Abstract
Auxin production and 1-aminocyclopropane-1-carboxylate (ACC) deaminase of rhizobacteria are very important plant growth promoting attributes. In the present study, Pseudomonas strains exhibiting these traits were evaluated for their growth promoting effects on Vigna mungo (L.). Colorimetric analysis revealed that Pseudomonas alcaliphila AvR-2, Pseudomonas sp. AvH-4 and Pseudomonas aeruginosa As-17, respectively, produced 40.30, 32.90 and 36.50 μg auxin ml−1 in the presence of 6% of glucose, sucrose and fructose. Similarly, Pseudomonas sp. AvH-4 expressed highest ACC-deaminase activity (355 nmol h−1) as compared to P. alcaliphila AvR-2 (115 nmol h−1) and P. aeruginosa As-17 (197 nmol h−1). Antibiotic sensitivity pattern of rhizobacteria also showed resistance against oxytetracyclin, erythromycin and penicillin. Inoculation of V. mungo with rhizobacteria positive for auxin production and ACC-deaminase activity enhanced plant growth in pot trials. In laboratory experiments (under axenic conditions), P. aeruginosa As-17 was the most effective at enhancing shoot length (70.90%), seedling fresh weight (185.70%) and root length (84.20%). Pot trials conducted under natural environmental conditions showed up to 45.60, 54.10 and 72.50% increases in shoot length, root length and number of pods, respectively, over control.
Similar content being viewed by others
Introduction
Bacteria inhabiting the rhizosphere may influence plant growth by a variety of mechanisms. Among them, bacterial syntheses of auxin and 1-aminocyclopropane-1-carboxylate (ACC) deaminase have been well characterized (Spaepen et al. 2007; Raddadi et al. 2008). Indole-3-acetic acid (IAA) represents one of the most extensively studied and abundant type of auxin in plants that regulates multidimensional developmental processes (Woodward and Bartel 2005). In addition to plants, IAA is also quantitatively the most abundant phytohormone secreted by rhizobacteria as secondary metabolite. L-tryptophan has been identified as the main precursor for IAA biosynthesis in bacteria. In vitro studies have demonstrated that some microbial cultures can produce small amounts of IAA in the absence of precursor. However, in the presence of L-tryptophan, microbes often release much greater quantities of IAA (Ali et al. 2009a). Root exudates of plants are considered to be the main source of tryptophan in rhizosphere that may result in microbial conversion to IAA (Kravchenko et al. 2004; Kamilova et al. 2006).
Ethylene is an important growth hormone that mediates a wide range of developmental processes in plants (Bleecker and Kende 2000). However, higher concentrations of ethylene are inhibitory to plant growth. ACC is the immediate precursor of ethylene that may be exuded from plant roots. For many plants, a burst of ethylene is required to break seed dormancy, but after seed germination a sustained level of ethylene inhibits root elongation. It has been reported that bacteria contain an enzyme ACC-deaminase that hydrolyses ACC into ammonia and α-ketobutyrate on the surface of roots and eliminates the potential inhibitory effects of higher ethylene concentrations and facilitates formation of longer roots (Penrose and Glick 2003; Glick 2005). Inoculation with specific rhizobacteria has been shown to alter the endogenous levels of ethylene which subsequently led to changes in the growth and development of inoculated plants (Shahroona et al. 2006). Bacterial ACC-deaminase activity increase plant tolerance to environmental stresses and promote legume nodulation (Grichko and Glick 2001; Ma et al. 2003; Saravanakumar and Samiyappan 2007). The main objective of this research work was to evaluate the potential of Pseudomonas strains to enhance the growth and yield of Vigna mungo (L.) Hepper. Pseudomonas strains were screened for plant growth promoting attributes, i.e. auxin production and ACC-deaminase activity. Bacterial auxin production was determined in the presence of glucose, sucrose and fructose. Pot trials were conducted to demonstrate the role of these two bacterial traits in plant growth promotion.
Materials and methods
Bacterial strains and biochemical characterization
Three PGPR strains used in this study were isolated by Ali et al. (2009b) from the rhizosphere of Amaranthus viridis (Pseudomonas alcaliphila AvR-2 and Pseudomonas sp. AvH-4) and Asphodelus tenuifolius (Pseudomonas aeruginosa As-17). Pseudomonas strains were characterized biochemically by using MicrobactTM Gram-negative identification system (Oxoid, UK) in accordance with the instructions of the manufacturer. It is a standardized micro-substrate system designed to simulate conventional biochemical substrates used for the identification of Enterobacteriaceae and common miscellaneous Gram-negative bacilli. It consists of dehydrated substrates for 24 different biochemical tests placed in the wells of a microtiter tray. A saline suspension of the test organism was added to each of the 24 wells and appropriate wells were overlaid with mineral oil and incubated overnight at 37°C. The results from biochemical tests were transcribed into an octal code and identification of Pseudomonas strains was confirmed by using the Microbact™ computer identification package.
Auxin production by Pseudomonas
Auxin production was estimated by growing strains in DF salts minimal medium (Dworkin and Foster 1958). About 50 ml medium was prepared in 100-ml Erlenmeyer flasks and supplemented with L-tryptophan to a final concentration of 500 μg ml−1. Medium was also supplemented with 2, 4 and 6% of glucose, sucrose and fructose to observe the effect of different carbon sources on auxin production. The flasks were inoculated with 100 μl of bacterial cell suspension adjusted to optical density of 0.5 measured at 600 nm by spectrophotometer. All flasks were incubated at 37°C for 72 h at 120 rpm in triplicate. After incubation, cells were removed from the culture medium by centrifugation at 2,300 g for 15 min and auxin was detected in 1 ml of supernatant using Salkowski reagent (Tang and Borner 1979). A standard curve was constructed by using different concentrations of standard IAA (Sigma) to determine auxin production by bacterial strains. Growth curve experiments were also conducted in 100 ml L-broth medium supplemented with 1,000 μg ml−I L-tryptophan. All flasks were incubated in duplicate and auxin production was quantified as mentioned above.
Screening rhizobacteria for ACC-deaminase activity
Induction of ACC-deaminase activity in Pseudomonas strains was carried out following the method of Penrose and Glick (2003). Bacterial cultures were grown in 25 ml L-Broth in triplicate and incubated overnight in an orbital shaker at 120 rpm at 37°C. Bacterial cells were harvested by centrifugation of the cultures at 8,000 g for 10 min at 4°C. Cells were washed with 5 ml DF salts minimal medium and centrifuged as mentioned above. Cells are then transferred to 7.5 ml DF salts minimal medium containing 45 μl ACC to a final concentration of 3 mM to induce the ACC-deaminase activity. The cultures were incubated in an orbital shaker at 120 rpm at 30°C for 24 h. After incubation, supernatant was removed and the cells were washed with 5 ml DF salts minimal medium. Finally, bacterial cells were suspended in 7.5 ml DF salts minimal medium in a fresh culture tube. The frozen 0.5 M ACC substrate solution was thawed, and an aliquot of 45 μl was added to the cell suspension as mentioned above. Cultures were incubated in an orbital shaker at 120 rpm at 30°C for 1 h. After 1 h, the liquid broth was centrifuged at 8,000g and liberated ammonia was measured in supernatant solution following the method of Nagatsu and Yagi (1966).
Antibiotic sensitivity of rhizobacteria
Antibiotic sensitivity of Pseudomonas strains was evaluated by the Kirby–Bauer method (Bauer et al. 1966). The susceptibility of the isolates was determined by using antibiotic discs of ampicillin (10 μg), chloramphenicol (30 μg), oxytetracyclin (30 μg), erythromycin (15 μg), penicillin (10 μg) and carbenicillin (100 μg). A lawn of test organisms was made on Muller–Hinton agar plates. Six discs for each antibiotic were placed on agar plate and experiment was repeated twice. All plates were incubated in an inverted position at 37°C for 24-h. After incubation, plates were examined for the presence and absence of zone of inhibition surrounding each disc. A measurement of the diameter of the zone of inhibition in millimeters was made and its size was compared to that contained in standardized chart as described in Cappuccino and Sherman (2002).
Plant growth experiments
Certified seeds of V. mungo were obtained from Punjab Seed Corporation, Lahore, Pakistan. Healthy seeds were surface sterilized with 0.1% HgCl2 for 5 min, with continuous shaking followed by repeated washing 3–4 times in sterilized glass-distilled water, so that all traces of HgCl2 were removed from the seeds. Sterilized seeds of V. mungo were incubated in bacterial cell suspension adjusted to optical density of 0.5 containing approximately 107 CFU ml−I for 15 min. The experiment was conducted under axenic conditions by inoculating seeds in pots (6 × 4 cm) filled with autoclaved soil and sand mixture in 1:1 ratio. All pots, plastic trays and covers used during the experiment were disinfected with 5% solution of sodium hypochlorite to maintain sterile conditions. Eight seeds were sown in each pot in triplicate and the experiment was repeated twice. After germination, thinning was carried out to leave 5 seedlings in each pot and growth parameters in terms of shoot length, shoot fresh weight, shoot dry weight and root length of plants were noted after 2 weeks. The experiment was conducted in the laboratory by incubating pots in Versatile Environmental Test Chamber (MRL-350 H; Sanyo, Osaka, Japan) at 25 ± 1°C under photoperiod of 16-h with light intensity of 50–55 μmol s−2 m−1. For the wire house experiment (at ambient light and temperature), seeds were treated with bacterial suspension as mentioned above and sown in larger pots (20 × 20 cm) containing 3 kg of unfertilized garden soil. Initially, 6 seeds were inoculated in each pot in six replicates. After germination, seedlings were thinned to 3 per pot. All pots were arranged in a completely randomized design. After 10 weeks, plants were harvested from each pot to measure shoot length, root length and number of pods.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) using SPSS 16, and means separated using Duncan’s multiple range test (P = 0.05). The correlation coefficients between bacterial auxin production and cell densities in growth curve experiment were also calculated (P = 0.01).
Results
Biochemical characterization
PGPR strains (AvR-2, AvH-4 and As-17) were identified by Microbact™ Gram-negative identification system (Table 1). All strains gave positive results for lysine, indole, citrate, TDA and arginine. None of the strains gave a positive result for Voges-Proskaüer, H2S, salicin fermentation and gelatin hydrolysis. Similarly, all strains were unable to ferment ornithine, mannitol, inositol, sorbitol, rhamnose and lactose into acids. Strains also showed variation for sucrose, arabinose, adonitol and raffinose utilization. Eight digit codes generated from positive results were used for the identification of strains by Microbact™ computer-aided identification package. On the basis of biochemical activities, strains showed maximum similarity with genus Pseudomonas. Biochemical results revealed that all strains gave positive results for indole and tryptophan deaminase (TDA). Strains showed cherry red color for TDA reaction that indicated the production of indolepyruvate by deamination of tryptophan. This is very important step in the transformation of tryptophan into IAA.
Auxin production by rhizobacteria
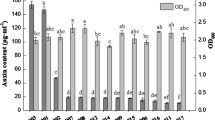
Colorimetric analysis showed that bacterial strains produced variable amounts of auxin when grown in the presence of different concentrations of carbon sources. Strains showed an increase in auxin content with increasing concentrations of sugars in all treatments (Fig. 1). For instance, P. alcaliphila AvR-2, Pseudomonas sp. AvH-4 and P. aeruginosa As-17, respectively, produced 40.30, 32.90 and 36.50 μg auxin ml−1 in minimal medium in the presence of 6% of glucose, sucrose and fructose. In the case of glucose (6%), up to 600% increase in auxin content was observed over control (Fig. 1a). When medium was amended with 6% sucrose, P. alcaliphila AvR-2, Pseudomonas sp. AvH-4 and P. aeruginosa As-17 showed 200, 400 and 460% increases in auxin content, respectively (Fig. 1b). Similarly, in the case of 6% fructose, strains showed up to 500% increase in auxin concentration (Fig. 1c).
Growth curve experiments
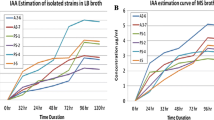
Bacterial strains produced maximum auxin levels at different time intervals as evident from growth curve experiments (Fig. 2). In all cases, maximum auxin synthesis was at stationary phase; nevertheless, variation exists between strains in early stationary, mid- or late stages. P. alcaliphila AvR-2 and Pseudomonas sp. AvH-4 showed maximum auxin production after 56 (32.4 μg IAA ml−1) and 64 h (72.3 μg IAA ml−1), respectively. However, P. aeruginosa As-17 showed an increase during the late stationary phase and expressed 106 μg IAA ml−I, after 80 h. Experiments also indicated that bacterial auxin production for each strain is dependent on bacterial cell density. This is evident from the highly significant positive correlation between bacterial cell densities and auxin production (Fig. 2).
Auxin production by rhizobacteria in L-broth medium supplemented with 1,000 μg ml−I L-tryptophan. a P. alcaliphila AvR-2, b Pseudomonas sp. AvH-4, c P. aeruginosa As-17. The results shown are representative of two repetitions of the experiment. Value r in each part indicates correlation between bacterial cell densities and auxin production. **P = 0.01
Antibiotic sensitivity
Generally, strains showed resistance against different antibiotics (Table 2). However, in some cases, strains also gave a zone of inhibition around the antibiotic discs. For instance, P. alcaliphila AvR-2 showed 16 and 14 mm zones of inhibition against ampicillin and oxytetracyclin, respectively. Pseudomonas sp. AvH-4 also showed a zone of inhibition against chloramphenicol (26 mm) and oxytetracyclin (12 mm). However, after comparison with a standard chart, P. alcaliphila AvR-2 showed intermediate sensitivity to ampicillin. On the other hand, Pseudomonas sp. AvH-4 was sensitive against chloramphenicol. Similarly, P. aeruginosa As-17 was sensitive to carbenicillin with a zone of inhibition of 17 mm.
ACC-deaminase activity and plant growth response
Pseudomonas sp. AvH-4 showed highest ACC-deaminase activity (355 nmol h−1), whereas P. alcaliphila AvR-2 and P. aeruginosa As-17 expressed 115 and 197 nmol h−1, respectively. Bacterization of V. mungo seeds resulted in increase of different growth parameters in pot trials. In the laboratory experiments (under axenic conditions), P. aeruginosa As-17 was the most effective that showed up to 70.90, 84.20, 185.70 and 228% increases in shoot length, root length, shoot fresh and dry weight, respectively (Fig. 3a, b). Similarly, in the wire house experiments, Pseudomonas sp. AvH-4 significantly enhanced shoot length (45.60%), over control (Fig. 4). In the case of root length, significant increases of 39.10, 54.10 and 33.30% were observed with P. alcaliphila AvR-2, Pseudomonas sp. AvH-4 and P. aeruginosa As-17, respectively. Inoculations with P. alcaliphila AvR-2, Pseudomonas sp. AvH-4 and P. aeruginosa As-17 recorded 35.80, 72.50 and 47.50% increases in the number of pods, over control.
Effect of Pseudomonas inoculations on growth of V. mungo in laboatory experiments under axenic conditions. a Shoot length (SL) and root length (RL). b Shoot fresh weight (SFW) and shoot dry weight (SDW). Bars represent means of 30 plants. Different letters on bars indicate significant difference between treatments, using Duncan’s multiple range test (P = 0.05)
Discussion
Bacterial strains showed variable potential for auxin production in the presence of different carbon sources. Increasing sugar concentrations also enhanced auxin production by bacterial strains. In growth curve experiments, maximum auxin production in bacterial culture supernatant was observed during the stationary phase. It has been previously reported that bacteria produced the highest concentrations of IAA during stationary phase cultures (Leveau and Lindow 2005). In other studies, it was noted that IAA biosynthesis is stimulated by stress factors including nutrient or carbon limitation, oxygen stress, and an entry into the stationary phase of growth (Ona et al. 2005; Malhotra and Srivastava 2009).
In our experiments, bacterization of V. mungo seeds significantly improved different growth parameter in laboratory and wire house experiments. In the laboraory experiments (under axenic conditions), strains improved shoot length (from 16.20 to 70.90%), seedling fresh weight (from 114 to 185%), seedling dry weight (from 128 to 228%) and root length (from 21 to 84%). Similarly, experiments conducted under natural environmental conditions showed up to 45.60, 54.10 and 72.50 increases in shoot length, root length and number of pods. It has been demonstrated that rhizobacteria with auxin production ability significantly enhanced plant growth (Ali et al. 2009a, b). However, in the present study, Pseudomonas sp. AvH-4 and P. aeruginosa As-17 showed maximum ACC-deaminase activity and they were also found to be most effective in stimulating root elongation. Shahroona et al. (2006) reported significant improvement in root elongation, shoot length and total biomass when plants were inoculated with rhizobacteria containing ACC-deaminase activity. In another study, inoculation of groundnut plants with Pseudomonas strains positive for ACC-deaminase significantly increased yield under salt stress (Saravanakumar and Samiyappan 2007).
Pseudomonas aeruginosa that is used in the present work is considered a potential human pathogen. The presence of Bacillus cereus, P. aeruginosa and Staphylococcus saprophyticus have been reported in the rhizosphere with several plant growth promoting traits including IAA (Egamberdieva et al. 2008). It has been reported that pathogenic species to animals and human are mainly transmitted through the food chain. Therefore, pathogenic bacteria can contaminate plant surfaces and actively interact and colonize them as an alternate host (Holden et al. 2009). Besides beneficial bacteria, the composition of root exudates also attracts potential human pathogens that also evolved to respond different plant signals. In this way, potentially pathogenic strains are supposed to survive and become enriched in the vicinity of roots where they rapidly utilize simple organic compounds (Ji and Wilson 2002; Egamberdieva et al. 2008). In conclusion, Pseudomonas strains produced maximum levels of auxin at high concentrations of glucose, sucrose and fructose. Strains also varied in their ability to exhibit ACC-deaminase activity. Inoculation of V. mungo with Pseudomonas strains enhanced different growth parameters in pot trials. Especially, Pseudomonas sp. AvH-4 significantly enhanced shoot length, root length and number of pods at final harvest. Hence, auxin production and ACC-deaminase activity of rhizobacteria are very important traits for the selection of effective plant growth promoting rhizobacteria.
References
Ali B, Sabri AN, Ljung K, Hasnain S (2009a) Quantification of indole-3-acetic acid from plant associated Bacillus spp. and their phytostimulatory effect on Vigna radiata (L.). World J Microbiol Biotechnol 25:519–526
Ali B, Sabri AN, Ljung K, Hasnain S (2009b) Auxin production by plant associated bacteria: impact on endogenous IAA content and growth of Triticum aestivum L. Lett Appl Microbiol 48:542–547
Bauer AW, Kirby WMM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Cappuccino JG, Sherman N (2002) Microbiology: a laboratory manual. Pearson, Signapore
Dworkin M, Foster JW (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–603
Egamberdieva D, Kamilova F, Validov S, Gafurova L, Kucharova Z, Lugtenberg B (2008) High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ Microbiol 10:1–9
Glick BR (2005) Modulation of plant ethylene levels by bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251:1–7
Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39:11–17
Holden N, Pritchard L, Toth I (2009) Colonization outwith the colon: plants as an alternate environment reservoir for human pathogenic enterobacteria. FEMS Microbiol Rev 33:689–703
Ji P, Wilson M (2002) Assessment of the importance of similarity in carbon source utilization profiles between the biological control agent and the pathogen in biological control of bacterial speck of tomato. Appl Environ Microbiol 68:4383–4389
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophan in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microb Int 19:250–256
Kravchenko LV, Azarova TS, Makarova NM, Tikhonovich IA (2004) The effect of tryptophan present in plant root exudates on the phytostimulating activity of rhizobacteria. Microbiology 73:156–158
Leveau JHJ, Lindow SE (2005) Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol 71:2365–2371
Ma W, Guinel FC, Glick BR (2003) Rhizobium leguminosarum Biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Micrbiol 69:4396–4402
Malhotra M, Srivastava S (2009) Stress-responsive indole-3-acetic acid biosynthesis by Azospirillum brasilense SM and its ability to modulate plant growth. Eur J Soil Biol 45:73–80
Nagatsu T, Yagi K (1966) A simple assay of monoamine oxidase and D-amino acid oxidase by measuring ammonia. J Biochem 60:219–221
Ona O, Impe JV, Prinsen E, Vnaderleyden J (2005) Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp 245 is environmentally controlled. FEMS Microbiol Lett 246:125–132
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Plant Physiol 118:10–15
Raddadi N, Cherif A, Boudabous A, Daffonchio D (2008) Screening of plant growth promoting traits of Bacillus thuringiensis. Ann Microbiol 58:47–52
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol 102:1283–1292
Shahroona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett Appl Microbiol 42:155–159
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Tang WY, Borner J (1979) Enzymes involved in synthesis and breakdown of indoleacetic acid. In: Paech K, Tracey MV (eds) Modern methods of plant analysis, vol. 7. Springer, Heidelberg, pp 238–241
Woodward AW, Bartel B (2005) Auxin: regulation, action and interaction. Ann Bot 95:707–735
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noreen, S., Ali, B. & Hasnain, S. Growth promotion of Vigna mungo (L.) by Pseudomonas spp. exhibiting auxin production and ACC-deaminase activity. Ann Microbiol 62, 411–417 (2012). https://doi.org/10.1007/s13213-011-0277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0277-7