Abstract
Plants for the phytoextraction of heavy metals should have the ability to accumulate high concentrations of such metals and exhibit multiple tolerance traits to cope with adverse conditions such as coexistence of multiple heavy metals, high salinity, and drought which are the characteristics of many contaminated soils. This study compared 14 succulent species for their phytoextraction potential of Cd, Cr, Cu, Mn, Ni, Pb, and Zn. There were species variations in metal tolerance and accumulation. Among the 14 succulent species, an Australian native halophyte Carpobrotus rossii exhibited the highest relative growth rate (20.6–26.6 mg plant−1 day−1) and highest tolerance index (78–93 %), whilst Sedum “Autumn Joy” had the lowest relative growth rate (8.3–13.6 mg plant−1 day−1), and Crassula multicava showed the lowest tolerance indices (<50 %). Carpobrotus rossii and Crassula helmsii showed higher potential for phytoextraction of these heavy metals than other species. These findings suggest that Carpobrotus rossii is a promising candidate for phytoextraction of multiple heavy metals, and the aquatic or semiterrestrial Crassula helmsii is suitable for phytoextraction of Cd and Zn from polluted waters or wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some plant species (defined as hyperaccumulators) can take up and accumulate extraordinarily higher (10–100 times) concentrations of heavy metals in shoots than do most plants (Brooks et al. 1977), and this has led to the development of a cleanup technology termed phytoextraction (Chaney 1983). As an environment-friendly and cost-effective approach compared to chemical and physical techniques (Moffat 1995; Salt et al. 1995a), this phytoextraction approach has attracted much attention in remediation research of soils contaminated with heavy metals. Selection of suitable plant species is the most important step in the development of a phytoextraction approach.
Most heavy-metal-contaminated sites are characterized by high concentrations of multiple heavy metals (Baun and Christensen 2004; Moffat 1995), especially landfills typically polluted with common heavy metals like Cd, Cr, Cu, Mn, Ni, Pb, and Zn. Although hyperaccumulators can accumulate high amounts of heavy metals in shoots, most hyperaccumulators are not effective for practical phytoextraction applications due to their specificity to a particular metal, low biomass production, and their requirement for specific management under field conditions (Gleba et al. 1999; Hassan and Aarts 2011). Recently, much interest has been focused on the utilization of crop plants capable of producing high biomass with high concentrations of heavy metals in shoots (Doty 2008) to improve phytoextraction efficiency. Indian mustard (Brassica juncea) has been considered as one such candidate species for phytoextraction (Belimov et al. 2005; Ebbs et al. 1997; Kochian and Ebbs 1998) because it is reasonably tolerant to and accumulate multiple heavy metals (Blaylock et al. 1997; Quartacci et al. 2006; Salt et al. 1995a) including Pb in shoots (Kumar et al. 1995) and produces high shoot biomass (Salt et al. 1995b; Singh et al. 2010). It is also tolerant to drought and salinity (Bauddh and Singh 2012; Moffat 1995; Novo et al. 2014).
More recently, two succulent species of Crassulaceae have been identified as potential candidates for the phytoextraction of heavy metals. These are Sedum alfredii which is a Cd/Zn cohyperaccumulator (Yang et al. 2004) and Pb accumulator and Sedum plumbizincicola as a Zn hyperaccumulator (Wu et al. 2012). As many succulent plant species are tolerant to drought, they are likely to be promising candidates for phytoextraction of metals in dry polluted sites like rural landfills in Australia, which have regular dry periods. This present study selected seven Sedum species and investigated their tolerance and shoot uptake of heavy metals. In addition, another seven succulent species were selected and investigated on the basis of their apparent metal tolerance (Baker 1984). Thus, 14 succulent species were exposed to multiple heavy metals (Cd, Cr, Cu, Mn, Ni, Pb, and Zn) at various concentrations. The objective was to assess their tolerance to these metals and to determine their ability to extract these metals from soil in their shoots.
Materials and methods
Soil
A silt loam soil was collected from the 0–25-cm surface layer at the agricultural reserve of La Trobe University. It contained 21.3 % clay, 54.5 % silt, and 24.1 % sand. The soil had 2.4 % organic C, 2.75 g kg−1 total N, 44 mg kg−1 Colwell P, 126 mg kg−1 Colwell K, 0.076 dS m−1 electrical conductivity (1:5 water), and pH 5.41 (1:5 soil/0.01 M CaCl2). The concentrations of total Cd, Cr, Cu, Mn, Ni, Pb, and Zn in the unamended soil were 0.55, 4.2, 18, 303, 5.0, 28, and 119 mg kg−1, respectively.
Experiment design and treatments
The experiment was conducted using a completely randomized block design with four replicates. It consisted of 15 plant species and three levels of heavy metals. The three levels of heavy metals (Cd, Cr, Cu, Mn, Ni, Pb, and Zn in a mixture) were selected based on the results of a preliminary experiment which grew geranium (Pelargonium zonal, tolerant) (Dan et al. 2002), radish (Raphanus sativus, sensitive) (Chaney 1983), and Indian mustard (B. juncea). These were T0 (without addition of heavy metals, as control), T1, and T2 (addition of designed concentrations of heavy metals as shown in Table 1).
The 15 species include 14 succulent plant species and geranium as a reference plant. Geranium showed some tolerance to the mixture of heavy metals in the preliminary experiment. The 14 succulent plant species belong to four families and six genera. The seven Sedum (Crassulaceae) species were collected from Royal Botanic Gardens Victoria, and these included Sedum rubrotinctum, Sedum × Graptosedum “Bert Swanwick”, Sedum stahlii, Sedum mexicanum, Sedum sediforme, Sedum spectabile, and Sedum “Autumn Joy”. Three Crassula species (Crassulaceae) (Crassula ovate, Crassula helmsii, and Crassula multicava), one Senecia [Senecia serpens (Asteraceae)], and a Portulacaria [Portulacaria afra (Portulacaceae)] species were collected from residential gardens. Two species of Aizoaceae were Carpobrotus rossii collected from a rural landfill site (37° 36′ S, 143° 35′ E) and Disphyma crassifolium collected from a Melbourne beach.
Plant growth
Uniform cuttings of each species were prepared for propagation in plastic nursery cells (5 × 5 × 8 cm) filled with the soil. Osmocote fertilizer (nutrient composition as N 15.3 %, P 3.56 %, K 12.6 %, Scotts Australia Pty Ltd) was mixed with the soil at 10 g kg−1, and an automatic water spray was used for irrigation. After 1 month, root systems of the cuttings were well developed and then transplanted into the experiment pots.
Each of experiment pots was prepared by mixing 1.5-kg soil in a plastic bag. Nutrients were added together with heavy metals at the desired concentrations. Copper and Mn were added as different metallic compounds (Table 1) to avoid excess of companion elements (Cl and S). All the chemicals were of analytical grade. Various amounts of KNO3, KCl, NH4Cl, (NH4)2SO4, K2SO4, and NH4NO3 were added to balance the amounts of Cl, S, N, and K between the treatments with final additions of 160, 234, 144, and 253 mg kg−1, respectively. This was followed by incubation at 80 % of field capacity in a constant-temperature room (25 ± 0.2 °C) for 2 weeks, and during this period, the soil was mixed daily.
Rooted cuttings of uniform size for each species were planted into the pots, and the number of plant cuttings in each pot varied from 2 to 6 depending on plant size of the individual species. Plants were grown in a glasshouse with minimum and maximum temperatures of 19 and 33 °C, respectively. The pots were irrigated with deionized water to 80 % of field capacity for 84 days.
Plant harvest
After 84 days of growth, the plants were harvested. Shoots and roots were separated except for Crassula helmsii and S. stahlii which produced very fine roots. The shoots were first rinsed with running tap water, then immersed in 0.01 M HCl for approximately 5 s in order to remove dust from the shoot surface (Papazoglou 2011), and then washed with deionized water twice. After removing the soil particles, the roots were given the same washing procedure as the shoots.
Measurements
Total root length was determined using a root scanner at 400 dpi (Epson Perfection 4990 Scanner, model J131B, Epson Inc.) with WinRhizo software (Regent Instruments Inc.). Shoots and roots were placed in paper bags and oven-dried at 80 °C for 72 h. Dry weight was recorded. The dried plant samples were then ground to pass a 0.25-mm sieve.
Ground plant samples were digested in a mixture of concentrated HNO3 and HClO4 (4:1 by volume) (Monsant et al. 2008). The samples were then diluted to 25 mL using 5 % HCl for further analysis. Metal concentrations in digests were determined using inductively coupled plasma–optical emission spectrometry (ICP-OES) analysis (Varian Vista AX CCD, Australia Pty Ltd.). For quality control, three reference samples and three blanks were used for every batch.
Assessment of tolerance and accumulation and statistical analysis
The relative growth rate (RGR) (mg plant−1 day−1) was calculated as
where W 1 and W 2 are the estimated initial (t 1) (an average biomass of five to ten cuttings per species at the beginning of the experiment) and final (t 2) shoot biomass (g) per plant, respectively.
The concentrations of individual heavy metals were compared between the species using one-way analysis of variance (ANOVA) using SPSS (version 18.0, Chicago, IL, USA). To assess the phytoextraction potential of these plant species, two indexes were calculated (Fitz and Wenzel 2002; Yoon et al. 2006).
The total phytoextraction potential of the chosen seven heavy metals was characterized by the following equation:
where i = 1, 2,…, 7 represent Cd, Cr, Cu, Mn, Ni, Pb, and Zn, respectively; Ri represents the ranking value of one species for each heavy metal.
Results
Biomass production
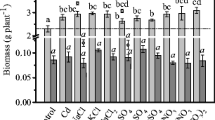
There was a large variation in growth parameters between the species grown in the control soil. Dry shoot weights ranged from 0.04 to 1.05 g plant−1 at the commencement of treatment and from 0.6 to 6.4 g plant−1 at harvest. The relative growth rate (RGR) of shoots ranged from 13.6 to 37.7 mg plant−1 day−1 during the experimental period (Table 2). Exposure to multiple heavy metals decreased shoot biomass and RGR, depending on species and the level of treatment (Table 2). At the highest level of treatment (T2), Crassula helmsii, D. crassifolium, Carpobrotus rossii, and S. mexicanum had higher RGR than other species, within a range of 20.6–24.6 mg plant−1 day−1, while Sedum “Autumn Joy” had the lowest RGR.
Exposure to multiple heavy metals also decreased root biomass production of all species and root length of selected species, except for Carpobrotus rossii at the low level of heavy metals (T1). Compared to the control (T0), root biomass and length of Carpobrotus rossii grown in T1 treatment increased by 31 and 17 %, respectively (Table 3) but, in T2 treatment, decreased by 15 and 18 %, respectively. Among the 14 succulent species grown in T2 treatment, Carpobrotus rossii and D. crassifolium (24 %) exhibited the lowest decrease in root biomass and length whilst Crassula multicava and Crassula ovate showed the great decrease in root biomass, i.e., 81 and 76 %, respectively.
Tolerance to multiple heavy metals
Overall, Crassula helmsii, Carpobrotus rossii, S. mexicanum, S. × Graptosedum, and D. crassifolium showed a higher tolerance to Cd, Cr, Cu, Mn, Ni, Pb, and Zn than the other species studied, with tolerance indices ranging from 88 to 93 % in T1 treatment and from 64 to 78 % in T2 treatment (Fig. 1). Carpobrotus rossii had the highest tolerance indices, i.e. 93 and 78 % in T1 and T2 treatments, respectively, whilst Crassula multicava showed the lowest tolerance indices, less than 50 %. However, the tolerance rankings of some species were inconsistent between T1 and T2 treatments.
Tolerance indices of 14 succulent species and Pelargonium zonal exposed for 84 days to low (T1) (a) and high levels (T2) (b) of multiple heavy metals. Tolerance index = (shoot RGR in treatment / shoot RGR in the control) × 100. Error bars represent ± SE (n = 4). The LSD bars are also shown. The species highlighted in a solid-line rectangle had the lowest reduction in RGR at T1 compared to at T0, and the species highlighted in a dash-line rectangle had the highest reduction in RGR at T1. The dotted lines are based on 50 and 90 % of the control (100 %), respectively
In the T1 treatment, there were seven species with significantly less tolerance than the reference species, and others had a similar tolerance to the reference species (Fig. 1). In the T2 treatment, only four species, namely S. sediforme, S. stahlii, S. spectabile, and Crassula multicava showed significantly less tolerance than the reference species (Fig. 1).
Concentrations of heavy metals in shoots and roots
The concentrations of heavy metals in shoots (Fig. 2) and roots (Fig. 3) showed a substantial species variation in the T1 and T2 treatments. The highest concentration of each metal varied with species, i.e., Cd 143 μg g−1 in shoots of Crassula helmsii and 571 μg g−1 in roots of S. rubrotinctum, Cr 3.0 μg g−1 in shoots of P. zonal and 561 μg g−1 in roots of S. rubrotinctum, Cu 30 μg g−1 in shoots of Carpobrotus rossii and 601 μg g−1 in roots of D. crassifolium, Mn 597 μg g−1 in shoots of D. crassifolium and 1087 μg g−1 in roots of D. crassifolium, Ni 55 μg g−1 in shoots of Carpobrotus rossii and 213 μg g−1 in roots of Crassula multicava, Pb 35 μg g−1 in shoots of Crassula helmsii and 714 μg g−1 in roots of Crassula multicava, and Zn 976 μg g−1 in shoots of Crassula helmsii and 1544 μg g−1 in roots of S. rubrotinctum.
Concentrations of Cd (a), Cr (b), Cu (c), Mn (d), Ni (e), Pb (f), and Zn (g) in the shoots of succulent species and Pelargonium zonal exposed for 84 days to low (T1) and high (T2) concentrations of multiple heavy metals. Error bars represent ±SE (n = 4). The species were ordered based on their concentrations at low level (T1). The species highlighted in a solid-line rectangle had the lowest reduction in RGR at T1 compared to at T0, and the species highlighted in a dash-line rectangle had the highest reduction in RGR at T1
Concentrations of Cd (a), Cr (b), Cu (c), Mn (d), Ni (e), Pb (f), and Zn (g) in the roots of succulent species and Pelargonium zonal exposed to low (T1) and high (T2) concentrations of multiple heavy metals. Error bars represent ±SE (n = 4). The roots of Crassula helmsii were too fine to be collected, and the roots of Sedum stahlii in T2 treatment were too small to be collected. The species highlighted in a solid-line rectangle had the lowest reduction in RGR at T1 compared to at T0, and the species highlighted in a dash-line rectangle had the highest reduction in RGR at T1
Concentrations of heavy metals in plants increased as their concentrations increased in the soil. An exception was that the Cu concentrations in the shoots of most species decreased with increasing the soil concentration of this metal (Fig. 2c) although the opposite was generally observed with the Cu concentrations in roots (Fig. 3c).
Bioaccumulation factor
The values for bioaccumulation factor (BF) showed a great variation between the heavy metals and the 14 succulent species grown in the two treatments (Fig. 4). Among the seven heavy metals, Cd and Zn had BF greater than 1.0 for most plant species, followed by Ni and Mn, while Cr, Cu, and Pb exhibited the lowest BFs of less than 1.0 for all selected species in the two treatments. Irrespective of plant species, BF values generally decreased with increasing the concentration of heavy metals in soil.
Bioaccumulation factor of Cd (a), Cr (b), Cu (c), Mn (d), Ni (e), Pb (f), and Zn (g) of 14 succulent species and Pelargonium zonal exposed for 84 days to low (T1) and high (T2) concentrations of multiple heavy metals. Error bars are ±SE (n = 4). Bioaccumulation factor = metal concentration in shoots / total metal concentration in soil (Baker et al. 1994). The species were ordered based on their concentrations at low level (T1). The species highlighted in a solid-line rectangle had the lowest reduction in RGR at T1 compared to at T0, and the species highlighted in a dash-line rectangle had the highest reduction in RGR at T1
In particular, Carpobrotus rossii, which was identified as tolerant to heavy metals, had BF of 2.3–2.7 for Cd, 2.3–2.8 for Zn, 1.2–1.6 for Ni, 0.8–1.1 for Mn, 0.2–0.7 for Cu, 0.06–0.11 for Cr, and 0.02–0.03 for Pb. Similarly, Crassula helmsii had BF of 6.9–7.4 for Cd, 2.0–2.3 for Zn, 0.5–1.1 for Ni, 0.3–1.0 for Mn, 0.3–0.4 for Cu, 0.05–0.07 for Cr, and 0.04–0.11 for Pb (Fig. 4).
Translocation factor
The translocation factor (TF) values of seven heavy metals varied among the 14 succulent species (Fig. 5). Similar to BF, the TF values of Cd, Mn, and Zn were greater than 1.0 in more than half of the species grown in the T1 treatment, while TF values of the other four heavy metals were less than 1.0 in almost all of the species grown in the T1 and T2 treatments.
Translocation factor (TF) of Cd (a), Cr (b), Cu (c), Mn (d), Ni (e), Pb (f), and Zn (g) of 13 succulent species and the reference plant Pelargonium zonal exposed for 84 days to low (T1) and high (T2) concentrations of multiple heavy metals. Error bars represent ±SE (n = 4). TF = metal concentration in shoots / metal concentration in roots (Baker et al. 1994). The roots of Crassula helmsii at T1 and T2, and of Sedum stahlii at T2 were too small to be collected. The species were ordered based on their concentrations at low level (T1). The species highlighted in a solid-line rectangle had the lowest reduction in RGR at T1 compared to at T0, and the species highlighted in a dash-line rectangle had the highest reduction in RGR at T1
Carpobrotus rossii had TF of 0.23–0.29 for Cd, 1.57–1.66 for Zn, 1.79–2.56 for Mn, 0.35–0.53 for Ni, 0.12–0.35 for Cu, 0.06–0.07 for Cr, and 0.03–0.05 for Pb. Due to insufficient root samples, TF was not determined for Crassula helmsii.
Shoot uptake of heavy metals and the phytoextraction potential
Shoot uptake of heavy metals was a function of shoot biomass and concentration of heavy metals in shoots and is related to phytoextraction efficiency. Carpobrotus rossii and Crassula helmsii had greater uptake of Cu, Mn, and Zn than the other species (Fig. 6c, d, g). Carpobrotus rossii showed the greatest uptake of Cr and Cu in the T1 treatment (Fig. 6c, d) while Crassula helmsii had the highest uptake of Cd, Pb, and Zn (Fig. 6a, f, g).
Amounts of Cd (a), Cr (b), Cu (c), Mn (d), Ni (e), Pb (f, and Zn (g) in the shoots of 14 succulent species and Pelargonium zonal exposed for 84 days to low (T1) and high (T2) concentrations of multiple heavy metals. Error bars represent ±SE (n = 4). Amounts of heavy metals in shoots (μg plant−1) = heavy metal concentration in shoot × shoot RGR × experimental period. The species highlighted in a solid-line rectangle had the lowest reduction in RGR at T1 compared to at T0, and the species highlighted in a dash-line rectangle had the highest reduction in RGR at T1
The phytoextraction potential of the plants grown in the T1 and T2 treatments varied widely between the 14 succulent species (Fig. 7). The ranking pattern was similar between the two treatments. Obviously, Carpobrotus rossii and Crassula helmsii had the highest phytoextraction potential whereas Crassula multicava had the lowest. Carpobrotus rossii exhibited the potential for phytoextraction as a result of its higher tolerance and biomass production as well as its higher concentrations of heavy metals than other species.
Phytoextraction potential of 14 succulent species and Pelargonium zonal exposed for 84 days to low (T1) (a) and high (T2) (b) concentrations of multiple heavy metals. Error bars represent ±SE (n = 4). The species highlighted in a solid-line rectangle had the lowest reduction in RGR at T1 compared to at T0, and the species highlighted in a dash-line rectangle had the highest reduction in RGR at T1
Discussion
Species variation in metal tolerance
The 14 succulent species showed significant differences in shoot (RGR) and root growth in response to the two treatments (Tables 2 and 3). Their tolerance to the combination of seven heavy metals, as indicated by the corresponding tolerance indices, also varied (Fig. 1). Moreover, the metal tolerance reflected cotolerance to multiple heavy metals rather than single-metal tolerance. It should be noted that the metal concentrations in shoots (Fig. 2) exceeded the critical toxic levels of Cd (5–10 μg g−1) (White and Brown 2010) in all species, of Zn (300–600 μg g−1) (Long et al. 2003) in some species, and of Ni (25 μg g−1) (Uren 1992) in two species. The cotolerance to multiple heavy metals has been shown in species (Baker et al. 1999) such as Deschampsia cespitosa which is cotolerant to Zn, Pb, Ni, and Cu (Cox and Hutchinson 1979), Silene vulgaris cotolerant to Cu, Zn, Cd, Ni, and Co (Schat and Vooijs 1997), and Chloris barbata cotolerant to Hg, Cd, and Zn (Patra et al. 1994). The cotolerance in plants may result from the sharing of transporters among these metals and their sharing of a similar fate in the plants (e.g., binding to cell walls) (Fernando et al. 2013; Guerinot 2000; Oomen et al. 2009; Thomine et al. 2000). But, it is still unclear why these cotolerant species still exhibit element-specific tolerances and differences in accumulation patterns. Further work is needed to elucidate whether the element-specific tolerance is related to some secondary metabolites in plants.
The four species with the highest shoot RGR, when grown in the two multi-metal treatments, were Crassula helmsii, D. crassifolium, Carpobrotus rossii, and S. mexicanum (Table 2). They also had higher tolerance indices than most of the other species studied. Additionally, it is interesting to note that among these four species, only Carpobrotus rossii showed significant increases in both root biomass and length in the T1 treatment (Table 3). The growth stimulus by heavy metals (Cox and Hutchinson 1980) has also been documented in many metal-tolerant species like Indian mustard, rapeseed, barley, and tumbleweed (Shi and Cai 2009 and literatures therein) and especially in hyperaccumulators like Arabidopsis halleri and S. alfredii (Hu et al. 2009; Tang et al. 2009; Yang et al. 2004; Zhao et al. 2000). In contrast, the root growth of other species generally decreased in the presence of heavy metals (Table 3), which was consistent with their shoot growth.
The reference plant P. zonal was a good indicator plant for distinguishing the metal tolerances among the 14 succulent species studied because it showed a moderate metal tolerance (Fig. 1). In previous studies, P. zonal showed high tolerance to Cd, Pb, and Ni (Arshad et al. 2008; Dan 2001; Dan et al. 2002). For example, when grown in a solution culture, 8.9 mM Cd did not decrease the photosynthesis in P. zonal (Dan et al. 2000). The high tolerance of P. zonal has been attributed mainly to a low TF and to increased lignification in its tissues leading to an increase in the formation of metal-lignin complexes on cell walls (Dan et al. 2002).
Although the amount of Cd added to the soil was moderate and the addition of other heavy metals exacerbated the metal toxicity effects, P. zonal still exhibited relatively higher metal tolerance (Fig. 1), which might be attributed to low translocation ability of these metals from roots to shoots (TF <1) (Fig. 5). The low translocation ability of P. zonal was also observed in other studies (Dan 2001; Dan et al. 2000, 2002; KrishnaRaj et al. 2000). By comparison with this reference plant grown in the T2 treatment, the 14 succulent species could be classified into three groups: (1) highly tolerant group with >78 % tolerance indices, Carpobrotus rossii and S. × Graptosedum; (2) sensitive group with 39–45 % tolerance indices, S. stahlii, S. spectabile, and Crassula multicava; and (3) moderately tolerant group with 53–65 % tolerance indices, the remaining species (Fig. 1b).
The species variation in metal tolerance could result from a number of different mechanisms. These include the metal detoxification by complexation of heavy metals with metabolites acting as high-affinity ligands. Other mechanisms involve compartmentalization in vacuoles or cell walls, their distribution to insensitive tissues (e.g. leaf epidermis) (Baker et al. 1999; Callahan et al. 2006), their exclusion from the roots, or their translocation to shoots (Baker 1981). In the present study, root and/or shoot growth of Crassula helmsii, D. crassifolium, Carpobrotus rossii, S. mexicanum, S. × Graptosedum, S. rubrotinctum, and S. stahlii were not affected by the T1 treatment. Among these seven species, the tolerance of S. × Graptosedum, Carpobrotus rossii, and D. crassifolium was associated with both high translocation ability of metals (e.g., Cd, Mn, and Zn) from roots to shoots, which was evidenced by TF values of >1, and high storage ability of metals (Cd, Cr, Cu, Ni, and Pb) (Fig. 5). D. crassifolium and Carpobrotus rossii are from the same family Aizoaceae and may share the same mechanisms of metal tolerance. It is interesting to note that Sedum species had higher TF values than most other species (Fig. 5), indicating that high translocation ability of heavy metals from roots to shoots plays an important role in their metal tolerance. However, the information on mechanisms of metal tolerance of individual species is limited.
Species variation in metal extraction
The present study showed that the 14 succulent species differed greatly in their ability to accumulate heavy metals. Interestingly, Carpobrotus rossii not only exhibited high metal tolerance (Fig. 1), but also had high concentrations and BFs for Cr, Cu, Mn, Ni, Pb, and Zn in its tissues (Figs. 2 and 3), indicative of the phytoextraction potential in soils contaminated with multiple heavy metals. However, due to competition of multiple heavy metals, the metal concentrations recorded in the present study are lower than those in plants with high accumulation capacity. Therefore, further experiments are needed to investigate tolerance and accumulation of the respective heavy metals in some promising species, especially Carpobrotus rossii, and to elucidate the associated mechanisms.
In comparison, most Sedum species in the T1 treatment had higher concentrations of metals in shoots (Fig. 2) and higher BFs for Cd, Cu, Ni, Pb, and Zn (Fig. 4). However, these Sedum species are not suitable for phytoextraction of Cu and Pb or Cr and Mn due to BFs of less than 1 (Fig. 4). Plants with lower metal concentrations in shoots than those in soils are not useful for phytoextraction (Lottermoser et al. 2008). Additionally, compared to two identified Sedum Cd hyperaccumulators S. alfredii (Yang et al. 2004) and S. plumbizincicola (Wu et al. 2012), the Sedum species in the present study had much lower concentrations of Cd, Pb, and Zn, which might partly result from the metal competition in the multiple heavy metal experiments. Similarly, Crassula helmsii has been reported as a Cu hyperaccumulator with Cu concentration of up to 9000 μg g−1 in shoots at 10 μM Cu2+ in a nutrient solution (Küpper et al. 2009), but the concentration of Cu in shoots ranged only from 15 to 30 μg g−1 in the present study (Fig. 2), indicating that Cu uptake and accumulation may have been inhibited by the other heavy metals present. The reduced accumulation due to competition of multiple heavy metals was also reported with a Cu accumulator (up to 355 μg g−1 in shoots) Hirschfeldia incana (L.) that did not have high Cu concentrations (7.5–12.2 μg g−1) in contaminated soils containing 35–72, 190–2345, and 68–2602 mg kg−1 of Cu, Pb, and Zn, respectively (Gisbert et al. 2006). The competition of heavy metals has been attributed to their sharing metal transporters in plants (Guerinot 2000; Rivetta et al. 1997; Sasaki et al. 2012; Zornoza et al. 2010) or to non-specific metal uptake (Baker et al. 1999).
The accumulation of heavy metals in shoots was proportional to the species translocation factor (TF). In the present study, most Sedum species had higher translocation ability than the other species studied, with Sedum “Autumn Joy” having the highest TF values for all heavy metals (Fig. 5). The high translocation ability of heavy metals in Sedum species is consistent with other studies involving S. alfredii (Yang et al. 2004) and S. plumbizincicola (Wu et al. 2012). Thus, the high TF in Sedum species was responsible for the high concentrations of Cd, Cu, Ni, Pb, and Zn in shoots relative to roots, but these species had a low uptake and, hence, low phytoextraction potential due to their shoot biomass (Table 3) being lower than that of the remaining succulent species. In comparison, although the higher metal-tolerant Carpobrotus rossii had lower TF for Cd and Ni, intermediate TF for Cr and Zn, and higher TF only for Cu, Pb and Mn, the combination of higher shoot biomass and higher concentrations of heavy metals (except for Cd) made it the species with the highest shoot uptake of heavy metals (Fig. 6) and, therefore, the species with the highest phytoextraction potential which was similar to that of the Crassula helmsii (Fig. 7).
Candidates for phytoextraction of multiple heavy metals
Most contaminated soils and sites are characterized by contamination with multiple heavy metals, high salinity, and deficiency of nutrients (Prasad et al. 2006). Therefore, potential plants used for phytoextraction should have at least five traits including (i) tolerance to multiple heavy metals;,(ii) fast growth and high biomass production, (iii) great accumulation of metals in harvestable parts, (iv) deep rooting, and (v) tolerance to soil constraints (Garbisu and Alkorta 2001; Lu et al. 2014). A high BF is also important for plants used for phytoextraction (1998). Based on these requirements and realistic performance in the field, we consider that Carpobrotus rossii and Crassula helmsii have phytoextraction potential for soils contaminated with multiple metals. Due to the presence of multiple heavy metals in the soil in the present study, further work is needed to investigate tolerance and accumulation of specific heavy metals by Carpobrotus rossii and to study the associated mechanisms. Additionally, in consideration of both high salinity in most polluted sites and the halophytic nature of Carpobrotus rossii (Pirie et al. 2013), experiments should be carried out to examine phytoextraction behavior of this species at sites with high salinity. It is worth noting that Crassula helmsii is aquatic or has semiterrestrial traits and thus may be suitable for remediation of polluted waters or wetlands.
Conclusions
This study showed that Carpobrotus rossii is a promising candidate for phytoextraction of multiple heavy metals in contaminated soils for a number of reasons. First, among the 14 succulent species, Carpobrotus rossii had the highest RGR and the highest tolerance index when grown in soil containing a mixture of multiple heavy metals. Second, Carpobrotus rossii showed the second highest phytoextraction potential ranking just behind aquatic or semiterrestrial Crassula helmsii at the two levels of the mixture of seven heavy metals. The latter may be suitable for phytoextraction of Cd and Zn in polluted waters or wetlands.
References
Arshad M, Silvestre J, Pinelli E, Kallerhoff J, Kaemmerer M, Tarigo A, Shahid M, Guiresse M, Pradere P, Dumat C (2008) A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 71:2187–2192
Baker AJM (1981) Accumulators and excluders—strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AJM (1984) Environmentally-induced cadmium tolerance in the grass Holcus lanatus L. Chemosphere 13:585–589
Baker AJM, Reeves RD, Hajar ASM (1994) Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol 127:61–68
Baker AJ, McGrath SP, Reeves RD, Smith JAC (1999) Metal hyperaccumulator plants. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishers, London, pp 85–107
Bauddh K, Singh RP (2012) Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol Environ Saf 85:13–22
Baun DL, Christensen TH (2004) Speciation of heavy metals in landfill leachate: a review. Waste Manag Res 22:3–23
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250
Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I (1997) Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol 31:860–865
Brooks RR, Lee J, Reeves RD, Jaffre T (1977) Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J Geochem Explor 7:49–57
Callahan DL, Baker AJM, Kolev SD, Wedd AG (2006) Metal ion ligands in hyperaccumulating plants. J Biol Inorg Chem 11:2–12
Chaney RL (1983) Plant uptake of inorganic waste constituents. In: Parr JF, Marsh PD, Kla JM. Park Ridge, NJ (eds), Land treatment of hazardous wastes. Noyes Data Corporation, pp 50–76
Cox RM, Hutchinson TC (1979) Metal co-tolerance in the grass Deschampsia cespitos. Nature 279:231–233
Cox RM, Hutchinson TC (1980) Multiple metal tolerances in the grass Deschampsia cespitosa (L.) Beauv. from the Sudbury smelting area. New Phytol 84:631–647
Dan TV (2001) Phytoremediation of metal contaminated soils metal tolerance and metal accumulation in Pelargonium sp. Ph. D. Thesis. The University of Guelph, Horticultural Science and Land Resource Division
Dan TV, KrishnaRaj S, Saxena PK (2000) Metal tolerance of scented geranium (Pelargonium sp. ‘Frensham: effects of cadmium and nickel on chlorophyll fluorescence kinetics’. Int J Phytoremed 2:91–104
Dan TV, KrishnaRaj S, Saxena PK (2002) Cadmium and nickel uptake and accumulation in scented geranium (Pelargonium sp. ‘Frensham’). Water Air Soil Pollut 137:355–364
Doty SL (2008) Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol 179:318–333
Ebbs SD, Lasat MM, Brady DJ, Cornish J, Gordon R, Kochian LV (1997) Phytoextraction of cadmium and zinc from a contaminated soil. J Environ Qual 26:1424–1430
Fernando DR, Marshall AT, Forster PI, Hoebee SE, Siegele R (2013) Multiple metal accumulation within a manganese-specific genus. Am J Bot 100:690–700
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil–rhizosphere–plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99:259–278
Garbisu C, Alkorta I (2001) Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour Technol 77:229–236
Gisbert C, Clemente R, Navarro-Aviñó J, Baixauli C, Ginér A, Serrano R, Walker DJ, Bernal MP (2006) Tolerance and accumulation of heavy metals by Brassicaceae species grown in contaminated soils from Mediterranean regions of Spain. Environ Exp Bot 56:19–27
Gleba D, Borisjuk NV, Borisjuk LG, Kneer R, Poulev A, Sarzhinskaya M, Dushenkov S, Logendra S, Gleba YY, Raskin I (1999) Use of plant roots for phytoremediation and molecular farming. Proc Natl Acad Sci U S A 96:5973–5977
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Hassan Z, Aarts MGM (2011) Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ Exp Bot 72:53–63
Hu P-J, Qiu R-L, Senthilkumar P, Jiang D, Chen Z-W, Tang Y-T, Liu F-J (2009) Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ Exp Bot 66:317–325
Kochian LV, Ebbs SD (1998) Phytoextraction of zinc by oat (Avena sativa), barley (Hordeum vulgare), and Indian mustard (Brassica juncea). Environ Sci Technol 32:802–806
KrishnaRaj S, Dan TV, Saxena PK (2000) A fragrant solution to soil remediation. Int J Phytoremed 2:117–132
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Küpper H, Gotz B, Mijovilovich A, Kupper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol 151:702–714
Long XX, Yang XE, Ni WZ, Ye ZQ, He ZL, Calvert DV, Stoffella JP (2003) Assessing zinc thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. Commun Soil Sci Plant Anal 34:1421–1434
Lottermoser BG, Ashley PM, Munksgaard NC (2008) Biogeochemistry of Pb–Zn gossans, northwest Queensland, Australia: implications for mineral exploration and mine site rehabilitation. Appl Geochem 23:723–742
Lu H, Li Z, Fu S, Mendez A, Gasco G, Paz-Ferreiro J (2014) Can biochar and phytoextractors be jointly used for cadmium remediation? PLoS One 9, e95218
Moffat AS (1995) Plants proving their worth in toxic metal cleanup. Science 269:302–303
Monsant AC, Tang C, Baker AJ (2008) The effect of nitrogen form on rhizosphere soil pH and zinc phytoextraction by Thlaspi caerulescens. Chemosphere 73:635–642
Novo LAB, Covelo EF, Gonzalez L (2014) Effect of salinity on zinc uptake by Brassica Juncea. Int J Phytoremed 16:704–718
Oomen RJFJ, Wu J, Lelievre F, Blanchet S, Richaud P, Barbier-Brygoo H, Aarts MGM, Thomine S (2009) Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol 181:637–650
Papazoglou EG (2011) Responses of Cynara cardunculus L to single and combined cadmium and nickel treatment conditions. Ecotoxicol Environ Saf 74:195–202
Patra J, Lenka M, Panda BB (1994) Tolerance and co-tolerance of the grass Chloris barbata Sw. to mercury, cadmium and zinc. New Phytol 128:165–171
Pirie A, Parsons D, Renggli J, Narkowicz C, Jacobson GA, Shabala S (2013) Modulation of flavonoid and tannin production of Carpobrotus rossii by environmental conditions. Environ Exp Bot 87:19–31
Prasad MNV, Sajwan KS, Naidu R (2006) Trace elements in the environment: biogeochemistry, biotechnology, and bioremediation. Taylor & Francis Group, LLC
Quartacci MF, Argilla A, Baker AJ, Navari-Izzo F (2006) Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 63:918–925
Rivetta A, Negrini N, Cocucci M (1997) Involvement of Ca2+-calmodulin in Cd2+ toxicity during the early phases of radish (Raphanus sativus L) seed germination. Plant Cell Environ 20:600–608
Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I (1995a) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology (N Y) 13:468–474
Salt DE, Prince RC, Pickering IJ, Raskin I (1995b) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24:2155–2167
Schat H, Vooijs RIET (1997) Multiple tolerance and co-tolerance to heavy metals in Silene vulgaris a co-segregation analysis. New Phytol 136:489–496
Shi G, Cai Q (2009) Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv 27:555–561
Singh S, Ramachandran V, Eapen S (2010) Copper tolerance and response of antioxidative enzymes in axenically grown Brassica juncea (L.) plants. Ecotoxicol Environ Saf 73:1975–1981
Tang YT, Qiu RL, Zeng XW, Ying RR, Yu FM, Zhou XY (2009) Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot 66:126–134
Thomine S, Wang RC, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci U S A 97:4991–4996
Uren NC (1992) Forms, reactions, and availability of nickel in soils. Adv Agron 48:141–203
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080
Wu LH, Liu YJ, Zhou SB, Guo FG, Bi D, Guo XH, Baker AJM, Smith JAC, Luo YM (2012) Sedum plumbizincicola X.H. Guo et S.B. Zhou ex L.H. Wu (Crassulaceae): a new species from Zhejiang Province, China. Plant Syst Evol 299:487–498
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Yoon J, Cao XD, Zhou QX, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Zhao FJ, Lombi E, Breedon T, McGrath SP (2000) Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ 23:507–514
Zornoza P, Sanchez-Pardo B, Carpena RO (2010) Interaction and accumulation of manganese and cadmium in the manganese accumulator Lupinus albus. J Plant Physiol 167:1027–1032
Acknowledgments
We thank the Royal Botanic Gardens Victoria for supplying Sedum species, Dr. Trevor Edwards for the identification of plant species, and Mr. Rob Evans for assistance in the experiment. We also thank two anonymous reviewers for constructive comments. This research was supported by the Australian Research Council Linkage Grant LP100100800.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Zhang, C., Sale, P.W.G., Clark, G.J. et al. Succulent species differ substantially in their tolerance and phytoextraction potential when grown in the presence of Cd, Cr, Cu, Mn, Ni, Pb, and Zn. Environ Sci Pollut Res 22, 18824–18838 (2015). https://doi.org/10.1007/s11356-015-5046-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5046-x