock (15 %w/v) of the isolate was prepared and stored at −70 °C until further use.
Biochemical characterization and identification of bacteria
The selected isolate was subjected to various basic microbiological and biochemical tests such as Gram staining, indole, methyl red, Voges-Proskauer (IMVIC), nitrate reduction and activities for some important enzymes such as lipase, oxidase, protease, pectinase, amylase, urease as per standard protocols ( Prescott and Harley 2002). The carbohydrate utilization ability and antibiotic resistance of the isolate were tested employing respective commercial kits (KB 009, and HTM 002, Himedia, India). Interpretation of results of antibiotic sensitivity test was done using zone inhibition chart provided by the manufacturer. The bacterial isolate SBP-6 was also tested for various motility behaviors namely swimming, swarming and twitching following the standard protocol (Connelly et al. 2004). Taxonomic affiliation of the test organism SBP-6 was identified based on analysis of 16S rRNA gene. Total genomic DNA of bacterium was isolated using a DNA extraction kit (Qiagen, USA) and used for amplification of 16S rRNA gene by polymerase chain reaction (PCR) using universal primers 27 F1 (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1494Rc (5′-TACGGCTACCTTGTTACGAC-3′) (Weisburg et al. 1991). Reaction was performed in a final volume of 25 µl mixture containing 50 ng template DNA, 125 µM of each dNTPs, 1 X Taq polymerase buffer with 1.5 mM MgCl2, 1 U of Taq DNA polymerase and 20 pmol of each primer with an initial denaturation at 94 °C for 3 min, 30 cycles of 1 min at 94 °C, 1 min at 54 °C, 1 min at 72 °C, and final extension for 3 min at 72 °C. The amplified product of 1.5 kb was gel extracted and purified with QIA-quick PCR purification kit (Qiagen, USA). The amplicons were sequenced at Xcelris Genomic Lab Ltd, Ahmedabad (India). The resulting sequence was analyzed by comparing with 16S rRNA gene sequences available on GenBank database using BLAST algorithm to identify the bacterial species. The sequence of test isolate was deposited in the NCBI database http://www.ncbi.nlm.nih.gov/BLAST. The taxonomic identification was assigned at 98 % threshold of 16S rRNA gene sequence using ribosomal database project (RDP) database (http://rdp.cme.msu.edu/seqmatch/seqmatch_intro.jsp). The 16S rRNA gene sequences of the test isolate and related strains were aligned using CLUSTAL-X and a phylogenetic tree was constructed in MEGA version 6.0 tool using neighbor joining method with the bootstrap of 500 replicates (Tamura et al. 2013).
ACC deaminase assay
The ACC deaminase activity was colorimetrically monitored by measuring the amount of α-ketobutyrate produced from hydrolytic cleavage of ACC following the protocol of Penrose and Glick (2003). For the test of ACCD activity, the isolated bacterium was cultured in tryptic soya broth at 200 rpm for 24 h at 30 °C. After centrifugation, cell pellets were suspended in DF salt minimal medium containing 3 mM ACC (Sigma-Aldrich, USA) as the sole source of nitrogen, and incubated at 30 °C for 72 h. The retrieved cell pellet was subjected to two subsequent washes each with 0.1 M Tris–HCl buffer pH 7.6 and pH 8.3, respectively. After adding 30 µl of toluene, the cell suspension was vortexed at highest setting for 30 s. Two hundred µl of toluenized cells were mixed with 20 µl of 0.5 M ACC, briefly vortexed, and incubated for 15 min at 30 °C. After the addition of 1 ml of 0.56 M HCl to the above mixture, it was vortexed and spun at 10,000 g for 5 min at room temperature. One ml of the resulting supernatant was mixed with 800 µl of 0.56 M HCl. Three hundred µl 2, 4-dinitrophenylhydrazine (prepared in 2 N HCl) was added to the reaction mixture and incubated for 30 min at 30 °C. Finally, 2 ml of 2 N NaOH was added, and the absorbance of the sample was measured at 540 nm in a spectrophotometer. The quantity of α-ketobutyrate in the reaction mixture was estimated by comparing absorbance of the sample at 540 nm with different concentrations of pure α-ketobutyrate (0.1 and 1.0 µmol, Sigma-Aldrich, USA).
Assay for PGP and other characteristic features
Biological nitrogen fixation ability of SBP-6 was tested by growing it in minimal medim devoid of nitrogen sources. The strain was inoculated in the nitrogen-free semisolid JNFb− agar medium and grown at 28 °C for 4 days (Dobereiner 1995). The production of indole-3-acetic acid was tested and quantified using colorimetric method of Gordon and Weber (1951). Phosphate solubilization ability of the isolate was determined employing method of Mehta and Nautiyal (2001). To detect siderophore production, the isolate was grown on chrome-azurole S agar (CAS) plate and observed for the development of yellow-orange halo zone around the spot inoculated colony as described by Schwyn and Neilands (1987). Test of ammonia production was determined as per the method of Cappuccino and Sherman (1992). Biocontrol ability of isolate was examined by HCN production according to the standard protocol (Millar and Higgins 1970). The well diffusion assay was used for in vitro test of the antagonistic activity of the bacterial isolate SBP-6 (Schillinger and Lucke 1989).
Screening for abiotic stress tolerance of the isolate SBP-6
The bacterial isolate SBP-6 was tested for its ability to tolerate salt and temperature stressors. For the test of tolerance to salt, the bacterial isolate was grown for 24 h in peptone broth supplemented with various concentrations of NaCl (0.5–10 %). Temperature tolerance of the isolate was tested by growing the isolate in peptone broth for 5 days at varying degree of temperature from 25 to 50 °C. The growth of the culture was determined by measuring absorbance at 600 nm in a UV–Vis spectrophotometer (Jasco, Japan) using un-inoculated broth as a blank. The experiment was performed in triplicate sets.
Test of ACCD activity in the isolate SBP-6 under various physiological conditions
Bacterial ACCD activity plays a significant role in plant growth promotion under diverse stress conditions like salinity, drought, heavy metals, etc. Therefore, ACCD activity was investigated under various physiological conditions namely salt, temperature, pH, substrate (ACC) and at different time intervals. The bacterial isolate was grown in DF media and ACCD enzyme activity was measured as described above. Suitable changes in growth condition were made for different variables. Different concentration of NaCl (2–6 %) was added in DF medium for estimating ACCD in salt stress condition, whereas the various concentration of ACC (1–5 mM) was used to estimate optimum substrate concentration for ACCD enzyme activity. For imposing different pH conditions, pH of the culture media was adjusted with 2 N HCl and 1 M NaOH to attain pH 5.0–10.0 using the pH meter (Eutech, pH 1100). Similarly, bacteria were grown at different temperature conditions ranging between 25 and 40 °C in an incubator. For the time course assay, ACCD enzyme activity was tested at different time intervals after adding an optimal concentration of ACC in the medium. Cultures of equal OD (OD600) were used for ACCD assay.
Assessment of wheat growth promotion under salt stress
Physiochemical characteristics of soil
Before performing the plant growth studies, the soil was analyzed for its various physio-chemical properties. The pH and electrical conductivity of soil were analyzed by digital pH and EC meter on a 1:2.5 ratio of soil and water suspensions. An estimation of organic carbon was done using 1 N potassium dichromate for titration and 0.5 N ferrous ammonium sulfate for back titration (Walkey and Black 1934). For determination of available phosphorous, the soil was extracted with 0.5 M sodium bicarbonate and the amount of phosphorus was quantified using chlorostannus-reduced molybdophosphoric blue color method (Olsen et al. 1954). Availability of other nutrients like nitrogen, potassium and micronutrients (Fe, Cu, Zn, and Mn) was estimated by the method of Jackson (1967). The soil used for plant growth experiment (described below) was autoclaved at 121 °C for 1 h for 3 consecutive days to eliminate the entire microorganism and 300 g of sterilized soil was filled in plastic pots.
Plant inoculation
Plant growth promotion ability of the isolate SBP-6 under varying salt stress was studied in a controlled environment. Wheat seeds were sterilzed by washing with 70 % ethanol for 2 min followed by washings twice with sterile distilled water. Then, seeds were treated with 1.5 % sodium hypochlorite (NaOCl) solution for 3 min followed by three times successive washing using sterile water to remove all traces of sterilants. To check the efficacy of sterilization process, few seeds were placed on plates containing MS medium for 4 days and observed for microbial growth if any. For bacterization of seeds, surface sterilized seeds were placed in bacterial suspension for 1 h under aseptic condition. The inoculum was prepared by suspending bacterial cell pellets in 0.5 ml sterile 0.03 M MgSO4 and diluted in the same solution to adjust the absorbance to 0.15 at 600 nm (Penrose and Glick 2003). Control seeds were suspended in 0.03 M MgSO4 solution for the same time period. Bacterized seeds were sown in plastic pots filled with sterilized soil and kept in a plant growth chamber (Labtech, India) with 16:8 photoperiods for 15 days after sprouting of seeds at 24 ± 2 °C. Hoagland media supplemented with salt (150, 175, 200 mM) was used for providing nutrient as well as imposing the salt stress at every alternate day (Hoagland and Boyer 1936). The experiment was conducted for 15 days, and seedling growth was measured by recording shoot length, root length, fresh weight and dry weight. For estimation of chlorophyll contents, fresh leaf samples were homogenized in 80 % acetone, and pigments were extracted and quantified (Duxbury and Yentsch 1956). The absorbance at 480, 510, and 663 nm was measured on a UV–Vis spectrophotometer (Jasco, Japan). All the experiments were done in triplicate.
Ionic analysis
Shoot tissues from above experimental set up were also used for determining the concentration of different ions namely Na+, K+ and Ca2+. Shoot tissues were ground in liquid nitrogen and dried at 70 °C. For estimation of ions, 1.0 g of shoot tissue was digested with perchloric acid, sulphuric acid and distilled water in 10:1:2 ratio and volume was made to 100 ml. A 10 ml sample was used for analysis by an Atomic Absorption Spectrophotometer (AAS 2380, Perkin Elmer, USA) at National Horticultural Research and Development foundation (Nashik, India).
Biochemical analysis of plant
Proline estimation
Proline content in the leaves was determined following the standard protocol of Bates et al. (1973) with minor modifications. A 0.5 g fresh leaves was homogenized in 3 % (w/v) sulfosalicylic acid and centrifuged at 8500g for 10 min. One ml of resultant supernatant was mixed with 1 ml freshly prepared ninhydrin reagent and 1 ml glacial acetic acid and boiled in a water bath for 1 h. After cooling, 4 ml toluene was added to the reaction mixture and mixed by vortexing for 20 s. Chromophore containing toluene phase was separated from the aqueous phase, and absorbance was measured at 520 nm against toluene blank. The proline content was calculated by comparing with a standard curve plotted from standard l-proline (Sigma-Aldrich USA).
Lipid peroxidation
Lipid peroxidation was determined by estimating the malondialdehyde (MDA) content produced by thiobarbituric acid reaction (Hodges et al. 1999) with minor modification. Briefly, 1 ml of alcoholic extract prepared with 0.5 g of the leaf was mixed with 1 ml of 0.5 % thiobarbituric acid containing 20 % trichloroacetic acid. The mixture was heated up to 90 °C for 30 min in a water bath. After cooling to room temperature, the sample was centrifuged at 5000g for 5 min and absorbance was measured at 400, 532 and 600 nm. After subtracting the nonspecific absorbance, the MDA concentration was determined by its molar extinction coefficient (155 nm−1 cm−1) and the results were expressed as mmol MDA g−1 FW.
Statistical analysis
Data from the seedling were collected for each treatment, and the mean values, and standard deviations were calculated. Data were analyzed by analysis of variance (ANOVA) and subsequently by Duncan’s multiple range test at p = 0.05.
Results
Isolation and PGP activity of isolate SBP-6
Isolate SBP-6 was selected based on its ability to grow on DF-ACC salt minimal medium which indicates the presence of ACC deaminase enzyme in bacteria. On quantification, ACC deaminase activity was found to be 284 ± 8.70 nmoles of α-KB mg−1 Pr.h−1 in the isolate. In addition to ACC deaminase activity, the isolate also showed production of IAA (0.4032 ± 0.025 µg ml−1), phosphate solubilization (9.50 ± 0.80 µg ml−1), ammonia production and nitrogen-fixation ability based on its growth on nitrogen free JNFb− media. However, it showed a negative result for siderophore production (Table 1). Antibiotic profiling revealed the sensitivity of the isolate to chloramphenicol and resistance to vancomycin, tetracycline, kanamycin and gentamycin (Suppl. Table 1). The isolate was found to show the swimming, swarming and twitching motility.
Biochemical analysis, identification, and phylogenetic analysis
The isolate SBP-6 was found to be positive for the test of catalase, Voges-Proskauer, pectinase and negative for indole, methyl red, amylase, lipase, urease, nitrate reductase, and cellulase. The optimum growth temperature and salt concentration was found to be 30 °C and 4 % respectively. In carbohydrate utilization test, the isolate was able to utilize lactose, xylose, maltose, fructose, dextrose, galactose, raffinose, trehalose, melibiose, sucrose, l-arabinose, mannose, inulin, glycerol, salicin, sorbitol, mannitol, adonitol, arabitol, rhamnose, cellobiose, ONPG, esculin hydrolysis, D-arabinose (Suppl. Table 2) as carbon source. In addition to plant growth promoting activities, the antagonistic activity of the isolate against pathogens was also tested. The isolate showed a certain degree of antagonism to fungal strain Candida albicans, Fusarium moniliforme and Aspergillus flavus only. Among pathogenic bacterial strains, it was found to be effective against Bacillus subtilis, E coli, Klebsiella sp., Staphylococcus aureus and Erwinia carotovora only (Suppl. Table 3). The strain was identified by partial sequence analysis of 16S rRNA gene to ascertain their taxonomic position. Based on the sequence of 16S rRNA gene, the strain showed a 99 % similarity with the 16S rRNA gene sequence of other Enterobacter sp. (Suppl. Fig 1). The obtained sequence was submitted to the NCBI Genbank under the accession number KJ950707.
Physiological characterization of ACCD activity
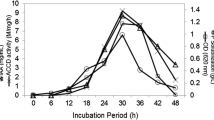
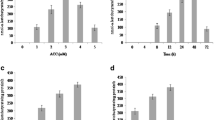
The isolate SBP-6 was screened for its ACCD activity under various physiological conditions. Among different concentration of substrate, 3 mM was observed to be optimal for enzyme activity (280.35 ± 19.87 nmol α-KB mg−1 Pr.h−1). Other measures used for evaluation of optimal ACCD activity was different pH and time period of bacterial growth which were demonstrated as pH 8.0 (276.40 ± 15.40 nmol α-KB mg−1 Pr h−1) and 48 h incubation (278.90 ± 11.90 nmol α-KB mg−1 Pr h−1) after the ACC induction respectively (Fig. 1). ACCD activity was also measured at different salt and temperature conditions which showed increase in ACCD activity with increasing salt concentration up to 6 %. The highest ACCD activity with 271.50 ± 18.70 nmol α-KB mg−1 Pr h−1 was observed in DF media supplemented with 6 % of NaCl (Fig. 1). Similarly, the highest enzyme activity of 282.30 ± 11.20 nmol α-KB mg−1 Pr h−1 was monitored at 30 °C of temperature.
Effect of isolate SBP-6 on plant growth under salt stress condition
Effects of inoculation of bacterial isolate SBP-6 on wheat growth was performed under different salt (NaCl) concentrations in the controlled environment. Treatment with a high concentration of NaCl adversely affected the growth of the plants in terms of decrease in shoot length, root length, fresh weight, and dry weight which reflects the toxicity of NaCl. However, inoculation of the isolate SBP-6 improved the growth of plants under salt stress (Fig. 2). It is evident from the Fig. 2a that treatment with 150 mM to 200 mM NaCl decreased the growth of shoot length by 10–30 %, whereas bacterial treatment significantly increased the shoot length. The most significant (p = 0.05) increase in shoot length (42.10 %) was observed at 200 mM as compared to respective control (Fig. 2a). Similarly, highest increase (p = 0.05) in root length (33.64 %) in inoculated plants was observed at 200 mM as compared to control plants treated with same salt stress (Fig. 2b). Bacterial inoculation improved the fresh weight in the range of 21–35 % with a highest significant (p = 0.05) improvement of 35.85 % at 200 mM followed by 33.15 % at 175 mM NaCl stress (Fig. 2c). Improvement in dry weight was observed in the range of 11–34 % in inoculated plants as compared to corresponding control (Fig. 2d). Similarly bacterial inoculation also significantly (p = 0.05) improved the photosynthetic pigment chlorophyll a (16–49 %) and chlorophyll b (17–39 %) as compared to corresponding plants treated with salinity stress (Fig. 2e, f).
Effect of inoculation with Enterobacter sp. SBP-6 on plant biomass and chlorophyll content under different salinity conditions (0, 150, 175, 200 mM NaCl) a shoot length, b root length, c fresh weight, d dry weight, e chlorophyll a, f chlorophyll b. Values are mean ± SD of triplicate sets (n = 15). Double asterisk represent the significant difference as compared to respective control according to Ducan’s multiple range test (p = 0.05)
Inoculation effect on ionic analysis under salt stress
Modulation of ionic accumulation Na+, K+, and Ca2+ in response to SBP-6 inoculation was measured at 0, 150, 175 and 200 mM NaCl. With the increase in salinity from 150 mM to 200 mM, a significant (p = 0.5) increase in Na+ in the range of 34–96 % was observed in uninoculated plants. However, bacterial inoculation remarkably decreased the level of Na+ content in treated plants. As compared to respective control plants, highest drop in Na+ content was noted by 41 % in bacteria-treated plants at 200 mM NaCl, while it was 22 and 19 % at 175 and 150 mM respectively (Fig. 3a). In addition to the change in the level of Na+, the concentration of other ions such as K+ and Ca2+ were also altered on treatment with NaCl. Salinity caused a decrease in K+ content by 22–41 % in salt-treated control plants. On the contrary, bacterial inoculation significantly (p = 0.5) improved K+ content, where highest increase 30.36 % was observed at 175 mM, while it was 27.37 and 22.94 % at 200 mM and 150 mM salt stress respectively (Fig. 3b). Similar to the result of K+, bacterial inoculation also improved the Ca2+ content in the range of 6–31 % (Fig. 3c).
Effect of inoculation with SBP-6 on ionic uptake by plants under salt stress, a Na+, b K+, c Ca2+. Error bar represents standard deviation of five measurements in triplicate sets (n = 15). Double asterisk represent the significant difference as compared to corresponding control as per Ducan’s multiple range test (p = 0.05)
Biochemical analysis of plants
Under salinity stress, a significant increase in proline content was observed in control (un-inoculated) plants in the range of 70-155 %. However, bacterial inoculation caused a significant reduction in proline content as compared to control plants subjected to salinity stress. The significant (p = 0.05) decrease in proline content (45.33 %) was observed at 175 mM NaCl, followed by 39.53 and 33 % as compared to control plants treated with 150 mM and 200 mM NaCl salt stress respectively (Fig. 4a). The increase in salinity induced the level of malondialdehyde (MDA) content, however bacterial inoculation significantly (p = 0.05) decrease the MDA content. Inoculation with SBP-6 caused a significant (p = 0.05) reduction in the MDA content (38–49 %) as compared to control plant treated with salt stress. Bacterial inoculation significantly (p = 0.05) decreased the 49.58 % MDA content at 175 mM NaCl followed by 43.07 and 37.29 % at 200 and 175 mM NaCl stress as compared to respective control plants (Fig. 4b).
Effect of bacterium inoculation on proline (a) and malondialdehyde (b) content under 0, 150, 175, 200 mM NaCl salinity conditions. The values are mean ± SD (n = 15). The significant difference compared to corresponding control has been denoted by double asterisk. Error bar represent the standard deviation of triplicate sets with five measurement in each set (n = 15)
Discussion
Application of plant growth promoting bacteria (PGPB) is one of the most promising approaches to ameliorate the salinity stress in plants. Certain PGPB can alleviate deleterious effects of salt stressors by producing ACC deaminase activity, which decreases the level of stress ethylene induced under stress conditions (Mayak et al. 2004; Singh et al. 2015). Therefore, the present study aimed to isolate and characterize plant growth stimulating potential of ACC deaminase bacteria. In present work, we have demonstrated the ability of an ACC deaminase producing bacterial isolate Enterobacter sp. SBP-6 to promote the growth of wheat plant treated with different concentration of salt (NaCl) under gnotobiotic conditions. In addition to ACC deaminase activity, the isolate SBP-6 also showed the amelioration of salt stress by lowering the toxic ionic effect in host plants resulted from the accumulation of salt.
PGPB can enhance the plant growth and increase their biomass under salt-stress conditions through various mechanisms. One of the most studied stress-ameliorating effects of PGPB is exhibited through ACC deaminase, which catabolizes ACC, a precursor of ethylene, and recovers plant growth inhibited due to stressors. Several ACC deaminase bacteria belonging to genera Arthobacter (Barnawal et al. 2014), Klebsiella (Singh et al. 2015) and Streptomyces (Palaniyandi et al. 2014) have been reported to mediate plant growth promotion under salt stress conditions. Since, many PGPB having ACCD activity are known to promote the plant growth under stress conditions, selection of isolate SBP-6 was primarily based on high ACCD activity under salt stress condition, as well as plant growth promoting properties, and its halo-tolerant behavior. The isolate showed enzyme activity >20 nmol of α-KB mg−1 h−1 which is enough to trigger induced systemic tolerance throughreduction of stress ethylene produced in plants under stress conditions (Penrose and Glick 2003). Moreover, ACCD activity of isolate SBP-6 was characterized under various physiological conditions. The optimal enzyme activity was observed at 3 mM substrate (ACC), pH of 8.0 and at 48 h of the incubation period. Similar results were obtained by Jha et al. (2012) who characterized ACC deaminase activity in Enterobacter sp. It was interesting to note that growth and enzyme activity increased with increasing salt concentration with the highest activity at 4 % salt concentration. It would be beneficial to use the bacterial inoculation for minimizing the salinity induced damages for plants growing in salt-rich regions. Our result is in agreement with the report of Tittabutr et al. (2013) who observed an increase in AcdS gene expression with increased salinity. The increased activity of ACCD may be correlated with the fact that the regulation of AcdS gene might be under the control of the stationary phase sigma factor that initiates expression of certain genes under stress condition (Saleh and Glick 2001).
Besides ACC deaminase activity, the isolate was also able to produce IAA and solubilize inorganic phosphate that is important contributors to plant growth (Glick 1995, 2010). The presence of these properties is known in several PGPB (Karthikeyan et al. 2012; Sachdev et al. 2009). PGPR able to produce IAA have a significant advantage to enhance root growth and development which in turn enhance nutrient uptake due to increased surface area of roots (Yang et al. 2009). According to previous reports, production of IAA is an important attribute of PGPB and promotes plant growth, especially root elongation (Glick 2014; Noreen et al. 2012). The microbially-produced auxin in the vicinity of plant roots might induce a physiological response in their associated plants (Upadhyay et al. 2011). Solubilization of phosphate by the isolate provides bio-available phosphate which can be a possible mechanism of improvement of plant growth (Podile and Kishore 2006).
Further, the ACC deaminase bacterial isolate SBP-6 was tested for its ability to promote the growth of wheat plants under salt stress conditions. Salt stress adversely affects the plant growth and productivity by reducing the photosynthetic efficiency, stomatal conductance, and leaf area, etc. (Wang et al. 2005; Colmenero-Flores et al. 2007). Physiological water deficit induced by salinity stress decreases the stomatal conductance and slows CO2 assimilation (James et al. 2002). In the present study, salinity significantly reduced the plant growth and biomass at varying concentrations. This might be due to adverse effect of salinity on plant metabolic and physiological processes, which decrease the plant growth and biomass (Munns and Termatt 1986). However, treating the wheat seeds with the isolate SBP-6 improved the plant growth and photosynthetic pigment chlorophyll, over the non-inoculated seeds under saline stress. The increase in photosynthetic pigments in bacteria-treated plants indicated improved photosynthetic activity in host plants. This is supported by the previous studies which reported enhancement in photosynthetic activity in host plants in response to PGPR (Nadeem et al. 2009; Han and Lee 2005). Our results are also in congruence with other studies where ACCD bacteria were observed to stimulate plant growth under salt stress conditions (Nadeem et al. 2010, Ramadoss et al. 2013). A significant increase in growth and macronutrient (N, P) concentration was recorded in the shoot of wheat plant treated with Enterobacter cloaceae MDSR9 possessing ACC deaminase activity (Ramesh et al. 2014). Similarly, significant increase in various growth parameters of the wheat plant was observed following inoculation of beneficial bacterial strains Pseudomonas aureantiaca TSAU22, P. extremorientalis TSAU6, and P. extremorientalis TSAU20 under NaCl stress (Egamberdiyeva 2007).
In plants, maintenance of ionic homeostasis especially Na+ and K+ also affects several aspects of plant growth and development. High internal Na+ concentration and low K+/Na+ ratio is correlated with inhibition of plant growth in response to increased soil salinity (Zhang et al. 2010). Therefore, plants tend to maintain desirable K+/Na+ ratio in the cytosol by regulating K+ uptake and restricting the Na+ uptake. This elevated level of K+/Na+ ratio is a good indicator of salinity tolerance (Hamdia et al. 2004). In the saline environment, certain, PGPR can mediate stress-tolerance in host plants through additional mechanisms such as regulation of nutrient uptake, maintenance of the ionic balance, modulation of osmolytes and phytohormones in plants. For example, Azospirillum-inoculated maize plants showed the higher uptake of K+ and reduced Na+, thereby maintaining the nutritional under saline conditions (Hamdia et al. 2004). Similarly, inoculation of wheat seeds with PGPR strains improved K+ content and reduced the Na+ uptake under saline conditions (Ashraf et al. 2004). The investigation of Upadhyay et al. (2011) suggested that increase in K+ following the PGPR (Bacillus subtilis SU47 and Arthrobacter sp. SU18) inoculation involved in the mitigation of salt induced oxidative stress in wheat plants. Our study also supports above observations as SBP-6 inoculation significantly decreased the Na+ content, while improved the K+, which in turn helps the plant to adapt to ameliorate the harmful effects of the salts.
Plants subjected to salt stress produce a higher amount of proline that function as an osmoregulant as well as stabilize the protein structures and cell membranes (Claussen 2005). Proline serves as an osmoprotectant that is required for maintaining an optimum osmotic potential in the plant cells with reference to their surroundings (Pollard and Wyn Jones 1979). Similarly, Lin and Kao (1995) proposed that accumulation of proline is considered as a contributor to the salinity-inhibited growth of the root. We observed that the plants treated with the isolate SBP-6 significantly produced less amount of proline as compared to uninoculated plants growing under salt stress. This might be due to alleviation of salt stress in the wheat plant by a different mechanism than proline accumulation (Palaniyandi et al. 2014). The lower level of proline in SBP-6 treated plants under salinity stress indicated that the bacterium-treated plants were less affected by salinity. Similar to our results, Nadeem et al. (2007) and Hamdia et al. (2004) also noticed decreased proline content in PGPR-treated plants exposed to salt stress.
The level of malondialdehyde (MDA) production is used as an indicator of oxidative damage under stress conditions and is directly linked to lipid peroxidation (Arbona et al. 2008). Therefore, the production of MDA content is a reflection of stress-induced damage of lipid membranes at the cellular level (Jain et al. 2001). The low level of MDA content in SBP-6 inoculated plants displays lower cell membrane damage or higher salt tolerance. The specific resistance mechanism of compatible solutes to a wide range of abiotic stresses is still not fully understood. However, these molecules significantly contribute to osmotic adjustment, stabilize lipid membranes, stabilizing protein during stresses, etc. (Colmer et al. 1995; Hincha et al. 2003; Yancey 2005).
In conclusion, the results of the present study illustrated that inoculation of wheat plants with soil bacterium Enterobacter sp. SBP-6 significantly mitigates the salinity-induced osmotic stress in wheat seedlings and also improved the plant growth, biomass and photosynthetic pigments. SBP-6-inoculated plants possess less accumulation of toxic Na+ and increased the uptake of K+, and therefore, able to maintain the ionic balance. The inoculated wheat seedlings counteracted the imposed salinity stress by reducing the salt-induced lipid peroxidation, and thus, confer protection against abiotic stressors. Therefore, application of such bacteria with these features could be useful to combat saline stress without any genetic manipulation of the plant.
Author contribution statement
Rajnish Prakash Singh performed all experiments, data analysis and writing the manuscript. Prabhat Nath Jha mentored the work and contributed to designing the work, writing and editing of the manuscript for publication.
References
Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in plant biology. Academic press, San Diego
Ait Barka E, Nowak J, Clement C (2006) Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmansstrain PsJN. Appl Environ Microbiol 72:7246–7252
Arbona V, Hossain Z, López-Climent MF, Pérez-Clemente RM, Gómez Cadenas A (2008) Antioxidant enzymatic activity is linked to water-logging stress tolerance in citrus. Physiol Plantarum 132:452–466
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Ashraf M, Berge SH, Mahmood OT (2004) Inoculating wheat seedlings with exopolysaccharide producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fert Soil 40:157–162
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2014) 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during water-logging stress via reduced ethylene generation. J Plant Physiol 58:227–235
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Cappuccino JG, Sherman N (1992) Biochemical activities of microorganisms. In: Microbiology, A Laboratory Manual. The Benjamin/Cummings Publishing Co. California, USA
Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168:241–248
Colmenero-Flores JM, Martinez G, Gamba G, Vazquez N, Iglesias DJ, Brumos J, Talon M (2007) Identification and functional characterization of cation-chloride co-transporters in plants. Plant J 50:278–292
Colmer TD, Epstein E, Dvorak J (1995) Differential solute regulation in leaf blades of various ages in salt-sensitive wheat and a salt-tolerant wheat xLophopyrum elongatum (Host) A. Love amphiploid. Plant Physiol 108:1715–1724
Connelly MB, Young GM, Sloma A (2004) Extracellular proteolytic activity plays a central role in swarming motility in Bacillus subtilis. J Bacteriol 186:4159–4167
Dardanelli MS, Fernandez de Cordoba FJ, Espuny MR, Carvajal MAR, Diaz MES, Serrano AMG, Okon Y, Megìas M (2008) Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol Biochem 40:2713–2721
De Zelicourta A, Al-Yousif M, Hirta H (2013) Rhizosphere microbes as essential partners for plant stress tolerance. Mol Plant 6:242–245
Diagne N, Thioulouse J, Sanguin H, Prin Y, Krasova-Wade T, Sylla S (2013) Ectomycorrhizal diversity enhances growth and nitrogen fixation of Acacia mangium seedlings. Soil Biol Biochem 57:468–476
Dobereiner J (1995) Isolation and identification of aerobic nitrogen-fixing bacteria from soil and plants. In: Alef K, Nanniperi P (eds), Methods Appl. Soil Microbiol. Biochem. Academic press, London, pp 134–141
Duxbury AC, Yentsch CS (1956) Plankton pigment monographs. J Marine Res 15:91–101
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–603
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36:184–189
El-Komy MH, Hamdia MA, Abd El-Baki GK (2003) Nitrate reductase in wheat plants grown under salinity and inoculated with Azospirillum spp. Biol Plant 46:281–287
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26:192–195
Grieve CM, Poss JP (2000) Wheat response to interactive effects of boron and salinity. J Plant Nutr 23:1217–1226
Hamdia MA, Shaddad MAK, Doaa MM (2004) Mechanism of salt tolerance and interactive effect of Azospirillum bransilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul 44:165–174
Han HS, Lee KD (2005) Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil stress. Res J Agri Biol Sci 1:210–215
Hincha DK, Zuther E, Heyer AG (2003) The preservation of liposomes by raffinose family oligosaccharides during drying is mediated by effects on fusion and lipid phase transitions. Biochim Biophys Acta (BBA) Biomemb 1612:172–177
Hoagland DR, Boyer TC (1936) General nature of the process of salt accumulation by roots with description of experimental methods. Plant Physiol 11:471–507
Hodges DM, Delong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acidreactive-substances assay for estimating lipid peroxidation in plant tissue containing anthocyanin and other interfering compounds. Planta 207:604–611
Jackson ML (1967) Soil chemical analysis. Prentice Hall of Indian Private Limited, New Delhi
Jain M, Mathur G, Koul S, Sarin NB (2001) Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Rep 20:463–468
James RA, Rivelli AR, Munns R, Caemmerer SV (2002) Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Funct Plant Biol 29:1393–1403
James RA, Blake C, Byrt CS, Munns R (2011) Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J Exp Bot 62:2939–2947
Jeschke WD, Wolf O (1988) External potassium is not required for root growth in saline conditions: experiments with Ricinus communis L. growth in a reciprocal split-root system. J Exp Bot 39:1149–1167
Jha Y, Subramanian RB, Patel S (2011) Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant 33:797–802
Jha CK, Annapurna K, Saraf M (2012) Isolation of Rhizobacteria from Jatropha curcas and characterization of produced ACC deaminase. J Basic Microbiol 52:85–95
Karthikeyan B, Joe MM, Islam MR, Sa T (2012) ACC deaminase containing diazotrophic endophytic bacteria ameliorate salt stress in Catharanthus roseus through reduced ethylene levels and induction of antioxidative defense systems. Symbiosis 56:77–86
Kohler J, Caravaca F, Carrasco L, Roldan A (2006) Contribution of Pseudomonas mendocina and Glomus intraradices to aggregates stabilization and promotion of biological properties in rhizosphere soil of lettuce plants under field conditions. Soil Use Manage 22:298–304
Kohler J, Hernandez JA, Caravaca F, Roldan A (2009) Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ Exp Bot 65:245–252
Lin CC, Kao CH (1995) NaCl stress in rice seedlings: Starch mobilization and the influence of gibberellic acid on seedling growth. Bot Bull Acad Sin 36:169–173
Liu W, Hou J, Wang Q, Ding L, Luo Y (2014) Isolation and characterization of plant growth-promoting rhizobacteria and their effects on phytoremediation of petroleum-contaminated saline-alkali soil. Chemosphere 117:303–308
Mayak S et al (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56
Millar RL, Higgins VJ (1970) Association of cyanide with infection of Birdsfoot trefoil by Stemphylium loti. Phytopathol 60:104–110
Munns R, Termatt A (1986) Whole-plant response to salinity. Aus J Plant Physiol 20:425–437
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2007) Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC-deaminase activity. Can J Microbiol 53:1141–1149
Nadeem SM, Zahir ZA, Nadeem M, Arshad M (2009) Rhizobacteria containing ACC deaminase confer salt tolerance in maize grown on salt affected soils. Can J Microbiol 55:1302–1309
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2010) Rhizobacteria capable of producing ACC deaminase may mitigate salt stress in wheat. Soil Sci Asoc Am J 74:533–542
Noreen S, Ali B, Hasnain S (2012) Growth promotion of Vigna mungo (L.) by Pseudomonas sp. exhibiting auxin production and ACC-deaminase activity. Ann Microbiol 62:411–417
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular 939 US Department of Agriculture, Washington DC, USA
Palaniyandi et al (2014) Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J Appl Microbiol 117:766–773
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plantarum 118:10–15
Podile AR, Kishore GK (2006) Plant-associated bacteria. In: Gnanamanickam SS (ed) Plant growth promoting rhizobacteria. Springer, Amsterdam, pp 195–230
Pollard A, Wyn Jones RG (1979) Enzyme activities in concentrated solutions of glycinebetaine and other solutes. Planta 144:291–298
Prescott L, Harley J (2002) Laboratory Exercises in Microbiology, 5th ed. McGraw-Hill, Boston, MA
Ramadoss D, Lakkineni VK, Bose P, Ali S, Annapurna K (2013) Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springer-Plus 2:6
Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP (2014) Plant growth promoting traits in Enterobacter cloaceae MDSR9 isolated from soyabean rhizosphere and its impact on growth and nutrition of soyabean and wheat upon inoculation. Agricultural Research 3:53–66
Rosegrant MW, Cline SA (2003) Global food security: challenges and policies. Science 302:1917–1919
Sachdev DP, Chaudhari HG, Kasure VM, Dahavale DD, Chopade BA (2009) Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Ind J Exp Biol 47:993–1000
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 2011:LSMR-21
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Saleh SS, Glick BR (2001) Involvement of gacS and rpoS in enhancement of the plant growth-promoting capabilities of Enterobacter cloacae CAL2 and Pseudomonas putida UW4. Can J Microbiol 47:698–705
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas Xuorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol 102:1283–1292
Schillinger U, Lucke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55:1901–1906
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Siddikee MA, Tipayno SC, Kim K, Chung JB, Sa T (2011) Influence of varying degree of salinity-sodicity stress on enzyme activities and bacterial populations of coastal soils of Yellow Sea, South Korea. J Microbiol Biotechnol 21:341–346
Singh RP, Jha P, Jha PN (2015) The plant growth promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J Plant Physiol 184:57–67
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6:molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729
Tester M, Munns R (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Tittabutr P, Piromyou P, Longtonglang A, Noisa-Ngiam R, Boonkerd N, Teaumroong N (2013) Alleviation of the effect of environmental stresses using co-inoculation of mungbean by Bradyrhizobium and rhizobacteria containing stress-induced ACC deaminase enzyme. Soil Sci Plant Nutr 59:559–571
TurkanI Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth promoting rhizobacteria under salinity condition. Pedosphere 21:214–222
Veerubommu S, Kanoujia N (2011) Biological management of vascular wilt of tomato caused by Fusarium oxysporum f. sp. lycospersici by plant growth-promoting rhizobacterial mixture. Biol Control 57:85–93
Walkey AE, Black JA (1934) An examination of the Degtiga Vett. Method for determining soil organic matter and proposed modification of the chromic acid titration method. Soil Sci 37:29
Wang LW, Showalter AM, Ungar IA (2005) Effects of intraspecific competition on growth and photosynthesis of Atriplex prostrata. Aquat Bot 83:187–192
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Yildirim E, Taylor AG, Spittler TD (2006) Ameliorative effects of biological treatments on growth of squash plants under salt stress. Sci Hortic 111:1–6
Zhang JL, Shi H (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosynth Res 115:1–22
Zhang Z, Rosenhouse-Dantsker A, Tang QY, Noskov S, Logothetis DE (2010) The RCK2 domain uses a coordination site present in Kir channels to confer sodium sensitivity to Slo2.2 channels. J Neurosci 30:7554–7562
Acknowledgments
We are grateful to Department of Biotechnology (File No. BT/PR14527/AGR/21/326/2010), Govt. of India, New Delhi to PNJ for their support by providing the fund for carrying out the research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by MJ Reigosa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, R.P., Jha, P.N. Mitigation of salt stress in wheat plant (Triticum aestivum) by ACC deaminase bacterium Enterobacter sp. SBP-6 isolated from Sorghum bicolor . Acta Physiol Plant 38, 110 (2016). https://doi.org/10.1007/s11738-016-2123-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2123-9