Abstract
Soluble acid invertase (SAI, EC 3.2.1.26), catalyzes the hydrolysis of sucrose into hexose sugars, and it has been considered a key enzyme for carbohydrate metabolism. In the present study, the activity of SAI enzyme was determined to establish a correlation between the change in transcript levels and enzyme activity in high and low sugar accumulating sugarcane cultivars, in various internodal tissues at different developmental stages. A decrease in SAI activity and transcript levels was observed with age, during all the developmental stages in both the cultivars. A negative correlation between SAI activity and sucrose content was observed in mature and immature internodes; however, there was a positive correlation between SAI activity and content of hexose sugars. These results imply that SAI plays a crucial role in sucrose partitioning in various intermodal tissues in high and low sugar cultivars. In addition to this, the changes in enzyme activity also resulted in changes in transcript level.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum officinarum L.) is the most important source of sucrose worldwide and accounts for >70% of global sucrose production (Singh et al. 2006). The high sucrose level (up to 650 mM) in storage tissues of some Saccharum spp. hybrids (Welbaum and Meinzer 1990) makes it an important model genus to study the interactions between source (leaf) and sink (culm) tissues. Commercial sugarcane cultivars are inter-specific hybrids with an estimated bio-physiological capability of storing up to 62% of the dry weight or 25% of the fresh weight of their stem as sucrose (Grof and Campbell 2001). This estimate is almost double the current commercial cultivars yields. Increases in sucrose yield and the incorporation of important agronomical traits have been achieved traditionally by using crossing and screening methods. However, it seems that the natural genetic potential has been exhausted as sucrose yields have reached a plateau (Grof and Campbell 2001; Moore 2005). Novel approaches, i.e., biotechnological interventions, are therefore needed to break through this apparent yield ceiling (Moore 2005; Grof and Campbell 2001). Unfortunately, despite a fair amount of research on the primary carbohydrate metabolism of sugarcane, our understanding of sucrose accumulation in the plant is still incomplete (Moore 1995, 2005).
Sucrose produced during photosynthesis in mature leaves is exported according to the demands of the sink tissues (McCormick et al. 2006). However, plant growth and productivity are not only determined by photosynthetic capacity, but also by the way in which the products of photosynthesis are partitioned and used in plant growth and development. Carbohydrate metabolism is orchestrated at the genetic level by proteins, which sense sucrose and hexoses and transduce these signals to modify transcription. Thus the whole carbon economy of the plant is sensitive to sugar-mediated regulation. There are two major enzymes in leaves, invertase (β-fructofuranosidase) and sucrose synthase, which produce free hexose from sucrose. In leaves, sucrose synthesized in the cytosol can be partitioned either for export or for storage in the vacuole (Winter et al. 1994). Acid invertase exists as a soluble form in the vacuole and as a cell wall-bound form in the cell wall, but totally absent in the cytosol (Walker and Pollock 1993). The hypothesis that vacuolar acid invertase activity regulates the amount of sucrose in leaves has been proposed on a number of occasions (Scholes et al. 1996). Under this scenario, high vacuolar invertase would decrease the amount of sucrose available for both storage and export, while low acid invertase activity would favor sucrose accumulation. Sugarcane storage tissue accumulates sugar against a concentration gradient using energy provided by respiration (Burg and Bieleski 1962). This is accompanied by a continuous cleavage and synthesis of sucrose during accumulation of sucrose in storage tissue (Batta and Singh 1986). Primary sucrose metabolism is governed by several enzymes (Quick and Schaffer 1996). Despite recent advances in the study of sucrose accumulation into the vacuole of plant cells (Echeverria et al. 1997), very little is known about its mobilization. From previous studies, it has been inferred that sucrose is enzymatically hydrolyzed in the vacuole prior to its mobilization (Martinoia 1992).
The improvement of modern cultivars could be achieved by identifying genes associated with important agronomic traits, such as sucrose content. These genes can then be used to generate transgenic plants or serve as molecular markers for map-assisted breeding (Menossi et al. 2008). Internodes have been expression-profiled during internodal development (Carson and Botha 2002; Carson et al. 2002; Casu et al. 2007), but differences between cultivars that contrast in sucrose content have not been extensively reported. Understanding differences in the regulation of genes related directly or indirectly to sucrose accumulation in different cultivars is an important step if we are to aid breeding for sugar yield improvement.
In this study, the sugar concentrations of various internodal tissues of four sugarcane cultivars (two high sucrose and two low sucrose accumulating) at different developmental stages were first compared to verify possible differences in sucrose concentrations. Thereafter, internodal tissues (immature and mature internodes) with significantly different sucrose concentrations were subjected to semiquantitative reverse transcriptase-PCR and changes in transcript levels of SAI gene were compared with the changes in SAI enzyme activity.
Materials and methods
Plant samples
Four elite sugarcane cultivars were used for this study, two early maturing, high sucrose-accumulating cultivars (CoS 96268, CoS 95255) and two late maturing, low sucrose-accumulating cultivars (CoS 97264, CoSe 92423). Sugarcane planting was performed in the first week of March 2006. During planting, two budded sets were transplanted at 60 cm within rows and 90 cm apart, replicated thrice at the Sugarcane Research Institute Farm, Shahjahanpur, India situated at 27.58°N latitude and 79.54°E longitude. The crop cultural practices were applied as per local recommendations. As a basal dose, 150 kg ha−1 N, 80 kg ha−1 P and 60 kg ha−1 K fertilizer and five irrigations were given to secure higher cane yield (t ha−1) and maintain soil fertility. In Shahjahanpur, the maturity phase starts from the month of October when the minimum and maximum temperatures range between 14–20 and 28–32°C, respectively, which is known to favor sugar synthesis and accumulation.
The collection of samples was started with 7-month-old sugarcane crop on the fifth day of each month from 240 to 390 DAP. Immature internodes (1/3rd part from top) and mature internodes (1/3rd part from bottom) of the stem were taken separately as samples. The harvesting procedure was performed quickly in the field so that the time elapse between excising the internodes and freezing the storage tissue was kept to a minimum, usually <1 min. All the samples were always collected at 9.00 a.m. to minimize the diurnal variation in enzyme activities and sugar levels.
Enzyme activity, sugar analysis and gene expression pattern
Until now, two types of soluble invertases, viz., cytoplasmic (neutral; pH 7.0) and vacuolar (acidic; pH 5.5), and one insoluble cell wall bound invertase (acidic; pH 4.5) have been reported in sugarcane. No other isoform of soluble invertase has been reported from the vacuole in sugarcane. In our studies, we have measured the vacuolar SAI activity at specific pH by assuming that no other isoform will show its activity at this pH. Further, for our expression studies, we have designed primers from conserved domains of SAI gene (Accession no. 31872117), specific for activity in the vacuoles. Therefore, the expressions are assumed to be solely of the vacuolar SAI gene.
Extraction of SAI enzymes
Stem (100 g) samples were ground into fine powder in liquid nitrogen, and 5 ml g−1 fresh weight of buffer A [50 mM di-sodium hydrogen phosphate adjusted to pH 7.5, containing 10 mM HCl, 1 mm β-mercaptoethanol, 5 μm MnSO4, modified from Vattuone et al. (1983)] was added. All subsequent procedures were performed at 4°C. The extract was filtered through nylon mesh and centrifuged for 5 min at 10,000g. The supernatant volume was recorded and the extract was desalted into buffer A using Sephadex G-50 (Sigma-Aldrich, St. Louis, MO, USA). Desalted extracts were frozen in liquid nitrogen and stored at −80°C until assay.
Assay of SAI activity
Soluble acid invertase activity was assayed by using the protocol of Batta and Singh (1986). The SAI activity assayed by employing the reaction mixture (1.0 ml) comprised 200 μl 0.5 M sucrose, 600 μl 0.2 M sodium acetate buffer (pH 5.5) and 100 μg desalted enzyme preparation. The reaction mixtures were incubated at 37°C for 1 h and the assay reaction was stopped by the addition of 3 M Tris base followed by boiling for 10 min. The amounts of hexose sugars produced were determined by Nelson’s arseno-molybdate reagent (Nelson 1944) method. Hexose sugar concentration was calculated by using glucose (Sigma-Aldrich) as a standard. Protein concentration was determined by the method of Lowry et al. (1951) using bovine serum albumin (Sigma-Aldrich) as a standard protein.
Extraction and estimation of endogenous free sugars
Endogenous free sugars were extracted from 100 g stem tissue. Small pieces of stem tissue were first boiled in 80% (v/v) ethanol followed by 70% (v/v) ethanol. The ethanol extracts were pooled and diminished pressure was applied at 40°C to get an aqueous syrup. Then sucrose content was determined according to the method of Roe (1934). To a test tube containing 500 μl of test extract, 500 μl 6% KOH was added. The tube was boiled in a water bath for 20 min. The tube was then allowed to cool down to room temperature and 10 ml 0.1% resorcinol solution and 3.0 ml 30% HCl were added. The tubes were incubated at 80°C for 10 min. OD of the solution was taken at 490 nm. The values were compared to a standard curve of sucrose (Sigma-Aldrich) treated in the same way. The content of total hexose sugars was determined by using Nelson’s arseno-molybdate reagent (Nelson 1944) method.
RNA isolation and cDNA synthesis
RNA isolation and cDNA synthesis was performed as described by Verma et al. (2010) for sugarcane. Total RNA was isolated from 100 mg of plant samples (mature and immature internodes) using total RNA isolation kit (Sigma). The integrity of isolated total RNA was checked by agarose gel electrophoresis (data not shown) and DNA contamination was removed by using a DNA-free kit (Ambion, Austin, TX, USA). The purified RNA was stored at −80°C for further experiments.
The cDNA was synthesized by using first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). In a 20 μl reverse transcription reaction, 1 μl of oligo (dT)18 primer was mixed with 2 μg total RNA, and DEPC treated water was added to a final volume of 16.2 μl. The RNA mixture was denatured for 10 min at 65°C and quenched on ice. cDNA-synthesis reaction buffer and 10 mM dNTP were added and the reaction mixture was incubated at 42°C for 2 min. Thereafter, the cDNA synthesis was initiated by adding 1 μl superscript II reverse transcriptase (20 U μl−1) and incubated at 42°C for 45 min. Thereafter, heating the reaction at 70°C for 10 min inactivated the reverse transcriptase.
Expression analysis of SAI gene by RT-PCR
Expression analysis of SAI gene was performed by following the method as described by Verma et al. (2010) for Sucrose phosphate synthase and sucrose synthase genes in sugarcane. Total RNA was isolated from the mature and immature internodes of the different cultivars and cDNAs were synthesized as described above. The sequences encoding the SAI and actin genes in sugarcane were downloaded from NCBI database. The accession numbers of the sequences were 31872117 and 53759188 for SAI and actin, respectively. Expression analysis of SAI gene was performed by semiquantitative RT-PCR. Primers (forward primer 5′CGTCCTCTCCGCCGCGCT3′, reverse primer 5′GGAGCCGGTGTAGAGCATGG3′) used for semiquantitative PCR of SAI were designed by using the sequence of accession no. 31872117 from NCBI to amplify the 519 bp of SAI gene. Primer for internal control actin (forward primer 5′GGACATCCAGCCTCTTGTC3′, reverse primer 5′GCAAGATCCAAACGAAGAATG G3′) were designed by using the sequence of accession no. 53759188 from NCBI to amplify the 534 bp actin gene. The respective cDNA was used for the amplification of both SAI and actin genes. Equal amount of template cDNAs were taken for the PCR reaction from each analyzed day of the respective cultivar and the amplification was carried by using taq DNA polymerase as follows: step 1, 95°C for 5 min, 58°C for 1 min, 72°C for 30 s; step 2, 95°C for 1 min, 58°C for 1 min, 72°C for 30 s. Step 2 was repeated for 10, 15, 20, 25 and 30 cycles for each sample and results were analyzed by densitometry after agarose gel electrophoresis. Semiquantitative RT-PCR was performed for the SAI enzyme activity in top and bottom portion of the cultivar CoS 96268 and CoS 95255 (high sucrose accumulating) and CoS 97264 and CoSe 92423 (low sucrose accumulating).
Data analysis
All the results of enzyme activities and sugar contents were average of three replicates of independent experiments, respectively. The correlation between enzyme activity and sugar content was determined by linear regression using the Excel™ software package (Microsoft) and by Pearson correlation using SPSS 11.0 for Windows™.
Results
Changes in endogenous free sugars of internodal tissues
Sugar accumulation levels in the immature and mature internodal tissues of sugarcane cultivars CoS 96268, CoS 95255 (high sugar accumulating) and CoS 97264, CoSe 92423 (low sucrose accumulating) were compared during the 240–390 DAP stages (grand growth to ripening stage). In high and low sucrose-accumulating cultivars, sugars (i.e., sucrose and hexoses) were accumulated differently in the immature and mature internodal tissues, with sucrose being the dominant sugar in different developmental stages. Sucrose concentration increased gradually from 240 to 390 DAP in both types of cultivars. Comparatively, higher concentration of sucrose was found in the different internodal tissues of high sucrose-accumulating cultivars than in low sucrose-accumulating cultivars from 240 to 390 DAP. At 240 DAP, sucrose concentration in high sugar accumulating cultivars was recorded as 93.0 and 97.0% of the total sugar, which increased to 97.0 and 98.0% at 390 DAP in immature and mature internodal tissues, respectively (Fig. 1a). However, sucrose concentration in low sucrose-accumulating cultivars was found 90.0 and 97.0% at 240 DAP which decreased to 75.0 and 86.0% at 390 DAP in immature and mature internodes, respectively (Fig. 1b). In both the high and low sucrose-accumulating cultivars, hexose sugars decreased with advancement of developmental stages (Fig. 1c, d).
Comparative analysis of two early (high sugar accumulating) and two late (low sugar accumulating) maturing sugarcane cultivars for endogenous free sugar [sucrose in early (a) and late (b) maturing cultivars; hexose sugars in early (c) and late (d) maturing cultivars] content in immature (T) and mature (B) internodal tissues from 240 to 390 DAP (data expressed in mg sugars g−1 fresh weight; bars indicating ±SD of mean values from three independent experiments, the SD values are below 5% in all the cases)
Changes in SAI enzyme activity and transcript levels
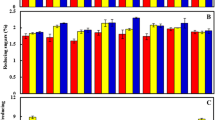
In this study, the relation between sucrose accumulation and SAI enzyme activity was established. The accumulation of sucrose was found to be inversely correlated with the SAI activity. The SAI activity decreased with the increase in DAP; however, the accumulation of sucrose increased with DAP as described above. The SAI activity was higher in immature internodes as compared to mature internodes. However, sugar accumulation was higher in mature internodes. A declining trend of SAI enzyme activity was observed from 240 to 390 DAP in immature and mature internodes of high and low sucrose-accumulating cultivars (Fig. 2a, b). The sugar concentrations in two different sucrose-accumulating cultivars differed predominantly in terms of their sucrose concentration at all developmental stages. There was a negative correlation between SAI enzyme activity and sucrose concentration, whereas a positive correlation with hexose sugars concentration was observed in both immature and mature internodal tissues (Fig. 3a–d).
Comparative analysis of two early (a; high sugar accumulating) and two late (b; low sugar accumulating) maturing sugarcane cultivars for specific SAI activities in immature (T) and mature (B) internodal tissues from 240 to 390 DAP (the unit of enzyme activity is expressed in μ mol g−1 protein min−1; bars indicating ±SD of mean values from three independent experiments, the SD values are below 5% in all the cases)
Relationship between mean sugar concentrations and mean specific SAI enzyme activities in immature and mature internodal tissues (a, b sucrose content and SAI activities in immature and mature internodal tissues, respectively; c, d hexose sugars and SAI activities in immature and mature internodal tissues, respectively)
Further, the changes in SAI enzyme activity were compared with the SAI transcript expression level using semiquantitative PCR. For transcript expression analysis, same cultivars of both groups were taken as were used in enzymatic activity analysis. However, we have showed the data of only one cultivar from each group (CoS 95255 and CoSe 92423), due to identical expression patterns of cultivars within the group. The SAI transcript expression level was observed at different developmental stages (240–390 DAP) in both mature and immature internodes of high and low sugar accumulating cultivars (Fig. 4). SAI transcript was found to be expressed differentially during all the developmental stages in mature and immature internodes. The decline in transcript level was observed with increase in DAP (240–390). This decline was very sharp in high sucrose-accumulating cultivars. However, it was relatively less prominent in low sucrose-accumulating cultivars (Fig. 4).
Transcript expression analysis of SAI genes from 240 to 390 DAP in sugarcane cultivar CoS 96268 (high sucrose accumulating) and CoS 97264 (low sucrose accumulating) using semiquantitative RT-PCR (a, b transcript levels of SAI gene in immature and mature internodal tissues of CoS 96268, respectively; c, d transcript levels of SAI gene in immature and mature internodal tissues of CoS 97264, respectively). All values for densitometric analysis are given in nanogram (ng)
Discussion
The dry matter accumulation and sucrose concentration increase sharply within the immature internodal tissues in developing sugarcane (Moore 1995). Sucrose accumulation is not merely a function of time, since the rate of accumulation significantly increases between young and more mature internodes (Botha and Black 2000). The increase in sucrose content during internodal maturation coincides with a re-partitioning of carbon from insoluble matter and respiration toward sucrose storage (Whittaker and Botha 1997). However, it is well known that significant difference in sucrose concentrations are obvious between different sucrose-accumulating sugarcane cultivars. After arrival of sucrose in stalk from the leaves, it can be hydrolyzed by reversible action of sucrose synthase or one of the three invertases: soluble acid invertase (high in apoplast and vacuoles of young internodes, but virtually less in mature tissue, pH optimum 5.5), cell wall bound insoluble acid invertase (found in all aged tissues; optimum pH 4.5) and a neutral invertase (found in cytoplasm at low concentrations in young tissue and greater concentrations in mature tissue; optimum pH 7.0) (Hawker and Hatch 1965; Glasziou and Gayler 1972). The close relationship between acid invertase activity and sucrose storage in young internodes suggested that sucrose uptake from the apoplast was dependent upon its hydrolysis prior to transfer to the storage compartment of the parenchyma tissue (Sacher et al. 1963). Sucrose synthase also hydrolyzes sucrose, but its activity remains low in storage parenchyma cells compared to vascular strands, in both immature and mature storage tissue. It has been suggested that this enzyme does not catabolize sucrose in the metabolic compartment of parenchyma cells, because a highly specific uridine diphosphatase rapidly hydrolyzes UDP rendering it unavailable for transfer to glucose by sucrose synthase (Moore 1995).
It has been reported that decreasing soluble acid invertase activity correlates with ripening in sink tissues and that the level to which sucrose accumulates may be regulated through the modulation of soluble acid invertase activity and the synthetic reaction of sucrose synthase (Kubo et al. 2001). SAI may play a role in the remobilization of stored sucrose from the vacuole (Sacher et al. 1963) and is also believed to regulate hexose levels in certain tissues. SAI activity shows striking seasonal variation, as it is high when growth is rapid and vice versa (Venkataramana et al. 1991).
In general, the expression of acid invertase genes correlates with the activity of the enzyme, although in certain instances post-translational mechanisms may regulate enzyme function (Rausch and Greiner 2004). To understand the roles of SAI gene expression in SAI enzyme activity and sucrose accumulation in different developmental stages of high and low sucrose-accumulating sugarcane cultivars, we assayed their transcripts by using semiquantitative RT-PCR. The SAI enzyme activity and sucrose contents were measured throughout the developmental stages and co-relation with gene expression was studied. A housekeeping actin gene was taken as internal control in semiquantitative PCR, because it is well characterized and reported in many plants as internal control. The amplification of actin gene for 20 cycles was sufficient to see that the amount of cDNA was taken equally for each analysis day of the respective cultivar. However, the amplification product of SAI transcript was analyzed after 25 and 30 cycles, because after 20 cycles, the concentration of the amplified product was not sufficient to study the differences in expression patterns. The semiquantitative PCR data of immature and mature portion of internode samples (low and high sugar accumulating cultivars) collected at different developmental stages was compared. Generally, the expression of the same gene may be different in different portions of the internodes of the same cultivars; therefore, it was possible that the level of transcript could be different in immature and mature internodes of the same cultivar at different developmental stages. All studied cultivars had their highest activities and transcript expression level of SAI associated with low levels of sucrose concentration in the immature internodal tissues; on the other hand, the lowest levels of SAI activity was found associated with the high levels of sucrose in the mature internodal tissues. The expression pattern of SAI transcript at different developmental stages was correlated with the SAI enzyme activity. The enzyme activity and transcript expression pattern both showed a similar trend. The increased enzyme activity with increase in transcript levels showed a direct correlation between the availability of enzyme and respective mRNA transcript. Further, the result also showed the possibility of in situ synthesis of SAI enzyme (i.e., synthesized in the respective tissue). The major differences among cultivars in SAI activity and sucrose storage included level of SAI activity in the immature internodes, the level of sucrose accumulated in the mature internodes, and developmental timing of the decline in SAI activity with the onset of sucrose accumulation. The relationship between SAI and sucrose was highly significant on the basis of internodal tissues, suggesting a simple, perhaps major, gene difference among the genotypes differing in the level of sucrose stored. The level and timing of sucrose accumulation in the whole stalk and within individual internodes was correlated with the downregulation of SAI activity (Zhu et al. 1997). The differences in the activity of SAI among early and late maturing sugarcane genotypes may be due to differences in the level of expression of essentially identical SAI genes (Zhu et al. 2000). Vacuolar SAI was also expressed highly during the early stage of development in Japanese pear fruit (Pyrus pyrifolia), which also stores sucrose in the vacuole and is similar to sugarcane. The level of SAI mRNA decreased during the fruit maturation stage when sucrose was accumulated (Yamada et al. 2007). Suppression of SAI gene by antisense RNA technology has resulted in increased sucrose content in tomato fruit (Klann et al. 1996), carrot (Tang et al. 1999) and sugarcane (Ma et al. 2000), confirming the role of vacuolar SAI in the control of sucrose accumulation. The limited decrease in SAI activity might be related to high polyploidy level of sugarcane, i.e., occurrence of large SAI gene family.
Thus, in conclusion, the differences were observed in the transcript expression levels of SAI genes and SAI enzyme activity when sugarcane cultivars with different sugar concentrations were compared. The direct correlation between SAI transcript levels and SAI enzyme activity in individual cultivars at different developmental stage suggested that SAI enzyme activity was controlled at the transcriptional level. Improved understanding of expression of SAI might allow the exploitation of their properties to specifically alter sucrose partitioning in internodes of different sucrose-accumulating sugarcane cultivars.
Abbreviations
- SAI:
-
Soluble acid invertase
- DAP:
-
Days after planting
- FW:
-
Fresh weight
References
Batta SK, Singh R (1986) Sucrose metabolism in sugarcane grown under varying climatic conditions: synthesis and storage of sucrose in relation to the activities of sucrose synthase, sucrose phosphate synthase and invertase. Phytochemistry 25:2431–2437
Botha FC, Black KG (2000) Sucrose phosphate synthase and sucrose synthase activity during maturation of internodal tissue in sugarcane. Aust J Plant Physiol 27:81–85
Burg SP, Bieleski RL (1962) The physiology of sugarcane. V. Kinetics of sugar accumulation. Aust J Biol Sci 15:429–444
Carson DL, Botha FC (2002) Genes expressed in sugarcane maturing internodal tissue. Plant Cell Rep 20:1075–1081
Carson DL, Huckett BI, Botha FC (2002) Sugarcane ESTs differentially expressed in immature and maturing internodal tissue. Plant Sci 162:289–300
Casu RE, Jarmey JM, Bonnett GD, Manners JM (2007) Identification of transcripts associated with cell wall metabolism and development in the stem of sugarcane by Affymetrix GeneChip Sugarcane Genome Array expression profiling. Funct Integr Genomics 7:153–167
Echeverria E, Gonzalez PC, Brune A (1997) Characteristics of proton and sugar transport at the tonoplast of sweet lime (Citrus limettioides) juice cells. Physiol Plant 101:291–300
Glasziou KT, Gayler KR (1972) Storage of sugars in stalks of sugarcane. Bot Rev 38:471–490
Grof CPL, Campbell JA (2001) Sugarcane sucrose metabolism: scope for molecular manipulation. Aust J Plant Physiol 28:1–12
Hawker JS, Hatch MD (1965) Mechanism of sugar storage by mature stem tissue of sugarcane. Plant Physiol 18:444–453
Klann EM, Hall B, Bennett AB (1996) Antisense acid invertase (T1V1) gene alters soluble sugar composition and size in transgenic tomato fruit. Plant Physiol 112:1321–1330
Kubo T, Hohjo I, Hiratsuka S (2001) Sucrose accumulation and its related enzyme activities in the juice sacs of Satsuma mandarin fruit from trees of different crop loads. Sci Hortic 91:215–225
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Ma H, Albert HH, Paull R, Moore PH (2000) Metabolic engineering of invertase activities in different subcellular compartments affects sucrose accumulation in sugarcane cells. Aust J Plant Physiol 27:1021–1030
Martinoia E (1992) Transport process in vacuoles of higher plants. Bot Acta 105:232–245
McCormick AJ, Cramer MD, Watt DA (2006) Sink strength regulates photosynthesis in sugarcane. New Phytol 171:759–770
Menossi M, Silva-Filho MC, Vincentz M, Van-Sluys MA, Souza GM (2008) Sugarcane functional genomics: gene discovery for agronomic trait development. Int J Plant Genomics 1:11
Moore PH (1995) Temporal and spatial regulation of sucrose accumulation in the sugarcane stem. Aust J Plant Physiol 22:661–679
Moore PH (2005) Integration of sucrose accumulation processes across hierarchical scales: towards developing an understanding of the gene to crop continuum. Field Crop Res 92:119–135
Nelson N (1944) A photometric adaptation of Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Quick WP, Schaffer AA (1996) Sucrose metabolism in sources and sinks. In: Zamski E, Schaffer AA (eds) Photoassimilate distribution in plants and crops. Marcel Dekker, NY, pp 115–156
Rausch T, Greiner S (2004) Plant protein inhibitors of invertases. Biochim Biophys Acta 1696:253–261
Roe JH (1934) A colorimetric method for the determination of fructose in blood and urine. J Biol Chem 107:15–22
Sacher JA, Hatch MD, Glasziou KT (1963) Sugar accumulation cycle in sugarcane. III. Physical and metabolic aspect of cycle in immature storage tissues. Plant Physiol 39:348–354
Scholes J, Bundock N, Wilde R, Rolfe S (1996) The impact of reduced vacuolar invertase on the photosynthetic and carbohydrate metabolism of tomato. Planta 200:265–272
Singh RK, Singh P, Singh SP, Mohapatara T, Singh SB (2006) Mapping QTLs for sugar content and segregation analysis in sugarcane. Sugar Cane Int 24:7–13
Tang GQ, Luscher M, Sturm A (1999) Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose portioning. Plant Cell 11:177–190
Vattuone MA, Fleischmacher OL, Prado FE, Vinals AL, Sampietro AR (1983) Localisation of invertase activities in Ricinus communis leaves. Phytochemistry 22:1361–1365
Venkataramana S, Naidu MK, Singh S (1991) Invertase and growth factors dependent sucrose accumulation in sugarcane. Plant Sci 74:65–72
Verma AK, Upadhyay SK, Verma PC, Solomon S, Singh SB (2010) Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biol. doi:10.1111/j.1438-8677.2010.00379.x
Walker RP, Pollock CJ (1993) The purification and characterisation of soluble acid invertase from coleoptiles of wheat (Triticum aestivum L. cv. Avalon). J Exp Bot 44:1029–1037
Welbaum GE, Meinzer FC (1990) Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol 93:1147–1153
Whittaker A, Botha FC (1997) Carbon partitioning during sucrose accumulation in sugarcane internodal tissues. Plant Physiol 115:1651–1659
Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolites in spinach leaves. Planta 193:530–535
Yamada K, Kojima T, Bantog N, Shimoda T, Mori H, Shiratake K, Yamaki S (2007) Cloning of two isoforms of soluble acid invertase of Japanese pear and their expression during fruit development. J Plant Physiol 164:746–755
Zhu YJ, Komor E, Moore PH (1997) Sucrose accumulation in the sugarcane stem is regulated by the difference between the activities of soluble acid invertase and sucrose phosphate synthase. Plant Physiol 115:609–616
Zhu YJ, Albert HH, Moore PH (2000) Differential expression of soluble acid invertase (SAI) genes correlates to differences in sucrose accumulation in sugarcane. Aust J Plant Physiol 27:193–199
Acknowledgments
The authors are grateful to the Sugar Development Fund, Ministry of Food and Consumer Affairs under the Government of India for the research grant provided for the research project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Sowinski.
Rights and permissions
About this article
Cite this article
Verma, A.K., Upadhyay, S.K., Srivastava, M.K. et al. Transcript expression and soluble acid invertase activity during sucrose accumulation in sugarcane. Acta Physiol Plant 33, 1749–1757 (2011). https://doi.org/10.1007/s11738-011-0711-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0711-2