Abstract
Airlift bioreactors were programmed for continuous and temporary immersion culture to investigate factors that affect the rhizome proliferation, shoot formation, and plantlet regeneration of Cymbidium sinense. During rhizome proliferation, the continuous immersion bioreactor system was used to explore the effects of activated charcoal (AC) in the culture medium, inoculation density, and air volume on rhizome differentiation and growth. The optimum conditions for obtaining massive health rhizomes were 0.3 g l−1 AC in the culture medium, 7.5 g l−1 inoculation density, and 150 ml min−1 air. In addition, the temporary immersion bioreactor system was used for both shoot formation and plantlet regeneration. Supplementing 4 mg l−1 6-benzylaminopurine and 0.2 mg l−1 naphthalene acetic acid (NAA) to the culture medium promoted shoot induction from the rhizome. Cutting the rhizome explants into 1 cm segments was better for massive shoot formation than cutting into 0.25 and 0.5 cm explant segments. NAA promoted plantlet regeneration and the rooting rate (94.7 %), with whole plantlets growing well in culture medium containing 1.0 mg l−1 NAA. Therefore, applying bioreactors in C. sinense micropropagation is an efficient way for scaling up the production of propagules and whole plantlets for the industrial production of high-quality seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cymbidium sinense is a traditional orchid in China that has attracted attention because of its graceful leaves and fragrant flowers (Weng et al. 2006). The species is propagated mainly through the division of pseudobulbs in vivo. However, one plant only generates three shoots each year (Chang and Chang 2000). Hence, tissue culture has been introduced to C. sinense for mass producing the seedlings and shortening the breeding time to meet market demand (Lee et al. 2011). The protocorm and rhizome are used for organogenesis from asymbiotic seed and shoot-tip culture of orchids (Chugh et al. 2009). C. sinense organogenesis occurs via the rhizome; however, rhizomes are recalcitrant to regeneration, and the shoots grow slowly and inconsistently even if they regenerate (Paek and Kozai 1998). To overcome these problems, a reliable culture method for efficient rhizome generation and plantlet regeneration needs to be developed. Recently, several studies have used bioreactors to micropropagate protocorm-like bodies (PLBs) (Park et al. 2000; Yang et al. 2010) and shoots (Wu et al. 2007; Yoon et al. 2007) of orchid. These studies indicate that bioreactor application is an efficient method for the rapid mass-propagation of orchid propagules. Our previous study also found that rhizome proliferation and shoot formation of C. niveo-maginatum in a simple bioreactor are significantly better than that in solid and flask suspension culture (Jin et al. 2007).
Numerous studies have applied bioreactors in plant cell (Ahmed et al. 2008; Huang and McDonald 2012) and organ (Wang and Qi 2010; Srivastava and Srivastava 2012) culture to obtain specific metabolites. In addition, a considerable number of researchers have cultured plant propagules in bioreactors to produce high-quality seedling (Hessami and Babaei 2012; Wang et al. 2012; Zhao et al. 2012). It is clear from these studies that temporary immersion bioreactor culture systems are appropriate for shoot multiplication and regeneration (Snyman et al. 2007; Watt 2012), and the continuous immersion system is suitable for the proliferation of propagules (without leaves) such as bublets (Lian et al. 2003; Kim et al. 2004), PLBs (Park et al. 2000; Yang et al. 2010) and rhizomes (Jin et al. 2007).
To our knowledge, no studies have investigated the micropropagation of C. sinense in bioreactors. Therefore, in the present study, continuous and temporary airlift bioreactor systems were used during different stages of C. sinense culture to explore several factors that affect rhizome proliferation, shoot formation, and plantlet regeneration, as well as to establish an efficient bioreactor culture protocol for mass-producing high-quality C. sinense seedlings.
Materials and methods
Rhizome proliferation
In vitro rhizomes of C. sinense were maintained on culture medium consisting modified Hyponex medium (Kano 1965), i.e., 2 g l−1 Hyponex I (N:P:K = 7:6:19, Jinan Plant Bio-Tech Co., Ltd., Shandong, China) and 0.5 g l−1 Hyponex II (N:P:K = 20:20:20, Jinan Plant Bio-Tech Co., Ltd., Shandong, China) supplemented with 1 g l−1 peptone + 2 mg l−1 6-benzylaminopurine (BA) + 0.2 mg l−1 naphthalene acetic acid (NAA) + 0.2 mg l−1 activated charcoal (AC) + 30 g l−1 sucrose + 7.0 g l−1 agar, and pH was adjusted to 5.4. The cultures were maintained at 25 °C with a 16-h photoperiod under 30 μmol m−2 s−1 white fluorescent lights. After 50 days of culture, the rhizomes were cut into 1 cm apical segments and used as experimental materials during rhizome proliferation in bioreactors.
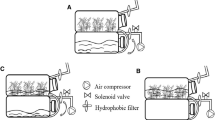
For rhizome proliferation, different activated charcoal concentrations, inoculation densities, and aeration volumes were tested in the continuous immersion culture system (Fig. 1) using 3-l airlift balloon-type bubble bioreactors with working volumes of 2 l. In the first experiment, 0.1, 0.2, 0.3, 0.4, and 0.5 g l−1 of AC powder were added into the culture medium [2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 2 mg l−1 BA + 0.2 mg l−1 NAA + 0.2 mg l−1 AC + 30 g l−1 sucrose (pH 5.4)]. Each bioreactor was inoculated with 5 g l−1 rhizome explants [fresh weight (FW), approximate 150 explants], and aerated at 100 ml min−1. In the inoculation density experiment, 5, 7.5, and 10 g l−1 FW of rhizome explants were transferred into separate bioreactors containing 2 l of culture medium [2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 2 mg l−1 BA + 0.2 mg l−1 NAA + 0.3 g l−1 AC + 30 g l−1 sucrose (pH 5.4)] and aerated at 100 ml min−1. Finally, 50, 100, and 150 ml min−1 air volumes were separately introduced into bioreactors with culture medium [2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 2 mg l−1 BA + 0.2 mg l−1 NAA + 0.3 g l−1 AC + 30 g l−1 sucrose (pH 5.4)] and inoculated with 7.5 g l−1 FW of explants. The rhizome numbers, length, and biomass of the three experimental sets were investigated after 50 days of culture.
To determine the kinetics of rhizome proliferation and growth, as well as the changes in culture medium, during bioreactor culture, a 3-l airlift bioreactor with a 2-l working volume (2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 2 mg l−1 BA + 0.2 mg l−1 NAA + 0.3 g l−1 AC + 30 g l−1 sucrose, pH 5.4) were inoculated with 7.5 g l−1 FW of explants per bioreactor and cultured at 25 °C under with a light intensity of 30 μmol m−2 s−1 (16 h day−1). The bioreactors were stopped at 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 days of culture, respectively. The rhizome cultures were collected from the bioreactors to investigate the biomass (fresh and dry weight) at every term, and approximately 10 ml of medium sample was simultaneously collected to determine its hydrogen ion concentration (pH), electrical conductivity (EC), and sugar content. The harvested rhizomes were patted dry with tissue paper, and their fresh weight was recorded, then the dry weight was determined after drying for 3 days at 55 °C. The culture medium samples were filtered with 0.2-μm membrane filters and their pH and EC were measured using pH and EC meters, respectively.
Shoot formation
Temporary immersion bioreactors (Fig. 1) were used to induce the shoots from the rhizome explants. First, the effect of BA and NAA in the culture medium was investigated. The rhizomes (7.5 g l−1 FW) were cut into 1 cm apical segments and transferred to 3 l bioreactor containing 2 l of medium (2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 30 g l−1 sucrose) supplemented with BA (4 mg l−1), with NAA (0.2 mg l−1), and a combination of BA (4 mg l−1) and NAA (0.2 mg l−1). The pH was adjusted to 5.4. In the second experiment, the rhizome inocula were cut into apical segments with different lengths (0.25, 0.5, and 1.0 cm). Then, the rhizome explants (225 explants per bioreactor) were inoculated into 3 l bioreactors with a working volume of 2 l. The medium consisted of 2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 4 mg l−1 BA + 0.2 mg l−1 NAA + 30 g l−1 sucrose (pH 5.4). In this shoot induction study, each temporary immersion bioreactor system was programmed using a timer and a solenoid valve to immerse the explants in the medium for 1 h and to dry for 1 h. The air volume was set to 150 ml min−1 and explants were cultured for 40 days.
Plantlet regeneration
The simplified culture method was used to obtain whole plantlets from shoots and investigated the effect of NAA concentration on plantlet growth. The bioreactors were programmed for temporary immersion (the explants were immersed in the medium for 1 h and allowed to dry for 1 h) to carry out the two-step culture in the same bioreactor; the air volume was adjusted to 150 ml min−1. In the first step, the rhizomes were cut into 0.5 cm apical segments to induce shoots from rhizomes. Then, 225 explants were inoculated into a 3-l bioreactor containing 2 l of shoot induction medium (2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 4 mg l−1 BA + 0.2 mg l−1 NAA + 30 g l−1 sucrose, pH 5.4). After 40 days of culture, the medium was replaced with plantlet regeneration medium containing different NAA concentrations [2 g l−1 Hyponex I + 0.5 g l−1 Hyponex II + 1 g l−1 peptone + 0.2 g l−1 AC + 30 g l−1 sucrose + NAA (0, 0.5, 1, 2 mg l−1), pH 5.4] to regenerate whole plantlets for 50 days.
Culture conditions and statistics
All of the bioreactor cultures were kept at 25 °C and 16-h photoperiod under white fluorescent light with 30 μmol m−2 s−1 intensity. Each treatment was performed three times, and all data were subjected to an analysis of variance and Duncan’s multiple range test using the SAS program (SAS Institute, Inc., USA), a probability of p < 0.05 was considered significant.
Results and discussions
Rhizome proliferation and growth
During rhizome proliferation in the bioreactor, the rhizome explants released phenolic compounds into the culture medium. The incised parts of the inocula turned brown after approximate 20 days of culture, and resulted in poor growth and proliferation (data not shown). To solve this problem, different amounts of AC were added to the culture medium to determine a suitable AC concentration. The maximum number of rhizomes was achieved in the 0.2 g l−1 AC treatment group whereas the maximum length was achieved in the 0.3 g l−1 AC treatment group. The rhizome biomass in the 0.3 g l−1 AC group was higher than that in the 0.2 g l−1 AC group. Rhizome differentiation and growth were restricted when the AC concentrations were less than 0.2 g l−1 or more than 0.3 g l−1 (Table 1; Fig. 2). Phenol is commonly exuded into the medium during orchid tissue culture, which influences cultures’ growth and differentiation. Hence, AC is often added to the culture medium for seed germination and growth (Shiau et al. 2005; Thompson et al. 2006), rhizome proliferation (Paek and Yeung 1991), and rooting (Yan et al. 2006) of orchids. Our results show that 0.3 g l−1 AC is the most effective AC concentration for C. sinense rhizome proliferation and growth in bioreactor cultures.
Inoculation density influences cultures’ growth during micropropagation (Yang et al. 2010). The present study also found that inoculation density affected the rhizome proliferation and biomass of C. sinense in bioreactors, but not during rhizome elongation (Table 2). The explants exhibited the highest differentiation in the 5.0 and 7.5 g l−1 inoculation density groups, up to 24.8–27.6 rhizomes per explant proliferated after 50 days of culture, but the total number of rhizome per bioreactor in the 7.5 g l−1 group was obviously more than that in the 5.0 g l−1 group. Inoculation densities of 7.5 and 10.0 g l−1 resulted in significantly higher rhizome biomass than with 5.0 g l−1. The relationship between inoculation density and cultures growth during in vitro culture has been studied repeatedly. Hahn and Paek (2005) reported that 20 nodes per 1 l medium was the best inoculation density for shoot multiplication during the bioreactor culture of Chrysanthemum. Piao et al. (2003) indicated that the maximum responses were recorded when 34 nodes per 1 l medium were included in the bioreactor. Yang et al. (2010) also found that an inoculation density of 6.6 g l−1 was optimal for Oncidium PLB growth. Cui et al. (2011) observed the greatest increase in Hypericum perforatum adventitious root biomass at an inoculum density of 3 g l−1. These results demonstrate that inoculation density affects culture differentiation and growth. The appropriate initial explant numbers depends on the plant species, organs, and culture methods. Plant micropropagation is used to obtain the maximum number of healthy cultures from a small quantity of inocula. Therefore, an inoculation density of 7.5 g l−1 was suggested for C. sinense rhizome proliferation culture in bioreactors.
The effect of air volume was also investigated in this study. During the initial 25 days of culture, rhizome explants were immersed in the culture medium and moved up and down in all groups of air volume (50, 100, and 150 ml min−1). However, after 25 days of culture, partial rhizomes dropped to the bottom of the bioreactor at air volumes of 50 and 100 ml min−1, restricting the airflow to the bioreactor. The less the air introduced into the bioreactor, the more the rhizomes dropped to the bottom. All rhizomes accumulated at the bottom of the bioreactor at 50 ml min−1 and partially at 100 ml min−1, whereas no rhizomes accumulated in the 150 ml min−1 group. The number, length, and biomass of the rhizome in the 150 ml min−1 group were significantly higher than in the 50 and 100 ml min−1 groups (Table 3). Aeration is an important parameter for plant culture in airlift bioreactors (Schlatmann et al. 1994). Dissolved oxygen is closely related to the air volume introduced into the culture medium in the airlift bioreactor, which affected culture growth (Jeong et al. 2006). However, excess air had a negative effect because of fluid dynamic stress (Zhang et al. 2007). A few studies were done on Cymbidium proliferation using bioreactors, Jin et al. (2007) found that the air volume of 100 ml min−1 promoted rhizome proliferation of C. niveo-maginatum in a simple bioreactor, this finding is a little different with our result. Consequently, air volume should be tested for the culture of every plant specials in the various kinds of bioreactor, and 150 ml min−1 was appropriate for rhizome proliferation of C. sinense in an airlift bioreactor.
To understand the rhizome growth kinetics during bioreactor culture, the changes in rhizome biomass were determined at regular intervals. Both the fresh weight and dry weight exhibited a similar pattern, and typically showed a lag phase (0–20 days), exponential phase (20–45 days), and stationary phases (45–50 days). Rhizome biomass increased sharply from 20 to 45 days, and peaked at 151.1 g fresh weight and 25.7 g dry weight on the 45th day, whereas no biomass changes were observed on the 50th day. Therefore, 45 days was considered as the optimum culture duration for rhizome proliferation culture in bioreactors (Fig. 3). The pH and EC of the culture medium are related to the inorganic salt concentrations, and indirectly reflect plant growth trend and nutritional needs. Figure 4 reveals that pH remained stable from day 0 to day 10, and then decreased rapidly from 5.3 to 4.3 from day 10 to day 40, followed by an increase to 5.1 until day 50 during the bioreactor culture. The EC values decreased with increasing culture duration, dropping from 2.6 to 2.0 mS cm−1 was observed during the initial 25 days of culture, and then gently decreased from 2.0 mS cm−1 to 1.8 mS cm−1 in the following days. Significant negative linear correlation was observed between EC and fresh weight (R 2 = 0.9602) or dry weight (R 2 = 0.9186) during the bioreactor culture (Fig. 5). The inverse correlation between EC and rhizome growth may be the result of the uptake of inorganic ions by plant cells (Hahlbrock and Kuhlen 1972). The pH of the culture medium did not affect rhizome biomass.
Shoot formation and growth
Continuous immersion culture system cannot induce shoots; hence, a temporary immersion bioreactor system was used for shoot induction from rhizomes in the present study. Shoots formed when the culture medium was supplemented with BA (with or without NAA); however, no shoot was induced upon the addition of NAA alone (Table 4). Compared with the medium containing BA alone, the medium containing both BA and NAA was more efficient for shoot proliferation and growth. The highest number of shoots (7.2) and highest fresh biomass (97.6 g explants−1) were observed in the group treated with 4 mg l−1 BA + 0.2 mg l−1 NAA, in which the shooting rate reached 89.2 %. The cytokinin to auxin ratio in the culture medium is one of the important factors for plant differentiation and growth during tissue culture (Kakani et al. 2009). For Cymbidium, higher cytokinin to auxin ratios stimulate shoot initiation (Lu et al. 2001). Cytokinins are essential for shoot formation from rhizomes in C. faberi (Hasegawa et al. 1985) and C. kanran (Shimasaki and Uemoto 1990), which supports our results. However, the types and concentrations of cytokinins and auxins need to be reasonably optimized according to plant species.
To test the effect of inoculum length on shoot formation, the rhizomes were cut into 0.25, 0.5, and 1.0 cm segments, and then inoculated into the bioreactors. Inoculum length affected shoot induction from the rhizomes, with more than 80 % of inocula forming shoots in the 0.5- and 1.0-cm groups and only 20.5 % of inocula forming shoots in the 0.25-cm group (Table 5). The 1.0 cm inoculum length favored the mass production of shoots, with 6.8 shoots per rhizome, and only 2.2 shoots per rhizome in the 0.5 cm group and 1.2 shoots per rhizome in the 0.25-cm group. Although the shoots in the 0.5-cm group were less than 1.0 cm, the 0.5-cm inoculum was beneficial for subsequent plantlet regeneration during the two-step bioreactor culture because only 1–2 shoots could form a health plantlet. This result suggests that rhizome explants need to be cut into lengths appropriate for the purpose of the culture, with 1.0 cm rhizome explants optimal for obtaining massive shoots and 0.5 cm rhizome explants suitable for obtaining whole plantlets during the two-step bioreactor culture.
Plantlet regeneration
To obtain whole plantlets using the bioreactor, 0.5-cm rhizomes were first inoculated into the bioreactors to induce the shoots. The medium was then replaced with medium containing different NAA concentrations and sequentially cultured for 50 days. Table 6 shows that NAA is a critical factor for shoot development and root formation, shoot length and fresh weight significantly differed among the NAA treatments. The 1-mg l−1 NAA treatment promoted shoot elongation and increased biomass, whereas concentrations higher and lower than 1 mg l−1 inhibited shoot development. NAA was more important for rooting. No roots formed in the culture medium without NAA. The highest rooting rate (94.7 %), root numbers (5.4), and root length (2.3 cm) were observed in the 1 mg l−1 NAA treatment. Although some shoots formed roots in the 0.5-mg l−1 NAA treatment (69.2 %) and the 1.5-mg l−1 NAA treatment (78.7 %), their root number and length were significantly lower than in the 1-mg l−1 NAA treatment. During plant micropropagation, indole-3-butyric acid (IBA) was used for rooting in many studies (Singh et al. 1994; Aktar et al. 2007; Melekber and Aysun 2013). However, some plant species also require NAA as rooting promoter (Mohsen 2001; Christos et al. 2010). Our results indicate that NAA promotes C. sinense rooting in bioreactor cultures. However, the effect of IBA and other auxins should be investigated in further studies.
In the present study, C. sinense rhizome proliferation, shoot formation, and plantlet regeneration were successfully performed in airlift bioreactors. The bioreactor culture revealed that AC in the culture medium, inoculation density, and air volume influence rhizome proliferation. The optimum conditions for massive rhizome production were 0.3 g l−1 AC, 7.5 g l−1 inoculation density, and 150 ml min−1 air volume. During shoot induction from rhizomes, the culture medium was supplemented with 4 mg l−1 BA with 0.2 mg l−1 NAA and the rhizomes were cut into 1 cm segments to maximize the number of shoots. To obtain whole plantlets, 1.0 mg l−1 NAA in the culture medium maximized plantlet growth, with 94.7 % of shoots forming roots. Further studies should focus on optimizing the two-step bioreactor culture for commercial Cymbidium seedling production.
Author contribution
All authors contributed extensively to the work presented in this paper (Micropropagation of Cymbidium sinense using continuous and temporary airlift bioreactor systems). Ri Gao, Song-Quan Wu, and Xuan-Chun Piao designed the experiments and wrote the paper under the guidance of Mei-Lan Lian. So-Young Park was responsible for viability tests and statistical analysis.
Abbreviations
- PLBs:
-
Protocorm-like bodies
- BA:
-
6-Benzylaminopurine
- NAA:
-
Naphthalene acetic acid
- AC:
-
Activated charcoal
- FW:
-
Fresh weight
- pH:
-
Hydrogenion concentration
- EC:
-
Electrical conductivity
References
Ahmed S, Hahn EJ, Paek KY (2008) Aeration volume and photosynthetic photon flux affect cell growth and secondary metabolite contents in bioreactor cultures of Morinda citrifolia. J Plant Biol 51:209–212
Aktar S, Nasiruddin KM, Huq H (2007) In vitro root formation in Dendrobium orchid plantlets with IBA. J Agr Rural Dev 5:48–51
Chang C, Chang WC (2000) Effect of thidiazuron on bud development of Cymbidium sinense Willd in vitro. Plant Growth Regul 30:171–175
Christos C, Chrysovalantou A, Ioannis P, Ioannis T, Kortessa D (2010) Effects of NAA and vitamin B2 on in vitro rooting of Citrus. Acta Agr Scand B-S P 60:189–192
Chugh S, Guha S, Rao IU (2009) Micropropagation of orchids: a review on the potential of different explants. Sci Hortic 122:507–520
Cui XH, Murthy HN, Jin YX, Yim YH, Kim JY, Paek KY (2011) Production of adventitious root biomass and secondary metabolites of Hypericum perforatum L. in a balloon type airlift reactor. Bioresource Technol 102:10072–10079
Hahlbrock K, Kuhlen E (1972) Relationship between growth of parsley and soybean cells in suspension cultures and changes in the conductivity of the culture medium. Planta 108:271–278
Hahn EJ, Paek KY (2005) Multiplication of Chrysanthemum shoots in bioreactors as affected by culture method and inoculation density of single node stems. Plant Cell Tissue Org Cult 81:301–306
Hasegawa A, Ohashi H, Goi M (1985) Effects of BA, rhizome length, mechanical treatment and liquid sharking culture on the shoot formation from rhizome in Cymbidium faberi Rolfe. Acta Hortic 166:25–40
Hessami S, Babaei A (2012) Banana micropropagation using an exclusive temporary immersion bioreactor. In Vitro Cell Dev Biol An 48:77
Huang TK, McDonald KA (2012) Bioreactor systems for in vitro production of foreign proteins using plant cell cultures. Biotechnol Adv 30:398–409
Jeong CS, Chakrabarty D, Hahn EJ, Lee HL, Paek KY (2006) Effects of oxygen, carbon dioxide and ethylene on growth and bioactive compound production in bioreactor culture of ginseng adventitious roots. Biochem Eng J 27:252–263
Jin H, Piao XC, Sun D, Xiu JR, Lian ML (2007) Mass production of rhizome and shoot of Cymbidum niveo-maginatum using simple bioreactor. J Northeast Forest Univ 35:44–48
Kakani A, Li GS, Peng ZH (2009) Role of AUX1 in the control of organ identity during in vitro organogenesis and in mediating tissue specific auxin and cytokinin interaction in Arabidopsis. Planta 229:645–657
Kano K (1965) Studies on the media for orchid seed germination. Mem Fac Agric Kagawa Univ 20:1–68
Kim EK, Hahn EJ, Murthy HN, Paek KY (2004) Enhanced shoot and bulblet proliferation of garlic (Album sativum L.) in bioreactor systems. J Hortic Sci Biotech 79:818–822
Lee OR, Yang DC, Chung HJ, Min BH (2011) Efficient in vitro plant regeneration from hybrid rhizomes of Cymbidium sinense seeds. Hortic Environ Biotechnol 52:303–308
Lian ML, Chakrabarty D, Paek KY (2003) Growth of Lilium oriental hybrid ‘Casablanca’ bulblet using bioreactor culture. Sci Hortic 97:41–48
Lu I, Sutter E, Burger D (2001) Relationships between benzyladenine uptake, endogenous free IAA levels and peroxidase activities during upright shoot induction of Cymbidium ensifoilum cv. Yuh Hwa rhizomes in vitro. Plant Growth Regul 35:161–170
Melekber S, Aysun C (2013) Micropropagation of cherry laurel Prunus laurocerasus L. Food Agric Environ 11:576–579
Mohsen KHE (2001) Comparison, determination and optimizing the conditions required for rhizome and shoot formation, and flowering of in vitro cultured calla explants. Sci Hortic 101:305–313
Paek KY, Kozai T (1998) Micropropagation of temperate Cymbidium via rhizome culture. HortTechnology 8:283–288
Paek KY, Yeung EC (1991) The effects of 1-naphthaleneacetic acid and N6-benzyladenine on the growth of Cymbidium forrestii rhizomes in vitro. Plant Cell Tissue Org Cult 24:65–71
Park SY, Murthy HN, Paek KY (2000) Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis. Plant Cell Tissue Org Cult 63:67–72
Piao XC, Chakrabarty D, Hahn EJ, Paek KY (2003) A simple method for mass production of potato microtubers using a bioreactor system. Curr Sci India 84:1129–1132
Schlatmann JE, Moreno PRH, Vinke JL, Tenhoopen HJG, Verpoorte R, Heijnen JJ (1994) Effect of oxygen and nutrient limitation on ajmalicine production and related enzyme-activities in high-density cultures of Catharanthus roseus. Biotechnol Bioeng 44:461–468
Shiau YJ, Nalawade SM, Hsai CN, Tsay HS (2005) Propagation of Haemaria discolor via in vitro seed germination. Biol Plantarum 49:341–346
Shimasaki K, Uemoto S (1990) Micropropagation of a terrestrial Cymbidium species using rhizomes developed from seeds and pseudobulbs. Plant Cell Tissue Org Cult 23:237–244
Singh S, Ray BK, Bhattacharyya S, Deka CP (1994) In vitro propagation of Citrus reticulata Blanco and Citrus limon Burm. f. HortScience 29:214–216
Snyman SJ, Meyer GM, Richards JR, Ramgareeb S, Banasiak M, Huckett B (2007) Use of the temporary immersion RITA (R) bioreactor system for micropropagation of sugarcane. S Afr J Bot 73:336–337
Srivastava S, Srivastava AK (2012) In vitro azadirachtin production by hairy root cultivation of Azadirachta indica in nutrient mist bioreactor. Appl Biochem Biotech 166:365–378
Thompson DI, Edwards TJ, van Staden J (2006) Evaluating asymbiotic seed culture methods and establishing Disa (Orchidaceae) germinability in vitro: relationships, requirements and first-time reports. Plant Growth Regul 49:269–284
Wang GR, Qi NM (2010) Influence of mist intervals and aeration rate on growth and second metabolite production of Pseudostellaria heterophylla adventitious roots in a siphon-mist Bioreactor. Biotechnol Bioproc E 15:1059–1064
Wang SM, Piao XC, Park SY, Lian ML (2012) Improved micropropagation of Gypsophila paniculata with bioreactor and factors affecting ex vitro rooting in microponic system. In Vitro Cell Dev Biol Plant 49:70–78
Watt MP (2012) The status of temporary immersion system (TIS) technology for plant micropropagation. Afr J Biotechnol 11:4025–14035
Weng JZ, Lin JG, Lin JB (2006) Influence of different activated carbon concentrations on culture in vitro of Cymbidium sinense. J Trop Subtrop Bot 35:37–38
Wu RZ, Chakrabarty D, Hahn EJ, Paek KY (2007) Micropropagation of an endangered jewel orchid (Anoectochilus formosanus) using bioreactor system. Hortic Environ Biotechnol 48:376–380
Yan N, Hu H, Huang J, Xu K, Wang H, Zhou Z (2006) Micropropagation of Cypripedium flavum through multiple shoots of seedlings derived from mature seeds. Plant Cell Tissue Org Cult 84:114–118
Yang JF, Piao XC, Sun D, Lian ML (2010) Production of protocorm-like bodies with bioreactor and regeneration in vitro of Oncidium ‘Sugar Sweet’. Sci Hortic 125:712–717
Yoon YJ, Murthy HN, Hahn EJ, Paek KY (2007) Biomass production of Anoectochilus formosanus Hayata in a bioreactor system. J Plant Biol 50:573–576
Zhang CH, Piao XC, Lian ML, Wang SM (2007) Application of bioreactors in rapid propagation of Gypsophila paniculata. Chinese Bull Bot 24:526–531
Zhao Y, Sun W, Wang Y, Saxena PK, Liu CZ (2012) Improved mass multiplication of Rhodiola crenulata shoots using temporary immersion bioreactor with forced ventilation. Appl Biochem Biotech 166:1480–1490
Acknowledgments
This research was supported by the National Science Foundation of China (30860176).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. van Staden.
Rights and permissions
About this article
Cite this article
Gao, R., Wu, SQ., Piao, XC. et al. Micropropagation of Cymbidium sinense using continuous and temporary airlift bioreactor systems. Acta Physiol Plant 36, 117–124 (2014). https://doi.org/10.1007/s11738-013-1392-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1392-9